Abstract
The present study examined the effects of a visual-based biofeedback training on improving balance challenges in autism spectrum disorder (ASD). Twenty-nine youth with ASD (7–17 years) completed an intensive six-week biofeedback-based videogame balance training. Participants exhibited training-related balance improvements that significantly accounted for postural-sway improvements outside of training. Participants perceived the training as beneficial and enjoyable. Significant moderators of training included milder stereotyped and ritualistic behaviors and better starting balance. Neither IQ nor BMI moderated training. These results suggest that biofeedback-based balance training is associated with balance improvements in youth with ASD, most robustly in those with less severe repetitive behaviors and better starting balance. The training was perceived as motivating, further suggesting its efficacy and likelihood of use.
Keywords: postural stability, motor, video game, technology-based interventions
As bipedal creatures, postural control is a fundamental aspect of human behavior, allowing us to stand, walk, play, and interact with the world around us. However, a number of individuals with autism spectrum disorder (ASD) struggle with postural stability (for a review see Lim et al. 2017). These postural stability difficulties have been linked to autism symptom severity (Radonovich et al. 2013; Travers et al. 2013) and have been shown to persist into adulthood, such that postural stability development in ASD is marked by an atypically early plateau during adolescence (Minshew et al. 2004). While postural stability challenges may affect function at any age, the timing of this plateau is particularly concerning given that postural stability challenges may detrimentally impact the development of independent-living skills that are gained in adolescence and adulthood. Therefore, a key question is whether we can improve postural stability in youth with ASD to avoid this early plateau in postural stability, and perhaps more importantly, if we can do so in a way that is motivating and likely to be utilized. The purpose of the present research was to investigate whether a visual-biofeedback balance training, implemented through a videogame, improves postural stability in youth with ASD.
Postural stability impairments in ASD have been found in terms of the amount of time individuals maintain a balance posture and the degree of postural sway that a person exhibits when asked to stand still (Lim et al. 2017). Differentially greater decreases in postural stability have been observed in ASD during more challenging balance poses, such as one-footed standing (Travers et al. 2013; Weimer et al. 2001), dual-task conditions (Chang et al. 2010; Memari et al. 2014), eyes-closed standing (Chen and Tsai 2016; Molloy et al. 2003), standing on a foam cushion (Minshew et al. 2004; Molloy et al. 2003), and standing during multiple sensory demands (Doumas et al. 2016). Therefore, balance challenge in ASD compared to typical development may be greater when task difficulty is increased and/or when sensory information is disrupted. Moreover, individuals with ASD may differentially rely on proprioceptive information relative to visual information during standing balance (Morris et al. 2015) and other motor tasks (Haswell et al. 2009). Therefore, one way of improving balance in ASD may be to emphasize the link between visual input and balance by having participants visually observe the effects of their balance.
There is limited evidence for visual biofeedback-based interventions that target postural stability in ASD, but several non-visual methods have been explored. Chen and Tsai (2016) found that touching one’s fingertip to a stationary bar, a technique that has been used to stabilize balance across multiple clinical populations, was able to reduce postural sway in children with ASD. This finding suggests that individuals with ASD may benefit from additional tactile information while balancing. By the same token, a small feasibility study (ASD n = 5) showed promise that 10 minutes of a vestibular intervention (swing) was able to reduce postural sway, although inferential statistics were not performed (Smoot Reinert et al. 2015). Longer-term changes in postural stability in ASD have additionally been reported from a 12-week hippotherapy intervention (Ajzenman et al. 2013) and a 6-week balance-exercise training (single-leg stance, double-leg stand, and walking along designated paths under different sensory conditions) (Cheldavi et al. 2014). These studies offer preliminary evidence that balance can be improved in individuals with ASD; however, adherence to long-term training may be difficult unless the training can be implemented in a fun and motivating way. Videogame training may be particularly motivating in children with ASD, as individuals with ASD spend a large amount of their free time playing video games (Mazurek et al. 2012).
In recent years, a number of videogame platforms, like the Nintendo Wii Fit, have implemented fitness-based training to improve balance. These commercially available video games have been shown to elicit greater energy expenditures in adolescents with ASD compared to adolescents with typical development (Getchell et al. 2012) and have been shown to lead to significant gains in fitness in children with ASD in a randomized control study (Dickinson and Place 2014). Thus, playing Wii Fit games appears to have fitness benefits for youth with ASD, but none of these studies investigated whether playing such games improved postural stability. In children with cerebral palsy, Wii gaming has led to improved postural stability (Tarakci et al. 2013; 2016), suggesting that video game training may be useful to target balance in pediatric populations. The balance challenges in ASD coupled with the high levels of interest in video gaming make videogame balance training a key area to explore.
