Abstract
The intestine is a critical site of immune cell development that not only controls intestinal immunity but extra-intestinal immunity as well. Recent findings have highlighted important roles for gut microbiota in shaping lung inflammation. Here, we discuss interactions between the microbiota and immune system including T cells, protective effects of microbiota on lung infections, the role of diet in shaping the composition of gut micro-biota and susceptibility to asthma, epidemiologic evidence implicating antibiotic use and microbiota in asthma and clinical trials investigating probiotics as potential treatments for atopy and asthma. The systemic effects of gut microbiota are partially attributed to their generating metabolites including short chain fatty acids, which can suppress lung inflammation through the activation of G protein-coupled receptors. Thus, studying the interactions between microbiota and immune cells can lead to the identification of therapeutic targets for chronic lower respiratory diseases.
Keywords: Asthma, Diet, Intestine, Microbiota, T cell
Introduction
Our body surfaces are inhabited by trillions of microorganisms collectively referred to as commensal and symbiotic microbiota. Recent advances in next-generation sequencing have led to a more complete profile of microbial species diversity and prevalence on the skin, mouth, respiratory, gastrointestinal (GI) and urogenital tracts. This has improved our understanding of host–microbe interactions as they relate to health and disease. Microbial dysbiosis in the GI tract contributes to several disorders, including inflammatory bowel disease (IBD), asthma and obesity [1]. Although the gut-lung axis has long been associated with respiratory diseases [2, 3], current studies are elucidating the mechanisms of how microbiota regulate lung inflammation. Such information is useful for considering the use of probiotic and/or prebiotic therapies for lung disease. In this review, we introduce the role of gut microbiota on intestinal immunity and lung infections, highlight human evidence supporting protective roles for the gut micro-biome in asthma, discuss dietary factors that may contribute to disease and review mechanistic data from pre-clinical animal models on how gut microbes regulate lung inflammation.
Asthma
Asthma is a complex disease involving intermittent wheezing, chest tightness, cough, dyspnea, airway obstruction and bronchial hyperresponsiveness [4]. Recently, the field has attempted to classify atopic and non-atopic asthma as being driven by type 2 or non-type 2 inflammation, respectively. Atopy is associated with an earlier onset of asthma and is measured by type I (immediate) hypersensitivity to allergens by skin testing, or increased frequencies of allergen specific or total serum IgE. Later-onset asthma is associated with more severe airflow limitation, less allergy, eosinophilia and Th2 cytokines [4]; however, there is significant overlap between atopic and non-atopic asthma and these two classifiers are not fully separated by type 2 cytokines. Asthma comorbidities include rhinitis, obesity [5], gastroesophageal reflux disease and vitamin D deficiency. Studies in the 1940s demonstrated that penicillin aerosol therapy could improve symptoms in patients with chronic inflammatory disorders such as bronchiectasis, non-atopic asthma, or chronic bronchitis [6, 7], indicating that airway microbes contribute to lung inflammation. The GI tract harbors the largest collection of microbes and immune cells in our body, and pulmonary manifestations are commonly associated with gut inflammation [8]. As described below, emerging evidence in mouse models and humans demonstrate that intestinal microbiota have a significant impact on lung inflammation.
Intestinal colonization
The microbiota we acquire soon following birth remains remarkably constant throughout our lives, and bacteria species are geospatially regulated. The stomach, duodenum and proximal jejunum are mainly colonized with aerobic bacteria including Streptococci spp. and Lactobacilli spp. [9], while the distal ileum has more anaerobes that resemble those found in the colon (Bacteroides, Bifidobacterium, and Clostridium spp.). We inherit our microbiomes from our mothers and the mode of delivery influences its composition. Babies born by conventional vaginal delivery have increased colonization with Prevotella and Lactobacillus spp. in the mouth and skin, linked to colonization of the mother’s urogenital tract with these species [13]. In contrast, Staphylococcus spp. are more prevalent in babies delivered by C-section, linked to skin colonization in mothers. Other bacteria increased in C-section babies include Bacillales, Propionibacterineae, Corynebacterineae and Acinetobacter spp [10]. The most profound differences in intestinal bacteria colonization between babies born by C-section or vaginal delivery are observed during their first year of life [11]. Vaginal delivery has also been linked to higher levels of fecal Clostridia [12]. Work in murine models has demonstrated that Clostridia spp. promote the development of anti-inflammatory regulatory T cells (Tregs) through the generation of short chain fatty acids (SCFAs) such as butyrate [13], and Tregs are critical for tolerance to inhaled allergens due to their suppression of pro-inflammatory CD4 T helper cell responses [14, 15]. The induction of Tregs may explain why intestinal Clostridia spp. are associated with protection against wheezing [16]. In addition, Escherichia colonization may protect against asthma by inducing monocytes to secrete IL-10 [17], a suppressive cytokine that promotes tolerance by inhibiting T cell costimulation [14]. Some intestinal pathogens may be associated with airway inflammation. For instance, intestinal colonization with C. difficile at 1 month of age was linked to wheezing and eczema throughout the first 6–7 years of life as well as childhood asthma [18].
During IBD, decreased colonization with Lachnospiraceae and Bacteroidales spp. are linked to reductions in SCFAs [19, 20]. Patients with diversion colitis were found to have negligible levels of SCFAs, and instillation of SCFAs (acetate, propionate, butyrate) resulted in the disappearance of symptoms [21]. A gnotobiotic mouse model revealed that the SCFA acetate can ameliorate colitis in a GPR43-dependent manner [22]. GPR43 is predominately expressed in immune cells and one of several G protein-coupled receptors that detect free fatty acids produced by fermentation, resulting in IP3 formation, intracellular Ca+2 mobilization, extracellular signal regulated kinase 1/2 activation and inhibition of cAMP accumulation [23]. Experimental allergy models also demonstrate therapeutic potential for SCFAs in decreasing allergic airway inflammation (AAI) [22, 24]; however, GPR41 rather than GPR43 was found to be required for the anti-inflammatory effects of SCFAs in lungs [24]. The mechanism of how SCFAs inhibit airway inflammation could be multi-factorial but may involve acetylation of the Foxp3 promoter in T cells [25], leading to increased Foxp3 expression and suppressive function of Tregs.
Local effects of intestinal bacteria on the immune system
Gut bacteria help to protect against pathogenic infections through competition, antimicrobial peptide secretion, innate immune cell stimulation, lymphoid tissue development, antibody production and T cell differentiation [26–28]. In addition, the microbiota contributes to food digestion and metabolism. For example, anaerobic bacteria ferment dietary fibers into SCFAs [29], which have many effects including stimulating incretin secretion [30], providing a source of energy, inhibiting fatty acid oxidation and cholesterol synthesis, and activating G protein-coupled receptors (GPR40-43) [29] which regulate immune function. For instance, GPR41 and GPR43 have been shown to inhibit immune cell recruitment to the intestine and lungs [22, 24]. Having a diverse microbiota is beneficial for promoting host defense and metabolism. Antibiotic use can disrupt the intestinal epithelial barrier and increase susceptibility to infections with Salmonella, Shigella and Clostridium difficile [28]. Impaired mucosal barriers also lead to microbial translocation and immune sensitization against innocuous micro-biota and subsequent IBD [31].