While videogame interventions may be promising, existing commercial video games, alone, may not be ideal for targeting balance challenges in ASD. Balance impairments in ASD are most evident in the presence of sensory challenges, and existing video games may not provide sufficient biofeedback to alter balance performance in ASD. Further, if biofeedback is provided, it may not be visual, which prevents a further coupling of visual-balance information that may be important for optimal postural stability in ASD. A second challenge is that many of the existing games verbally deliver instructions which may present another layer of difficulty for individuals on the autism spectrum, given the social communication challenges in ASD. Third, for a population known to struggle with balance, many of the existing games may start off as too challenging, thus limiting the time and intensity of balance training that a person with ASD is willing to tolerate. To overcome these limitations, we developed a prototype of a balance training game that was specifically designed for youth with ASD to have a straightforward interface with visual biofeedback, minimal verbal instructions, and games that can be made easier or harder based on each individual’s starting balance ability. In addition, our game was designed to use both a Microsoft Kinect camera and a Nintendo Wii balance board to monitor balance and posture, as well as track fine-tuned improvements in balance over time. We theorized that by combining the refinement and variety of commercially available games with the simplicity of our in-house game, a multi-week balance training protocol may enhance motivation and lead to robust balance improvements. Therefore, the present pilot study combined our specially designed video game with off-the-shelf Nintendo Wii Fit games to create a six-week, videogame balance training for children and adolescents with ASD. The four aims of this study were to 1) characterize session-by-session balance changes in youth with ASD, 2) identify whether balance-training changes accounted for pre-post changes in postural stability measured outside of the game, 3) identify moderators of balance training (i.e., characteristics of participants who benefited most from the training), and 4) characterize the perceived training benefit and enjoyment from the perspective of the participants and their families.
Method
Design
To address Aim 1, the present study used a repeated-measures design to examine the rate of change in balance times in youth with ASD over the course of six weeks of biofeedback-based balance training implemented through a videogame interface. We assessed the amount of time (in seconds) the participant held one-footed and two-footed poses during the game as a function of the number of completed sessions of training. To address Aim 2, postural sway area was measured outside of the context of the game under different sensory conditions both before and after training. Post-training postural sway was examined as a function of training-related progress after accounting for pre-training postural sway. To address Aim 3, we examined training-related progress as a correlate of individual differences in age, cognitive, social, repetitive-behavior, and motor variables. To address Aim 4, we asked questions regarding the perceived benefit and enjoyment of the training to participants (in person) and one of their family members (via questionnaire).
Participants
The study was approved by the University of Wisconsin-Madison Institutional Review Board. All parents provided written informed consent, and all children provided informed assent. Twenty-nine community-ascertained youth with ASD (ages 7–17 years; two females) participated in this study. A subset (n=7) of these participants additionally completed pre- and post-training magnetic resonance imaging (MRI); however, the brain imaging data are not reported here. All participants were recruited through fliers in the community, the Waisman Center registry database, and word of mouth. The participants with ASD had a prior clinical diagnosis of ASD that was supported by meeting criteria for ASD on Modules 3 or 4 of the Autism Diagnostic Observation Scale, 2nd editions (ADOS-2) (Lord et al. 2012). One participant did not meet criteria for autism spectrum on the ADOS-2. However, this participant had a well-documented, previous clinical diagnosis of ASD and scores on parent-report measures that were indicative of ASD symptoms. Therefore, this participant was included in the analysis. Participants were required to not have tuberous sclerosis, fragile X, a history of severe head injury, hypoxia ischemia, or intellectual disability. Participants were asked to not to start any new exercise or treatment programs during the course of the six weeks of training. The demographic form further inventoried the number of hours each week that participants were receiving occupational and physical therapy services that could potentially lead to balance-related improvements. See Table 1 for more detailed participant demographics.
Table 1.
Demographic characteristics of the sample.
| N=29 participants with ASD | ||
|---|---|---|
| Mean(SD) | Range | |
| Age (years) | 12.38(3.15) | 7.80–17.85 |
| Height (inches) | 61.96(8.98) | 45–77 |
| Weight (kilograms) | 51.5(23.11) | 18.36–112.00 |
| BMI | 32.08(10.81) | 16.07–62.99 |
| Full Scale IQ | 104.14(13.24) | 73–135 |
| Verbal IQ | 101.69(14.97) | 72–130 |
| Performance IQ | 105.59(15.76) | 79–139 |
| BOT-2 Percentile | 13.97(10.44) | 1st–50th |
| Social Responsiveness Scale raw score | 93.41(35.92) | 28–166 |
| Repetitive Behaviors Scale-Revised scores | ||
| Total raw score | 34.59(23.79) | 0–91 |
| Stereotyped Behavior | 4.90(4.00) | 0–13 |
| Self-Injurious Behavior | 2.90(2.88) | 0–11 |
| Compulsive Behavior | 5.79(5.77) | 0–21 |
| Ritualistic Behaviors | 6.17(4.12) | 0–14 |
| Sameness Behavior | 9.72(7.20) | 0–24 |
| Restricted Behavior | 5.10(3.62) | 0–12 |
| Current weekly Occupational Therapy (hours) | 0.97(2.65) | 0–10 |
| Current weekly Physical Therapy (hours) | 0.22(0.86) | 0–4 |
ASD= Autism Spectrum Disorder; BMI = body mass index; BOT-2 = Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition
Measures
Bruininks-Oseretsky Test of Motor Proficiency, 2nd Edition Short Form (BOT-2 SF; Bruininks and Bruininks 2005)
The BOT-2 is a standardized motor test that assesses stability, mobility, strength, coordination, and object manipulation in individuals four to 21 years of age. Good content and construct validity for the BOT-2 have been established (Bruininks and Bruininks 2005). The short form consists of 14 items selected from all eight subtests of the full BOT-2 and takes approximately 20–25 minutes to administer. A single score of motor proficiency is calculated, with higher scores pointing towards greater motor proficiency. The BOT-2 SF has strong internal consistency reliability (.82−.87), test-retest reliability (.80−.87), and interrater reliability (.98). In addition to the short form, the full balance subtest was administered to participants to get a balance raw score before starting the training.
Repetitive Behavior Scale-Revised (RBS-R) (Bodfish et al. 2000; Lam and Aman 2007)
The RBS-R is a 43-item parent/caregiver questionnaire that assesses the presence and severity of repetitive behaviors in the previous month. The RBS-R has been found to be a reliable measure of multiple types of repetitive behaviors and restricted interests for people three to 48 years of age (Lam and Aman 2007). Higher RBS-R scores are indicative of higher intensity repetitive behaviors/restricted interests. Internal consistency for all of the subscales was high (Crohnbach’s alphas of 0.78 to 0.91, M = 0.83).
Social Responsiveness Scale (SRS) (Constantino 2002)
The SRS is a 65-item parent/caregiver questionnaire assessing the presence of ASD symptoms over the past six months. Each item on the scale asks about an aspect of observed reciprocal social behavior and is rated on a scale from “0” (never true) to “3” (almost always true). Higher scores on the SRS indicate greater severity of autism symptoms. The SRS has demonstrated excellent reliability and validity (Constantino et al. 2003).
Wechsler Abbreviated Scale of Intelligence-2nd Edition (WASI-II; Wechsler and Hsiao-pin 2011)
The WASI-II is an abbreviated measure of the intelligence of individuals six to 90 years of age, taking approximately 30 minutes to administer. The WASI-II includes four subtests: block design, vocabulary, matrix reasoning, and similarities to establish intellectual functioning in verbal comprehension (VCI) and perceptual reasoning (PRI), as well as approximate general intellectual ability (Full Scale IQ [FSIQ-4]). Average reliability coefficients of the subtests were high, ranging from .91−.97 for the children and .90−.92 for adults. In terms of validity, the four-subtest version of the WASI-II was found to be highly consistent with both the IQ scores of the Wechsler Intelligence Scale for Children, 4th edition (WISC-IV) and the Wechsler Adult Intelligence Scale, 4th edition (WAIS-IV).
Procedures
Intake and post-training sessions
Participants completed an intake session, including IQ, diagnostic, symptom severity, and BOT-2 assessments. Following the intake, 60-minute video game training sessions took place three times per week for six consecutive weeks. All procedures were videotaped in order to check data accuracy.