Genetic determinants impact host-microbe interactions on several levels, including the mucosal barrier, phagocytosis and bacteria killing, inflammatory cytokine secretion, adaptive immunity or immunosuppression. The host maintains a physical separation of intestinal microbiota and the epithelium in part due to the production of large amounts of secretory IgA [32], defensins and regenerating islet-derived proteins [33]. Host-microbe interactions are facilitated by pattern recognition receptors, several of which are implicated in IBD [34]. For example, mutations in NOD2, which recognizes the bacterial product muramyl dipeptide, are associated with Crohn’s disease (CD) [35], decreased α-defensin release by Paneth cells and microbial dysbiosis.
Proinflammatory cytokine production by innate immune cells in response to toll-like receptor (TLR) ligands or TNF family receptors may also contribute to IBD. Tumor necrosis factor (TNF)-α has been strongly implicated in IBD pathogenesis and is the foundation of current biologic therapy [36]. CD40 stimulation resulting in IL-23 production is capable of driving a T cell-independent form of colitis [37]. The anti-inflammatory cytokine IL-10 has a protective role against IBD in part by suppressing inflammatory responses to TLR ligands [38], and IL-10-deficiency results in spontaneous enterocolitis in a microbiota-dependent manner [39, 40]. Mechanistically, the direct action of IL-10 on macrophages is necessary for suppressing enterocolitis [38].
In addition to innate immune activation, tissue damage in Crohn’s Disease (CD) is also thought to be caused by pro-inflammatory T cell responses against microbes [41]. For instance, T cells from inflamed intestines of IBD patients proliferate in response to intestinal bacteria antigens [42]. Murine models show that several CD4+ T cell subsets (Th1, Th2, Th17) cause gut inflammation [43–46], while CD4+ CD25+ Tregs are protective against disease [47]. The resistance of germ-free mice to IBD demonstrates a microbial component to disease induction [31]. Towards this, microbiota helps to strengthen the intestinal epithelial barrier. Enteroinvasive pathogens may compromise the barrier and introduce microbiota into the draining mesenteric lymph nodes, resulting in CD4+ T cell activation against the flora and gut inflammation [48]. Thus, maintaining a healthy barrier may be critical in reducing susceptibility to IBD.
Th17 cells are of particular interest due to their roles in mucosal immunity. While IL-17 receptor signaling on gut epithelium induces inflammation and neutrophil recruitment [46], it also promotes secretory IgA transcytosis [49], strengthening the microbe-epithelial barrier. Another cytokine produced by Th17 cells, IL-22, is important for epithelial repair following injury [50]. Many species of microbiota in mice and humans have been shown to positively or negatively regulate Th17 cell development in the intestine [51–53]. The reciprocal relationship between Th17 cells and Tregs suggest the balance of intestinal CD4+ T helper subsets influences susceptibility to IBD [54]. The pathogenesis of CD depends upon complex interactions between host genetics, environmental triggers, immune responses and microbiota. Chronic infections or dysbiosis, i.e. an altered composition or metabolic function of enteric microbes, are thought to underlie CD pathogenesis. The same changes caused by these factors on intestinal health also influence susceptibility to chronic lower respiratory diseases.
Systemic effects of gut microbiota on lung infections (animal studies)
There is emerging evidence that the role of gut microbiota on immunity extends beyond the GI tract. Antibiotic drinking water is commonly used to study the systemic effects of gut micro-biota during lung infections, and increases viral titers during experimental Lymphocytic choriomeningitis virus (systemic) or influenza (lung) infections [55]. The increased viral load was associated with fewer viral antigen-specific T cells and IgG antibodies. In the influenza model, antibiotic-treated mice also had greater weight loss and mortality compared to non-antibiotic treated mice. This was attributed to decreased expression of type I interferon-dependent genes in response to infection, suggesting that microbial products epigenetically modified macrophages/dendritic cells regulate anti-viral immunity [55]. Further, GF mice have been shown to be more susceptible to pulmonary infection with K. pneumoniae [56]. This increased susceptibility could be reversed by TLR activation, suggesting that microbial stimuli set the tone of the innate immune response, consistent with “trained” immunity [57]. During acute fungal infection in the lung, antibiotic containing drinking water was found to decrease lung Th17 cell accumulation, which correlated with decreased intestinal segmented filamentous bacteria (SFB) colonization [58]. Serum from SFB-colonized mice increased lung Th17 cell numbers when transferred to SFB-negative recipients during infection, demonstrating a role for soluble factors driving the lung Th17 response. This effect of serum was dependent on IL-1, as pre-incubation of serum from SFB-colonized mice with an IL-1 receptor antagonist significantly decreased lung Th17 cell accumulation [58]. In this model, the respective contributions of IL-α or β remain to be determined. During pulmonary Staphylococcus aureus infection, intestinal SFB colonization was found to be protective by augmenting IL-22 secretion in bronchoalveolar lavage [59]. Additionally, in a murine model of pneumococcal pneumonia, a diverse gut micro-biota was shown to protect against mortality [60]. In that study, antibiotic-treated mice had increased pneumococcal burdens in the lungs and blood, and decreased levels of TNF and IL-10 compared to control mice. Alveolar macrophages from the microbiota-depleted mice were hyporesponsive to TLR ligands and also had a diminished capacity for phagocytosing S. pneumoniae. Thus, gut microbiota were essential for normal macrophage function in this model. Overall, these findings demonstrate a critical role for gut microbes in pulmonary host defense, and suggest that tonic signaling of pattern recognition receptors by microbial products in the steady state may also contribute to chronic lower respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD) and chronic bronchitis.