In-lab videogame setup
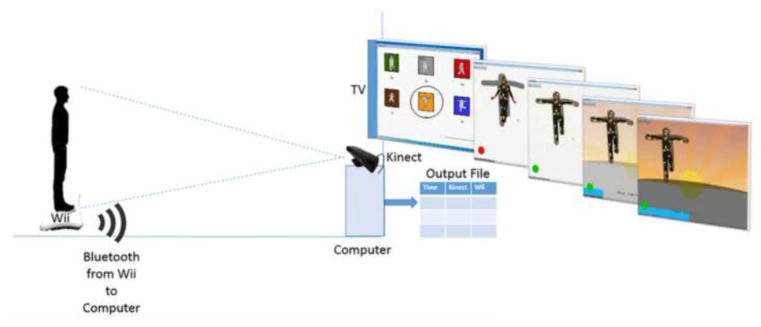
The in-lab video game was specifically designed for children with ASD to have minimal verbal instructions and to have balance tasks that can be customized based on each individual’s starting balance ability. The game software was developed for a Windows platform using Adobe Air, and the software integrated a Microsoft Kinect camera and a Nintendo Wii balance board to monitor balance and posture, as well as track fine-tuned improvements in balance over time. See Figure 1 for a depiction of the game setup. To play the game, the participant stood on the balance board in front of a large television (a Samsung 51” plasma 720p HDTV) that was mounted on the wall. Once the Kinect camera detected the participant, the participant would see him or herself on a blank television screen with 16 dots projected on the participant’s image to represent joint coordinates. A research assistant manually adjusted a shadow of the to-be-copied pose to the size of the participant. When the participant’s body was positioned within the shadow, all joint dots turned yellow. However, any joint dot not within the shadow turned red to alert the participant of the error. In this way, our game provided continuous, visual, and nonverbal feedback about how to hold the pose. To reward the participant for holding the pose as long as possible, a background scene (mountain, beach, or tree landscapes) slowly appeared on the screen and darkened with each second the pose was maintained. When any two dots were not within the shadow, the background paused but would resume once all dots were back in the shadow. There were a total of six “ninja” poses to choose from (see Figure 1): three two-footed poses and three one-footed poses. These poses were selected based on their presence in existing balance practices, such as tai chi and yoga.
Figure 1.
Schematic of the in-lab videogame setup. The Kinect camera connected to the computer via a USB cord. The Wii balance board connected to the computer via Bluetooth. Participants trained one-at-a-time on the six poses shown. There were three two-footed poses (Standing Side Bend [gray], Energy Ball [red], and Hug the Tree [brown]), and there were three one-footed poses (Tree [green], Karate Kid [orange], and Arm to Knee [blue]). For the one-footed poses, participants completed the pose with the right and left feet separately Using the software designed in Adobe Air, participants saw themselves on the screen with joint dots projected onto their image. Joint dots were yellow when within the shadow but red when outside the shadow. When all joint dots were within the shadow, the background would slowly come into view with each passing second that the participant held the pose correctly. The force data from the Wii balance board and the Kinect camera joint data were synchronized and output to a .csv file.
Training protocol
The session-by-session training protocol can be seen in Supplemental Appendix A. Every session began and ended with the in-lab balance-training video game (“Ninja Training Game”). In this game, participants completed a series of 3–6 poses, while the game collected kinematic and postural sway data using the Wii balance board and Kinect camera. The postural sway and kinematic measures gained from these devices are not reported in the present study, although they were used by the game to determine the balance times in seconds for each pose.
To individualize the difficulty of holding the balance pose and promote self-efficacy, the participant worked with the research assistant to set a goal for how long to hold each pose. This goal could range from 5–120 seconds, although the participant was allowed to hold the pose for double the goal (i.e., a goal of 120 seconds could be held for up to 240 seconds). During pilot testing, we realized that goal-setting could be made more accurate by adding a “max testing” protocol at the first, middle (session 8), and last sessions of the training. For the max testing, the participant held a two-footed pose (i.e., energy ball) and a one-footed pose (karate kid, left and right foot separately) for as long as possible. These times were recorded and used to assist the participant in future goal setting, but they were not included in the data analysis because of the different set of instructions.
After the first set of 3–6 ninja poses, participants played Wii Fitness games. These games provided variety compared to the static balance poses and included balance training mini-games (soccer heading, ski jump, penguin slide, tightrope walk, table tilt, and balance bubble), aerobic games (hula hoop, basic step), yoga poses (downward facing dog, chair pose, and triangle pose), and strength training games (rowing squats, sideways-leg lift, and single-leg extension). In the middle of the training session, a snack break was given. Sessions concluded with a second set of 3–6 ninja poses. Participants received $10 per hour for participation.