Diet, microbiota, and asthma (pre-clinical evidence)
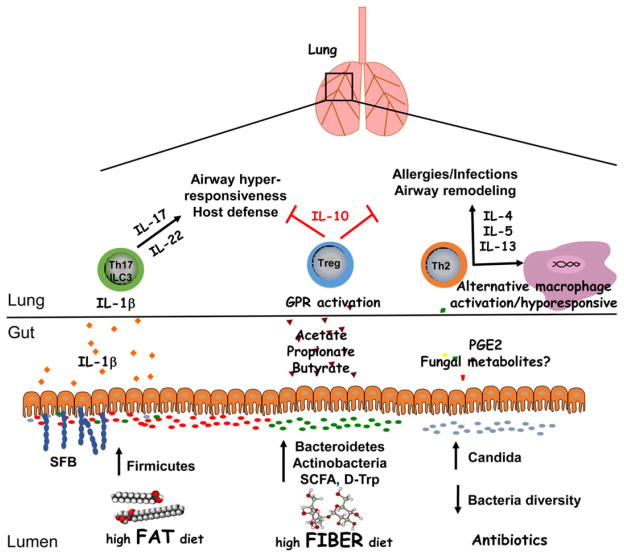
Obesity is associated with an abnormal microbiome and has been strongly linked to asthma. Using a murine model of dietary fat-induced obesity, Kim et al. found that a high-fat diet increased the number of group 3 innate lymphoid cells (ILCs) producing IL-17 in the lung and increased airway hyperresponsiveness (AHR) to cholinergic stimuli (Fig. 1) [61]. Although the NLRP3 inflammasome was required to increase group 3 ILC numbers, the specific role of the microbiota was not assessed. Interactions between microbiota and intestinal epithelial cells (IECs) have also been shown to regulate lung pro-inflammatory responses, including IL-17 production, in response to aero-allergens [62]. IEC-specific deletion of IKKβ, an inhibitor of the NF-κB transcriptional complex, was associated with an increased ratio of Clostridia to Bac-teroida and increased expression of Il17a and Ifng in lung tissue following allergen challenge. In contrast to a high-fat diet, high fiber diets suppress airway allergic responses, including type-2 responses such as lung eosinophilia, goblet cell metaplasia, as well as allergen-specific IgE and Th2 cytokines [63]. Protection in this model was associated with increased colonic Bacteroidetes and Actinobacteria species and decreased Firmicutes and Proteobacteria, suggesting that a high fiber diet may mitigate airway inflammation through modulation of the gut microbiota (Fig. 1). In support, administration of probiotics containing Bifidobacterium breve with or without non-digestible oligosaccharides was shown to attenuate AHR, Th2 activation, eosinophilia, and allergen-specific antibodies (IgE, IgG1) [64, 65]. Supplementation with B. breve increased expression of Il10 and Foxp3 transcripts in lung tissue. Administration of Bacteroides fragilis capsular polysaccharide PSA during experimental asthma produced similar results, suppressing cellular inflammation, IFN-γ production from T cells and increasing IL-10 [66]. These findings suggest that dietary-induced changes in microbiota regulate lung inflammation.
Figure 1.
Regulation of lung inflammation by intestinal microbiota. A diverse intestinal microbiota supports immune functions that are critical for maintaining homeostasis in the lungs. High fiber diets can increase the prevalence of Bacteroidetes and Actinobacteria species as well as the production of short chain fatty acids, which protect against airway inflammation through the induction of Tregs. Dysbiosis resulting from dietary fat or antibiotic use enhances lung inflammation in response to allergens or infections. Notably, an increased ratio of Firmicutes/Bacteroidetes species as well as segmented filamentous bacteria (SFB) colonization is associated with increased lung IL-17 and IL-22 responses. While these cytokines are important for host defense, they may contribute to AHR when directed against innocuous antigens. Antibiotic use can result in intestinal fungal overgrowth and increased blood concentrations of PGE2, leading to Th2 cell differentiation and alternative macrophage activation. Thus, antibiotics may inhibit the phagocytic capacity of alveolar macrophages, increasing susceptibility to opportunistic infections in the lungs, and promote Th2 responses to allergens. Overall, a lack of intestinal bacteria diversity may contribute to airway remodeling in patients with asthma. Future studies into the mechanistic relationship between diet, microbiota, genetics and lung inflammation may involve gnotobiotic models as well studying the effects of microbial metabolites on cell populations.
Several studies have examined the role of metabolites in experimental asthma. Microbial changes induced by a high fiber diet increase serum levels of SCFAs (Fig. 1), resulting in the suppression of AAI [24]. This anti-inflammatory effect of high fiber diet could be transferred in utero [25]. Notably, treatment of pregnant mice with acetate increased acetylation of the Foxp3 promoter in both adults and their offspring. This enhanced Treg function was due to the inhibition of histone deacetylase activity. Another microbial metabolite, D-tryptophan (Fig. 1), increases gut and lung Treg cell numbers when fed to mice, and has been shown to decrease lung Th2 responses and block AAI and AHR [67]. Kefir, a symbiont mixture containing Lactobacillus, Lactococcus, Leuconostoc, Acetobacter and Streptococcus spp. as well as yeasts has also been shown to attenuate AAI in the ovalbumin-induced model of airway allergic inflammation [68]. Overall, these data demonstrate that diet and probiotics regulate lung inflammation through the induction of metabolites and Tregs.
Antibiotics have profound effects on the gut microbiota and their use in children has been implicated in asthma. To model this, Russell et al. used neonatal mice treated with oral streptomycin or vancomycin prior to induction of murine allergic asthma [69]. Vancomycin reduced microbial diversity and Treg cell numbers in the colon, enhancing allergic disease severity, whereas streptomycin had minimal effects. In contrast to neonatal mice, antibiotic administration in adult mice had no effect on the development of allergic asthma, suggesting a critical window of susceptibility. One mechanism by which dysbiosis caused by broad-spectrum antibiotic treatment promotes AAI may be by inducing alternatively activated macrophages in bronchoalveolar lavage [70]. Antibiotic use has been linked to intestinal overgrowth of Candida species and increased plasma levels of PGE2 (Fig. 1). In this model, administration of a Cox-2 inhibitor suppressed macrophage polarization and decreased airway inflammation [70]. Antibiotic treatment, followed by colonization with Candida albicans, resulted in GI fungal overgrowth and enhanced pulmonary Th2 responses to another fungal species upon intranasal challenge, Aspergillus fumigatus [71]. The ovalbumin model of allergic asthma revealed that iNKT cells accumulate in the colonic lamina propria and lungs of GF mice, exacerbating disease [72]. This was associated with increased levels of CXCL16 in the colon, serum and lungs. Overall, these findings demonstrate that a diverse gut microbiota helps to suppress AAI, and age-sensitive contact with microbiota may be critical for establishing iNKT tolerance to later environmental exposures to allergens.
Regulatory T cells (Tregs) are critical for preventing allergic inflammation and maintaining self-tolerance, and can develop in the thymus or extrathymically from mature CD4+ T cells, known as inducible Tregs (iTregs). Mice deficient in iTregs spontaneously developed Th2 pathologies in the GI tract and lungs, with hallmarks of AAI and asthma [44]. iTreg deficiency has been shown to increase the ratio of Bacteroidetes to Firmicutes species, demonstrating that extra-thymic Treg differentiation regulates micro-biota composition and restrains allergic type inflammation at mucosal surfaces. In support of this, animal models of asthma have found that feeding with Bifidobacterium or C. leptum increases Tregs and attenuates AAI [73–75].