Pre-post postural sway measures
Outside of the gaming setup, postural sway measures were assessed at the beginning of the training (start of session 1) and at the end of the training (start of session 18) under three different conditions: 1) eyes-open standing, 2) eyes-closed standing, and 3) visual-feedback standing (i.e., seeing one’s center of pressure on the screen). Participants were instructed to stand on a Wii balance board with feet together on the center line of the board and arms crossed at the chest. The Wii balance board has demonstrated excellent reliability and validity compared to research-grade force platforms (Clark et al. 2010; Monteiro-Junior et al. 2015), and it has been previously used to measure postural sway in individuals with ASD (Travers et al. 2013). However, because of the Wii board’s inherent decreased sensitivity (only four sensors), it may be best when used in repeated measures contexts (Bartlett et al. 2014), as was done in the present study. The Wii balance board was connected via Bluetooth to a Linux-based computer. In the eyes-open condition, participants looked forward at a red dot on the wall (from the BOT-2). In the visual-feedback condition, the computer screen was placed in front of the participant to view one’s center of pressure. Across all three conditions, participants were asked to hold as still and as centered as possible for 60 seconds. After the participant demonstrated understanding of the instructions and was standing on the balance board in the correct position, a button was pressed to begin data collection. Force on each of the four balance board sensors was recorded at 35 Hz and used to calculate Center of Pressure (COP), accounting for the distance between the four sensors on the Wii balance board (Bartlett et al. 2014). To control for possible movement at the beginning and end of the trial, we omitted the first and last five seconds, thus modeling 50 seconds of data.
While many different COP metrics are available to measure postural control during static balance, we decided a priori to use COP area (area of ellipse [mm2] that contains 95% of the data) (Winter, 2009). This decision was made because COP area is one of the most commonly used and easily interpretable methods of measuring postural sway. We calculated the x and y COP coordinates for each time point. We then calculated the eigenvectors and eigenvalues of the covariance matrix formed from all the x and y coordinate pairs. From these eigenvectors and eigenvalues, we calculated the semimajor and semiminor axes of the ellipse with Equations 1 and 2.
| (1) |
| (2) |
where λ1 and λ2 correspond to the first and second eigenvalues, respectively. We then calculated the area of the best fit ellipse that encapsulates 95% of the data with Equation 3:
| (3) |
Unfortunately, not all 29 participants had complete postural sway data. Specifically, the first participant in the study only completed eyes-open postural sway measurements (before we changed the protocol to include more sensory-based measures). Another participant had complete pre-training measures, but the computer malfunctioned during the post-training feedback postural sway measurements and prevented an additional participant’s post-training data collection. Therefore, eyes-open postural sway analyses include 28 participants, eyes-closed analyses include 27 participants, and visual-feedback analyses include 26 participants.
Post-study questionnaire
Throughout the study, participants and their families shared their thoughts about the training with us, which provided the impetus for us to collect these perceptions in a more systematic way. Therefore, we designed a post-study questionnaire that asked whether the participant enjoyed the games, perceived a benefit from the games, and would play these games outside the study. Once this questionnaire was officially implemented as part of our research protocol, 11 participants and 11 family members who accompanied the participants to the last session separately completed the questionnaires. The family-member version of the questionnaire differed in that it provided a Likert scale for enjoyment (0 = no enjoyment, 3 = some enjoyment, 6 = much enjoyment), rather than just a yes-or-no response. After each question, there was space to provide comments.
Data analysis
All statistical analyses were performed in R version 3.2.2 (R Core Team 2015). Prior to data analysis, data collected via the game were checked and corroborated by researcher notes and videos of the training sessions to ensure accuracy. On a small minority of trials (41 of 3953 trials [1%]), the game timing sped up to double the speed. To ensure that these errors would not affect our analyses, those trials were checked and omitted prior to running the analyses. To examine balance changes as a function of training session, we fit linear mixed-effects models, accounting for the repeated measurements over the training sessions. The model examined balance time (seconds) as function of session number (1–18), while accounting for random effects due to repeated measures (Balance time ~ intercept + session number + random effects). Because we hypothesized individual differences not only in the starting balance times but also in the rate of learning across the participants with ASD, the random effects in our model accounted for separate intercepts and slopes for each participant. Separate models were fit for one-footed and two-footed poses, where the balance time was the average balance time of one-footed (or two-footed poses) for each session. Additionally, all linear models were tested against a quadratic session model (intercept + session + session2+random effects), and a model that accounted for the order of the pose within the session (assuming that the fifth pose held may be more challenging to hold than the first pose held). The linear models had a better fit according to AIC (Akaike 1974), and the order of the pose within the session did not account for significant variance. Therefore, the model was fit linearly, and the order of the pose in the session was not included in the model. After the group-level models were calculated, a linear model was performed on each individual’s data to estimate the balance-training starting point (intercept) and the rate-of-change over the course of the training (slope).