Helicobacter pylori colonizes the upper GI tract in some people, and oral or intraperitoneal delivery of H. pylori extract prevented AHR, eosinophilia, lung inflammation, goblet cell metaplasia and cytokine production from both Th2 and Th17 cells [76, 77]. Protection in this model was most robust in mice infected neonatally and the protective effect was abrogated by antibiotic treatment. Further, protection could be adoptively transferred by Tregs from infected mice, implicating these cells as critical effectors. Lung CD103+ DCs have been reported to induce Treg differentiation through retinoic acid production [78]. Adults with asthma have been found to have lower levels of IgG antibodies against H. pylori, suggesting that H. pylori is a marker for protection against asthma [79]. Humans infected with H. pylori have significantly higher Treg frequencies in peripheral blood compared to uninfected controls [80]. H. pylori can also inhibit Th2-mediated bronchial inflammation through the secretion of the TLR2 agonist, neutrophil activating protein, which promotes Th1 responses [81]. These findings may explain the inverse relationship between H. pylori infection and asthma in U.S. adults [82], and identifying products from H. pylori that induce Tregs could have therapeutic value.
Microbiota and asthma (human evidence)
Several human studies have demonstrated a correlation between gut microbial diversity and asthma. Maternal antibiotic use during pregnancy, including cephalosporins, macrolides, and penicillins, is associated with an increased risk of childhood asthma [83–85]. In children, the use of any antibiotic during the first year of life also increased the risk of asthma. Specifically, macrolide use between ages 2–7 resulted in a long-lasting shift in microbiota (decreased Actinobacteria, increased Bacteroidetes and Proteobacteria), metabolism (decreased bile salt hydrolase), and increased prevalence of asthma [86]. Colonization with fewer bacteria species during the first month of life was found to correlate with an asthma diagnosis at age 7 [87]. While the prevalence of Bifidobacterium longum was higher in samples from non-wheezing infants, B. breve was more abundant in wheezing infants [88]. Infants at risk of asthma had a transient reduction in Lachnospira, Veillonella, Faecalibacterium and Rothia spp. during the first 100 days of life, reduced fecal acetate and increased urinary microbe-derived metabolites [89]. A gnotobiotic mouse model demonstrated that these four species can ameliorate allergic airway inflammation (AAI), suggesting that gut microbiota regulate the threshold of allergic sensitization. Another study found that a neonatal group at high risk for atopy and asthma had increased intestinal colonization with Candida and Rhodotorula species and decreased colonization with Bifidobacterium, Akkermansia and Faecalibacterium [90]. This microbial profile was associated with increased production of the pro-inflammatory metabolite 12,13 DiHOME. In a different cohort of children ages 6–17, there was no association between gut microbiota and asthma [91]. Adults with long-term asthma were found to have a decreased abundance of Bifidobacteria spp. [92]. Together, these data support the hypothesis that microbial colonization during a developmental window early in life protects against childhood asthma.
The hygiene hypothesis states that infections during a developmentally protective time window in infancy will prevent asthma from developing several years later. Asthma was inversely related to bacterial species in mattress dust and to a lower extent in nasal samples [93], suggesting that inhalation of bacteria that do not colonize nasal passages contribute to airway inflammation. Another study found that Cyanobacteria and Proteobacteria were abundant in dust from the homes of patients with asthma [94]. Although this does not demonstrate a cause-effect relationship, the data suggest environmental triggers may contribute to the initiation or development of asthma in susceptible individuals. Although having older siblings is associated with increased intestinal microbial diversity and richness during childhood [95], there were no associations found between gut microbiota and asthmatic bronchitis during early childhood.
Due to the complexity of asthma, its etiology is usually multifactorial. The relationship between these factors was studied in a population-based birth cohort study as part of the Prevention of RSV: Impact on Morbidity and Asthma study [96]. This study found that several factors including maternal urinary tract infection during pregnancy, antibiotic use, C-section delivery, or Group B streptococcus colonization were associated with an increased risk for childhood asthma, highlighting the complexity of disease. This study found that having older siblings at home decreased the risk, and infant antibiotic use was the greatest predictor of childhood asthma. In another birth cohort study in Belgium, colonization with Clostridium coccoides XIVa and Bacteroides fragilis was associated with a positive asthma predictive index [97].
As mentioned above, a critical factor that separates host cells from interacting with the gut microbiota is secretory IgA. To better understand how sIgA regulates the microbiota, labs have used Ig-seq to identify which microbial species are recognized. Dzidic et al. used this technique in children’s stool at 1 and 12 months of age, and found that lower amounts of bacteria-specific IgA was associated with increased allergic symptoms and asthma at 7 years of age [98]. These data indicate that an aberrant mucosal IgA response may be another deciding factor in controlling the susceptibility to asthma.
Human studies on probiotics, prebiotics and helminths
Microorganisms are well known to have beneficial effects in our GI tract. Some of these species, termed probiotics, are consumed with hopes of improving digestive health or counteracting inflammatory diseases associated with dysbiosis. In 2012, the World Allergy Organization concluded that probiotics do not have an established role in prevention or treating allergy [99], and no probiotic has been demonstrated to efficiently influence the course of any allergic manifestation or longterm disease. Since then, several studies have assessed the therapeutic efficacy of probiotics and/or prebiotics, referred to as synbiotic treatments, on chronic lung inflammation. Although a comprehensive discussion of this is outside the scope of our review, we highlight some recent studies to illustrate the current trends in clinical research.
Prebiotics contain dietary fibers, including fructo- and galacto-oligosaccharides, that are selectively fermented by beneficial intestinal bacteria [100]. Infants at risk for atopy were fed a formula containing prebiotic oligosaccharides or the digestible sugar maltodextrin as a control [101]. The prebiotic mixture was found to significantly decrease plasma concentrations of immunoglobulin free light chains, a marker for atopic dermatitis [101]. Another study found that prebiotic treatment attenuated exercise-induced bronchoconstriction and serum levels of CCL17 and TNF [102]. In asthmatic subjects, a single dose of inulin with probiotics significantly decreased airway inflammation markers and increased expression of the SCFA receptors GPR41 and GPR43 in sputum four hours later [103]. This correlated with significantly increased FEV1 measurements. Mouse models have corroborated these results by demonstrating that prebiotic diets decrease AAI, AHR and inflammatory cells [104–106]. Therefore, soluble fiber appears to have acute anti-inflammatory effects in asthmatic airways.
Some studies have found that probiotic supplementation improves correlates of protection for asthma. For instance, feeding human volunteers with Bifidobacterium infantis increased the expression of Treg markers (IL-10, Foxp3) in peripheral blood and during in vitro culture with B. infantis [107]. This was dependent on TLR and IDO expression by dendritic cells. Another study found that treating children with mild persistent asthma, ages 6–14, with L. reuteri for 60 days reduced bronchial inflammation as measured by FeNO [108]. Although several mouse studies demonstrate that probiotic species (Bifidobacterium lactis, Saccharomyces cerevisiae, Clostridium butyricum, Lactobacilli) inhibit AAI [109–114], most human data suggests they are not sufficient to reduce asthma-related events [115, 116]. Perhaps individuals already sensitized to allergens are less likely to have the symptoms of asthma and/or allergy reversed by probiotic treatment.
Synbiotic supplementation, in which probiotics are used in combination with food products, has been shown to significantly improve lung function in children with asthma while reducing asthma-like symptoms and the use of medications [117, 118]. Some data suggests that the efficacy of synbiotics is age-dependent. While L. reuteri use during pregnancy and throughout the first year of life significantly reduced the incidence of IgE-associated eczema in children at age 2 [119], there was no effect on asthma prevalence at age 7 [120]. Other studies have also found no significant effect of perinatal synbiotic use on airway inflammation, lung function or asthma at ages 5 to 6 [121, 122]. These data suggest the beneficial anti-inflammatory effects of synbiotics are transient and may require continuous use. In adults with established asthma, the combination of non-digestible oligosaccharides and B. breve significantly increased peak expiratory flow (PEF), correlating with decreased serum IL-5 concentrations, although eosinophilia and forced expiratory volume 1 (FEV1) measurements were unaffected [123]. Thus, synbiotics can improve some but not all of the parameters associated with asthma pathogenesis, and the best results might be obtained through its use as an adjunctive therapy rather than stand-alone treatment. Consistent with this, Clostridium butyricum supplementation with antigen-specific immunotherapy reduced total asthma clinical score and specific IgE, while increasing IL-10 production from B cells [124]. This effect of C. butyricum could be mimicked by butyrate. Another study found that using probiotics in combination with sublingual immunotherapy in children (ages 5–12) sensitive to grass pollen with allergic rhinitis significantly increased Treg induction [125]. Overall, some studies have shown that synbiotics hold promise to suppress allergic responses and asthmatic inflammation in conjunction with other immunotherapies.
Parasitic helminths have immunosuppressive effects in the intestine and have been investigated as treatments for inflammatory disorders including IBD, multiple sclerosis, asthma and atopy [126]. Schistosoma mansoni antigens can increase Treg frequencies and IL-10 production from PBMCs isolated from patients with asthma [127]. Likewise, stimulation of PBMCs with an excretory/secretory antigen from Marshallagia marshalli decreased expression of IFN-γ and IL-4 and increased expression of IL-10 and TGF-β [128]. A murine model demonstrated that chronic infection with Heligmosomoides polygyrus bakeri attenuates AAI [129]. Infection altered the intestinal microbiota and increased SCFA production. Further, helminth-modified micro-biota could transfer protection against allergic asthma in GF recipient mice [129]. The profound anti-inflammatory effects of helminth infection on AAI required GPR41 signaling, suggesting a role for SCFAs. Thus, helminths may directly or indirectly suppress inflammation through Treg induction or modulating the balance of microbial species, respectively. These data also suggest that products from helminths have the potential for development as therapeutics.
Concluding remarks/future directions
Our gut microbiota is part of a complex ecosystem that regulates systemic inflammation in addition to other vital processes. Identifying the mechanisms of how microbiota influence asthma susceptibility requires a holistic approach to account for the many factors driving the allergen sensitization, re-exposure, hyper-responsiveness and airway remodeling phases of disease. Studies described in this review point towards a model in which our environment and genetics determine the composition of our micro-biota, which directly or indirectly stimulate our immune system through TLR ligands or metabolic products, respectively (Fig. 1). For instance, the increased ratio of Bacteroidetes to Firmicutes species in response to high fiber diets will increase the production of SCFAs that suppress inflammation by activating GPR40–43 [22, 24, 29, 63]. In future studies, it will be important to delineate which cell types must express individual GPRs in order to suppress airway inflammation and induce the generation of Tregs. In contrast, high fat diets are associated with activation of the NLRP3 inflammasome in lung tissue and increased IL-1β which promotes AHR [61]. Although the mechanistic link between high fat diets and lung inflammation remains unclear, it is intriguing to hypothesize that the gut microbiota may influence the tone of the acute phase response. Quinton et al. reported that liver expression of RelA, encoding a component of the NF-κB transcriptional complex, and Stat3 are required for the acute phase response and host defense during pulmonary S. pneumoniae infection [130]. Airway macrophages from mice unable to elicit an acute phase response had decreased expression of Il6 and Cxcl1 [131], demonstrating a role for hepatocytes in conditioning alveolar macrophages to respond to lung infections. Thus, microbial products that activate NF-κB in hepatocytes could impact alveolar macrophage function for example. Other data show that a lack of microbial diversity promotes alternative macrophage activation in the lungs and type 2 inflammation, possibly due to intestinal Candida overgrowth [70]. Thus, factors that contribute to the gut epithelial barrier and control microbial translocation across the GI tract may have unappreciated roles in systemic inflammation and host defense.
There has been significant progress in understanding the development of the intestinal microbiota and intestinal immune system. There is strong epidemiologic evidence that this development also impacts allergic diseases including atopic dermatitis and asthma. The influence of intestinal microbiota on exacerbations that are distinct from disease development remains an understudied area. As cited above, there have been several interventional trials to manipulate the microbiota, and these studies report varied effects on allergic diseases as well as asthma. Future studies will likely need to precisely define disease endotypes in order to detect which patients may benefit from prebiotics/synbiotics in addition to other ongoing therapies. Although microbial diversity in the GI tract promotes health, specific functions for individual species are not clear. Towards this, metagenomics studies may reveal important metabolic pathways that can be manipulated for treating or preventing the development of allergic lung disease.
Acknowledgments
This work was supported in part by a grant from the NIH R37 HL079142 (JKK) and funding from Marshall University School of Pharmacy (JPM).