To examine whether balance training accounted for changes in postural stability outside of the video game, we performed an ANCOVA examining whether the balance rate-of-change over the course of the training related to post-training postural sway area, while controlling for pre-training postural sway area. Postural sway area was measured under three different sensory conditions (i.e., eyes opened, eyes closed, and visual feedback/seeing your postural stability on the computer screen). Separate models were run for each sensory condition because of the different sample sizes. Prior to running analyses, we examined the data to make sure that they met the assumptions for ANCOVA. All postural sway area data violated the assumptions by demonstrating substantial positive skew. Therefore, we log-transformed all postural sway area data prior to analysis, and then the data met the assumptions.
To explore potential moderators of the balance training, we performed Pearson correlations among the one-footed balance training measures (estimated training slopes and intercepts), participant age, BMI, IQ, standardized motor scores from the BOT-2, SRS total score, and the overall and subdomain scores of the RBS-R. To account for multiple comparisons in these exploratory analyses, results of the correlations are reported with and without FDR correction.
Results
Session-by-session balance training changes
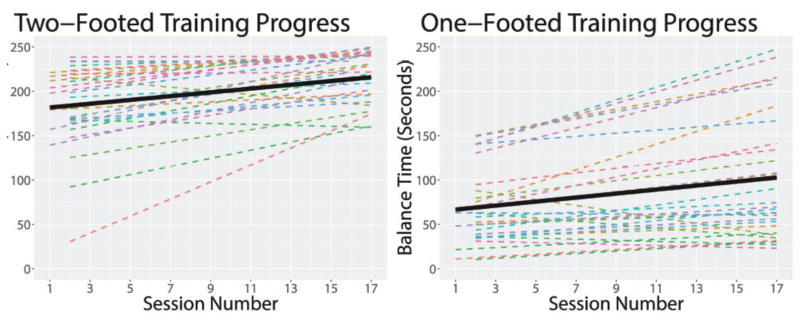
Although there was substantial variability in the starting balance times and progress in the ASD participants, 27 of the 29 participants demonstrated improvements in two-footed balance over the course of the training (Figure 2). One of the two individuals who did not show progress, started the training at ceiling. A linear mixed-effects model demonstrated a significant main effect for session number, such that each session demonstrated an average 2.41-second increase in balance times for two-footed poses, b = 2.41, SE = 0.40, p < .001. Using the calculated slope and intercept for each participant, participants showed an average 1.77-fold increase in the time they were able to stand in a two-footed pose over the course of the training (0.83–16.23 [<1.00 is a decrease]). Ceiling effects were observed in this analysis. Therefore, a follow-up survival analysis was conducted using linear mixed-effects logistic regression to see what percentage of participants reached the maximum 240 seconds balance times as a function of session number. This analysis confirmed a significant effect of session number on balance times, p = .04.
Figure 2.
Fitted linear smoothed lines for balance improvements over the course of the training sessions at both the level of the individual participants (dashed lines) and at the level of the group (black solid lines). Overall, the participants demonstrated significant training progress in both two-footed and one-footed poses (p’s < .001). However, there was significant variability in the performance of individual participants.
For one-footed balance times, 24 of 29 participants demonstrated improvements over the course of the training (Figure 2). A linear mixed-effects model demonstrated a significant main effect for session number, such that each session demonstrated an average 2.13-second increase in the amount of time a participant was able to balance in the one-footed poses, b = 2.13, SE = 0.47, p < .001. Using the calculated slope and intercept for each participant, participants showed an average 1.72-fold increase in the time they were able to stand on one foot over the course of the training (0.36–4.10 [<1 is a decrease]). A one-sample t-test confirmed that this pre-post change was significantly different from no change (μ =1), t(28) = 4.70, p < .001.
Follow-up analyses examined Wii Fit game performance over the course of training and found that participants significantly increased the number of stars received for each Wii Fit game as a function of session number, b = 0.03, SE = .004, p < .001. Therefore, participants demonstrated session-by-session improvements in both the Wii Fit and in-lab games.