Abbreviations
- AAI
Allergic Airway Inflammation
- AHR
Airway Hyper-responsiveness
- CD
Crohn’s Disease
- COPD
Chronic Obstructive Pulmonary Disease
- FEV1
forced expiratory volume 1
- GF
germ-free
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- PEF
peak expiratory flow
- SCFAs
short chain fatty acids
- SFB
segmented filamentous bacteria
- Treg
regulatory T cell
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Ferreira CM, Vieira AT, Vinolo MA, Oliveira FA, Curi R, dos Martins FS. The central role of the gut microbiota in chronic inflammatory diseases. J Immunol Res. 2014;2014:689492. doi: 10.1155/2014/689492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraft SC, Earle RH, Roesler M, Esterly JR. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976;136:454–459. [PubMed] [Google Scholar]
- 3.von Wichert P, Barth P, von Wichert G. Tracheal and bronchial involvement in colitis ulcerosa - a colo-bronchitic syndrome? A case report and some additional considerations. Ger Med Sci. 2015;13:Doc03. doi: 10.3205/000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corren J. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discov Med. 2013;15:243–249. [PubMed] [Google Scholar]
- 5.Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther. 2013;26:455–458. doi: 10.1016/j.pupt.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barach AL, Garthwaite B. Physiologic and antibiotic therapy of intractable bronchial asthma. Ann Allergy. 1947;5:297–316. [PubMed] [Google Scholar]
- 7.Garthwaite B, Barach AL. Penicillin aerosol therapy in bronchiectasis, lung abscess and chronic bronchitis. Am J Med. 1947;3:261–293. doi: 10.1016/0002-9343(47)90158-7. [DOI] [PubMed] [Google Scholar]
- 8.Vutcovici M, Brassard P, Bitton A. Inflammatory bowel disease and airway diseases. World J Gastroenterol. 2016;22:7735–7741. doi: 10.3748/wjg.v22.i34.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balfour Sartor R. Bacteria in Crohn’s disease: mechanisms of inflammation and therapeutic implications. J Clin Gastroenterol. 2007;41(Suppl 1):S37–S43. doi: 10.1097/MCG.0b013e31802db364. [DOI] [PubMed] [Google Scholar]
- 10.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 11.Stokholm J, Thorsen J, Chawes BL, Schjorring S, Krogfelt KA, Bonnelykke K, Bisgaard H. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138:881–889. e882. doi: 10.1016/j.jaci.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53:1388–1389. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 14.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3:216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126:2394–2403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A longitudinal analysis on the association between antibiotic use, intestinal microflora, and wheezing during the first year of life. J Asthma. 2008;45:828–832. doi: 10.1080/02770900802339734. [DOI] [PubMed] [Google Scholar]
- 17.Orivuori L, Mustonen K, de Goffau MC, Hakala S, Paasela M, Roduit C, Dalphin JC, et al. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin Exp Allergy. 2015;45:928–939. doi: 10.1111/cea.12522. [DOI] [PubMed] [Google Scholar]
- 18.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, Reijmerink NE, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–955. e941–943. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treem WR, Ahsan N, Shoup M, Hyams JS. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994;18:159–164. doi: 10.1097/00005176-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 22.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–1851. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 24.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 25.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. doi: 10.1038/ncomms8320. https://doi.org/10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarchum I, Pamer EG. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol. 2011;23:353–360. doi: 10.1016/j.coi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15:189–196. doi: 10.1007/s11154-014-9288-6. [DOI] [PubMed] [Google Scholar]
- 31.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 32.Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh D, McCarthy J, O’Driscoll C, Melgar S. Pattern recognition receptors–molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev. 2013;24:91–104. doi: 10.1016/j.cytogfr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Al Nabhani Z, Dietrich G, Hugot JP, Barreau F. Nod2: The intestinal gate keeper. PLoS Pathog. 2017;13:e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugliese D, Felice C, Papa A, Gasbarrini A, Rapaccini GL, Guidi L, Armuzzi A. Anti TNF-alpha therapy for ulcerative colitis: current status and prospects for the future. Expert Rev Clin Immunol. 2017;13:223–233. doi: 10.1080/1744666X.2017.1243468. [DOI] [PubMed] [Google Scholar]
- 37.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 38.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 39.Eun CS, Mishima Y, Wohlgemuth S, Liu B, Bower M, Carroll IM, Sartor RB. Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10−/ − mice. Infect Immun. 2014;82:2239–2246. doi: 10.1128/IAI.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 42.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iijima H, Takahashi I, Kishi D, Kim JK, Kawano S, Hori M, Kiyono H. Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor alpha chain-deficient mice. J Exp Med. 1999;190:607–615. doi: 10.1084/jem.190.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 47.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J Autoimmun. 2001;16:115–123. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 48.Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jeng RR, Velardi E, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A. 2016;113:E8141–E8150. doi: 10.1073/pnas.1617460113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanabe S. The effect of probiotics and gut microbiota on Th17 cells. Int Rev Immunol. 2013;32:511–525. doi: 10.3109/08830185.2013.839665. [DOI] [PubMed] [Google Scholar]
- 54.Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, Macdonald TT, et al. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 55.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, et al. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol. 2012;188:1411–1420. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 57.Arts RJ, Joosten LA, Netea MG. Immunometabolic circuits in trained immunity. Semin Immunol. 2016;28:425–430. doi: 10.1016/j.smim.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 58.McAleer JP, Nguyen NL, Chen K, Kumar P, Ricks DM, Binnie M, Armentrout RA, et al. Pulmonary Th17 Antifungal Immunity Is Regulated by the Gut Microbiome. J Immunol. 2016;197:97–107. doi: 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauguet S, D’Ortona S, Ahnger-Pier K, Duan B, Surana NK, Lu R, Cywes-Bentley C, et al. Intestinal microbiota of mice influences resistance to staphylococcus aureus pneumonia. Infect Immun. 2015;83:4003–4014. doi: 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnegarde-Bernard A, Jee J, Fial MJ, Aeffner F, Cormet-Boyaka E, Davis IC, Lin M, et al. IKKbeta in intestinal epithelial cells regulates allergen-specific IgA and allergic inflammation at distant mucosal sites. Mucosal Immunol. 2014;7:257–267. doi: 10.1038/mi.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z, Shi L, Pang W, Liu W, Li J, Wang H, Shi G. Dietary fiber intake regulates intestinal microflora and inhibits ovalbumin-induced allergic airway inflammation in a mouse model. PLoS One. 2016;11:e0147778. doi: 10.1371/journal.pone.0147778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hougee S, Vriesema AJ, Wijering SC, Knippels LM, Folkerts G, Nijkamp FP, Knol J, et al. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: a bacterial strain comparative study. Int Arch Allergy Immunol. 2010;151:107–117. doi: 10.1159/000236000. [DOI] [PubMed] [Google Scholar]
- 65.Sagar S, Vos AP, Morgan ME, Garssen J, Georgiou NA, Boon L, Kraneveld AD, et al. The combination of Bifidobacterium breve with non-digestible oligosaccharides suppresses airway inflammation in a murine model for chronic asthma. Biochim Biophys Acta. 2014;1842:573–583. doi: 10.1016/j.bbadis.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Johnson JL, Jones MB, Cobb BA. Bacterial capsular polysaccharide prevents the onset of asthma through T-cell activation. Glycobiology. 2015;25:368–375. doi: 10.1093/glycob/cwu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kepert I, Fonseca J, Muller C, Milger K, Hochwind K, Kostic M, Fedoseeva M, et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol. 2016;139:1525–1535. doi: 10.1016/j.jaci.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Lee MY, Ahn KS, Kwon OK, Kim MJ, Kim MK, Lee IY, Oh SR, et al. Anti-inflammatory and anti-allergic effects of kefir in a mouse asthma model. Immunobiology. 2007;212:647–654. doi: 10.1016/j.imbio.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Nunez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2) Cell Host Microbe. 2014;15:95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang F, Qiao HM, Yin JN, Gao Y, Ju YH, Li YN. Early-life exposure to clostridium leptum causes pulmonary immunosuppression. PLoS One. 2015;10:e0141717. doi: 10.1371/journal.pone.0141717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li YN, Huang F, Liu L, Qiao HM, Li Y, Cheng HJ. Effect of oral feeding with Clostridium leptum on regulatory T-cell responses and allergic airway inflammation in mice. Ann Allergy Asthma Immunol. 2012;109:201–207. doi: 10.1016/j.anai.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 75.MacSharry J, O’Mahony C, Shalaby KH, Sheil B, Karmouty-Quintana H, Shanahan F, Martin JG. Immunomodulatory effects of feeding with Bifidobacterium longum on allergen-induced lung inflammation in the mouse. Pulm Pharmacol Ther. 2012;25:325–334. doi: 10.1016/j.pupt.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Muller A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engler DB, Reuter S, van Wijck Y, Urban S, Kyburz A, Maxeiner J, Martin H, et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci U S A. 2014;111:11810–11815. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, Ray A. Cutting edge: inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J Immunol. 2013;191:25–29. doi: 10.4049/jimmunol.1300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, Blaser MJ. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hussain K, Letley DP, Greenaway AB, Kenefeck R, Winter JA, Tomlinson W, Rhead J, et al. Helicobacter pylori-mediated protection from allergy is associated with IL-10-secreting peripheral blood regulatory T cells. Front Immunol. 2016;7:71. doi: 10.3389/fimmu.2016.00071. https://doi.org/10.3389/fimmu.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D’Elios MM, de Bernard M. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10:2355–2363. doi: 10.1111/j.1462-5822.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 83.Lapin B, Piorkowski J, Ownby D, Freels S, Chavez N, Hernandez E, Wagner-Cassanova C, et al. Relationship between prenatal antibiotic use and asthma in at-risk children. Ann Allergy Asthma Immunol. 2015;114:203–207. doi: 10.1016/j.anai.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy. 2015;45:137–145. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- 85.Mulder B, Pouwels KB, Schuiling-Veninga CC, Bos HJ, de Vries TW, Jick SS, Hak E. Antibiotic use during pregnancy and asthma in preschool children: the influence of confounding. Clin Exp Allergy. 2016;46:1214–1226. doi: 10.1111/cea.12756. [DOI] [PubMed] [Google Scholar]
- 86.Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, de Vos WM. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 88.Nermes M, Niinivirta K, Nyland L, Laitinen K, Matomaki J, Salminen S, Isolauri E. Perinatal pet exposure, faecal microbiota, and wheezy bronchitis: is there a connection? ISRN Allergy. 2013;2013:827934. doi: 10.1155/2013/827934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 90.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arrieta MC, Sadarangani M, Brown EM, Russell SL, Nimmo M, Dean J, Turvey SE, et al. A humanized microbiota mouse model of ovalbumin-induced lung inflammation. Gut Microbes. 2016;7:342–352. doi: 10.1080/19490976.2016.1182293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hevia A, Milani C, Lopez P, Donado CD, Cuervo A, Gonzalez S, Suarez A, et al. Allergic Patients with Long-Term Asthma Display Low Levels of Bifidobacterium adolescentis. PLoS One. 2016;11:e0147809. doi: 10.1371/journal.pone.0147809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C, Loss GJ, et al. Environmental and mucosal microbiota and their role in childhood asthma. Allergy. 2017;72:109–119. doi: 10.1111/all.13002. [DOI] [PubMed] [Google Scholar]
- 94.Ciaccio CE, Barnes C, Kennedy K, Chan M, Portnoy J, Rosenwasser L. Home dust microbiota is disordered in homes of low-income asthmatic children. J Asthma. 2015;52:873–880. doi: 10.3109/02770903.2015.1028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laursen MF, Zachariassen G, Bahl MI, Bergstrom A, Host A, Michaelsen KF, Licht TR. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol. 2015;15:154. doi: 10.1186/s12866-015-0477-6. https://doi.org/10.1186/s12866-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu P, Feldman AS, Rosas-Salazar C, James K, Escobar G, Gebretsadik T, Li SX, et al. Relative Importance and Additive Effects of Maternal and Infant Risk Factors on Childhood Asthma. PLoS One. 2016;11:e0151705. doi: 10.1371/journal.pone.0151705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vael C, Vanheirstraeten L, Desager KN, Goossens H. Denaturing gradient gel electrophoresis of neonatal intestinal microbiota in relation to the development of asthma. BMC Microbiol. 2011;11:68. doi: 10.1186/1471-2180-11-68. https://doi.org/10.1186/1471-2180-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dzidic M, Abrahamsson TR, Artacho A, Bjorksten B, Collado MC, Mira A, Jenmalm MC. Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J Allergy Clin Immunol. 2017;139:1017–1025. e1014. doi: 10.1016/j.jaci.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 99.Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle RJ, Cocco R, Dreborg S, et al. Clinical use of probiotics in pediatric allergy (CUPPA): a world allergy organization position paper. World Allergy Organ J. 2012;5:148–167. doi: 10.1097/WOX.0b013e3182784ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collins S, Reid G. Distant site effects of ingested prebiotics. Nutrients. 2016;8:523. doi: 10.3390/nu8090523. https://doi.org/10.3390/nu8090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schouten B, Van Esch BC, Kormelink TG, Moro GE, Arslanoglu S, Boehm G, Knippels LM, et al. Non-digestible oligosaccharides reduce immunoglobulin free light-chain concentrations in infants at risk for allergy. Pediatr Allergy Immunol. 2011;22:537–542. doi: 10.1111/j.1399-3038.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 102.Williams NC, Johnson MA, Shaw DE, Spendlove I, Vulevic J, Sharpe GR, Hunter KA. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstric-tion and markers of airway inflammation. Br J Nutr. 2016;116:798–804. doi: 10.1017/S0007114516002762. [DOI] [PubMed] [Google Scholar]