Training as a predictor of pre-post changes in postural stability
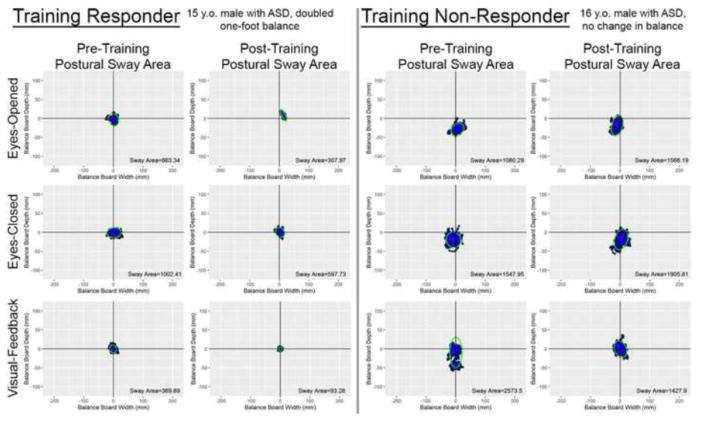
In addition to balance improvements being measured within the context of the game, postural stability was measured outside of the game both before and after training. These pre-post measures were not trained during the game, making them an index of whether balance-training gains generalized to better postural stability outside of the game context. As an example, Figure 3 shows pre- and post-training postural sway area for a participant who did not show training-related balance gains compared to a participant who showed substantial training-related balance gains.
Figure 3.
Pre- and post-training postural sway area data for a participant who made substantial balance improvement during the training (“Training Responder”) compared to a similarly aged participant who did not make substantial balance improvements during the training (“Training Non-Responder”). Importantly, the postural sway area measures were taken outside of the game context, and these poses were not explicitly trained during the game. The blue dots represent the center of pressure over the course of the trial. The green ellipse represents the 95% confidence ellipse for the blue dots, from which postural sway area was calculated.
For eyes-open postural stability measures, balance-training improvements (i.e., the estimated slope of balance-training progress) significantly predicted post-training postural sway area, F(1,25) = 7.97, p = .009, after controlling for pre-training postural sway area. Importantly, estimated starting balance times were not a significant predictor of post-training postural sway, F(1,25) = 2.12, p = .16, indicating that it was the training progress and not the training starting times that predicted postural sway area.
For eyes-closed postural stability measures, there was one extreme outlier whose post-training data were 3.5 standard deviations above the mean. The assumptions for the statistical model were only met when this participant’s data were omitted from the model, leaving a final n = 26 for this analysis. Balance-training improvements significantly predicted post-training postural sway area, F(1,23) = 11.99, p = .002, after controlling for pre-training postural sway area. Again, estimated starting-balance times were not a significant predictor of post-training postural sway area, F(1,23) = 1.96, p = .18.
For visual-feedback postural stability measures, balance-training improvements significantly predicted post-training postural sway area, F(1,23) = 6.09, p = .02, after controlling for pre-training postural sway area. Estimated starting-balance times were not a significant predictor of post-training postural sway, F(1,23) = 2.62, p = .12.
Even though participants improved in Wii-Fit performance over the course of the training, follow-up analyses found that Wii Fit game improvements were not able to significantly predict post-training postural sway area in eyes-opened standing, F(1,25) = 0.17, p = .69, eyes-closed standing, F(1,23) = 1.36, p = .25, nor visual-feedback standing, F(1,23) = 0.07, p = .76. Therefore, training gains in the in-lab videogame but not the Wii Fit games were related to improvements in postural sway area.
Moderators of balance-training gains
The rate of improvements in one-footed balance learning were examined as a function of age, BMI, IQ, pre-training motor measures, and ASD symptom severity. These correlations are reported in Table 2. Greater improvements in one-footed balance training were associated with higher pre-training balance scores on the BOT-2, older age, and less-severe ritualistic, stereotyped, self-injurious, and overall repetitive behaviors. After FDR correction, remaining significant correlates of balance improvement rate included the BOT-2 pre-training balance scores, ritualistic behaviors, and stereotyped behaviors.
Table 2.