- 103.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients. 2017;9:57. doi: 10.3390/nu9010057. https://doi.org/10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verheijden KA, Willemsen LE, Braber S, Leusink-Muis T, Delsing DJ, Garssen J, Kraneveld AD, et al. Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model. Respir Res. 2015;16:17. doi: 10.1186/s12931-015-0171-0. https://doi.org/10.1186/s12931-015-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verheijden KA, Willemsen LE, Braber S, Leusink-Muis T, Jeurink PV, Garssen J, Kraneveld AD, et al. The development of allergic inflammation in a murine house dust mite asthma model is suppressed by synbiotic mixtures of non-digestible oligosaccharides and Bifidobacterium breve M-16V. Eur J Nutr. 2016;55:1141–1151. doi: 10.1007/s00394-015-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vos AP, van Esch BC, Stahl B, M’Rabet L, Folkerts G, Nijkamp FP, Garssen J. Dietary supplementation with specific oligosac-charide mixtures decreases parameters of allergic asthma in mice. Int Immunopharmacol. 2007;7:1582–1587. doi: 10.1016/j.intimp.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 107.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, Quigley EM, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 108.Miraglia Del Giudice M, Maiello N, Decimo F, Fusco N, D’ Agostino B, Sullo N, Capasso M, et al. Airways allergic inflammation and L. reuterii treatment in asthmatic children. J Biol Regul Homeost Agents. 2012;26:S35–S40. [PubMed] [Google Scholar]
- 109.Blumer N, Sel S, Virna S, Patrascan CC, Zimmermann S, Herz U, Renz H, et al. Perinatal maternal application of Lactobacillus rhamnosus GG suppresses allergic airway inflammation in mouse offspring. Clin Exp Allergy. 2007;37:348–357. doi: 10.1111/j.1365-2222.2007.02671.x. [DOI] [PubMed] [Google Scholar]
- 110.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 111.Fonseca VM, Milani TMS, Prado R, Bonato V, Ramos SG, Martins FS, Vianna EO, et al. Oral administration of Saccharomyces cerevisiae UFMG A-905 prevents allergic asthma in mice. Respirology. 2017;22:905–912. doi: 10.1111/resp.12990. [DOI] [PubMed] [Google Scholar]
- 112.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 113.Hong HJ, Kim E, Cho D, Kim TS. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. J Clin Immunol. 2010;30:449–458. doi: 10.1007/s10875-010-9375-8. [DOI] [PubMed] [Google Scholar]
- 114.Juan Z, Zhao-Ling S, Ming-Hua Z, Chun W, Hai-Xia W, Meng-Yun L, Jian-Qiong H, et al. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. 2017;22:898–904. doi: 10.1111/resp.12985. [DOI] [PubMed] [Google Scholar]
- 115.Jensen MP, Meldrum S, Taylor AL, Dunstan JA, Prescott SL. Early probiotic supplementation for allergy prevention: long-term outcomes. J Allergy Clin Immunol. 2012;130:1209–1211. e1205. doi: 10.1016/j.jaci.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 116.Rose MA, Stieglitz F, Koksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy. 2010;40:1398–1405. doi: 10.1111/j.1365-2222.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- 117.Lee SC, Yang YH, Chuang SY, Huang SY, Pan WH. Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: a randomised controlled trial. Br J Nutr. 2013;110:145–155. doi: 10.1017/S0007114512004692. [DOI] [PubMed] [Google Scholar]
- 118.van der Aa LB, van Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, Ben Amor K, et al. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy. 2011;66:170–177. doi: 10.1111/j.1398-9995.2010.02416.x. [DOI] [PubMed] [Google Scholar]
- 119.Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, Oldaeus G. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174–1180. doi: 10.1016/j.jaci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 120.Abrahamsson TR, Jakobsson T, Bjorksten B, Oldaeus G, Jenmalm MC. No effect of probiotics on respiratory allergies: a seven-year follow-up of a randomized controlled trial in infancy. Pediatr Allergy Immunol. 2013;24:556–561. doi: 10.1111/pai.12104. [DOI] [PubMed] [Google Scholar]
- 121.Kukkonen AK, Kuitunen M, Savilahti E, Pelkonen A, Malmberg P, Makela M. Airway inflammation in probiotic-treated children at 5 years. Pediatr Allergy Immunol. 2011;22:249–251. doi: 10.1111/j.1399-3038.2010.01079.x. [DOI] [PubMed] [Google Scholar]
- 122.Simpson MR, Dotterud CK, Storro O, Johnsen R, Oien T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi: 10.1186/s12895-015-0030-1. https://doi.org/10.1186/s12895-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van de Pol MA, Lutter R, Smids BS, Weersink EJ, van der Zee JS. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy. 2011;66:39–47. doi: 10.1111/j.1398-9995.2010.02454.x. [DOI] [PubMed] [Google Scholar]
- 124.Liao HY, Tao L, Zhao J, Qin J, Zeng GC, Cai SW, Li Y, et al. Clostridium butyricum in combination with specific immunotherapy converts antigen-specific B cells to regulatory B cells in asthmatic patients. Sci Rep. 2016;6:20481. doi: 10.1038/srep20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jerzynska J, Stelmach W, Balcerak J, Woicka-Kolejwa K, Rychlik B, Blauz A, Wachulec M, et al. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. 2016;37:324–334. doi: 10.2500/aap.2016.37.3958. [DOI] [PubMed] [Google Scholar]
- 126.Helmby H. Human helminth therapy to treat inflammatory disorders - where do we stand? BMC Immunol. 2015;16:12. doi: 10.1186/s12865-015-0074-3. https://doi.org/10.1186/s12865-015-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Almeida TV, Fernandes JS, Lopes DM, Andrade LS, Oliveira SC, Carvalho EM, Araujo MI, et al. Schistosoma mansoni antigens alter activation markers and cytokine profile in lymphocytes of patients with asthma. Acta Trop. 2017;166:268–279. doi: 10.1016/j.actatropica.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 128.Jabbari Azad F, Kiaee F, Rezaei A, Farid Hosseini R, Soleimani N, Borji H. Downregulation of immune responses in asthmatic humans by ES products of Marshallagia marshalli. Clin Respir J. 2017;11:83–89. doi: 10.1111/crj.12309. [DOI] [PubMed] [Google Scholar]
- 129.Zaiss MM, Rapin A, Lebon L, Dubey LK, Mosconi I, Sarter K, Piersigilli A, et al. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity. 2015;43:998–1010. doi: 10.1016/j.immuni.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, Zhang X, et al. Hepatocyte-specific mutation of both NF-kappaB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest. 2012;122:1758–1763. doi: 10.1172/JCI59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, Jones MR, et al. The lung-liver axis: a requirement for maximal innate immunity and hepatoprotection during pneumonia. Am J Respir Cell Mol Biol. 2015;53:378–390. doi: 10.1165/rcmb.2014-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]