Pearson correlations examining the relation between balance-training gains and individual characteristics across cognitive, motor, and symptom domains. More robust balance improvements during training were associated with better pre-training balance and less-severe stereotyped and ritualistic behaviors.
| Balance-Training Rate-of-Change | |
|---|---|
| Pearson Correlations | |
| Balance-Training Starting Point | +.44* |
| BOT-2 Balance Scores | +.50*** |
| Age (Years) | +.41* |
| BMI | +.30 |
| Verbal IQ | −.04 |
| Performance IQ | +.20 |
| Social Responsiveness Scale | −.26 |
| BOT-2 Percentile | +.22 |
| Repetitive Behavior Scale-Revised | |
| Overall | −.42* |
| Stereotyped Behavior | −.50*** |
| Self-Injurious Behavior | −.42* |
| Compulsive Behavior | −.31 |
| Ritualistic Behaviors | −.51*** |
| Sameness Behavior | −.25 |
| Restricted Behavior | −.36 |
p < .05 uncorrected,
p < .05 FDR-corrected
Perceptions of Training Benefit and Enjoyment
Responses to the post-training questionnaire can be seen in Table 3. The ratings by the participants and family members indicated that the vast majority perceived a benefit from playing the games, enjoyed the games, and would play the games outside of the study context. Additional comments provided by the participant’s and their families indicated that one aspect of the training that was helpful was the engagement with the researchers during the training. Another theme was the desire for the in-lab game (“Ninja Training Game”) to have better graphics and more action.
Table 3.
Reponses to questions from the participants and one of their family members regarding the perceived training benefit and enjoyment.
| Do you think you have benefitted from playing these games? / Do you think your child has benefited from playing these games? | |
|---|---|
| 9 of 11 participants thought they benefited | 11 of 11 family members thought the participant benefited |
| • I feel a little more balanced. | • I see increased strength and overall fitness. |
| • It benefitted my balance and my patience. It definitely helped improve my autism. | • He loves games, so this was the perfect opportunity for him to get to do what he loves while getting paid. |
| • I became so tired and painful but it helped me to balance more, I mastered all of them. Can’t wait to try it in sports, like tennis or soccer. | • He hadn’t played video games before. Now he operates a TV remote with increased dexterity. There is also less hesitation in trying new physical activities. |
| • Add more stuff happening in the background (action). | • It makes him more aware of how a body progresses with practice. |
| • I can stand on one leg better without wobbling. I can do more stuff on one leg, like skate on one leg in hockey. | • He enjoyed explaining what he did to anyone who asked. |
| • Very fun, got to meet a lot of people, made me think in a different way. | • He enjoyed the game itself and also hanging out with grown-ups. |
| • I feel like I’ve been able to hold poses longer | • Yes, better balance. |
| Did you enjoy playing these games? / Overall, how much do you think your child enjoyed playing these games? | |
| 10 of 11 participants enjoyed the games | Family members rated participant’s enjoyment on average at 4.8 (mode = 6) on scale of 0 = no enjoyment to 6 = much enjoyment (range: 3–6) |
| • Make an NX version. | • He talks about the game at home too. |
| • The Wii games were fun but the ninja games could have been more enticing. | • He generally looks forward to these sessions and doesn’t mind the times when he has to hold his position steady. |
| • The nurses (researchers) were being nice, the ninja games were a pain in the butt, and Wii fit is annoying, but there are games that I like, bubble, and super hula hoop, etc. | • He enjoyed the games he played, however, he frequently complained about the graphics on the Wii. |
| • Please add more video games that kids like. | • It is hard to get a sense of how he felt about it--the canned response was “it’s okay.” |
| • I liked energy ball and hug the tree (two Ninja Training games) | • Loved it! Very sad that it is over. |
| Would you play these games outside of this study? / Do you think your child would play these games outside of this study? | |
| 8 of 11 participants said “yes,” 1 said “maybe,” and 2 said “no” | 11 of 11 family members said “yes” |
| • Play them anywhere! | • We’ll most probably get him a game console to see if such games can help in the future. |
| • Meh. • If it is for free. |
• He is, however, since our Wii is broken, it might be a while until we play these games again. |
| • Don’t know, maybe yes, maybe not, it depends on homework and school life. | • Maybe if not so busy at school. |
| • Would play ninja and Wii. | • He used to actually play these games when he was 10–12 years old. |
| • Would not play ninja games but would play Wii games. | • It will be a good activity over the winter. |
| • Probably. | • He tries to find similar games on our Wii at home to compete. |
Discussion
The present study investigated changes in balance and postural stability as a function of a six-week videogame training in youth with ASD. This balance training included both a novel in-lab video game that provided visual biofeedback and a commercially available Nintendo Wii Fit game. Over the course of training, participants significantly improved balance times in the novel in-lab video game. On average, participants almost doubled the amount of time they were able to balance on one foot, which is important given that one-foot standing is particularly challenging for individuals with ASD (Travers et al. 2013; Weimer et al. 2001). While participants also improved performance in the Wii Fit game, only improvements in the in-lab video game related to post-training postural sway area measurements, suggesting that only the in-lab videogame training generalized to outside measures of postural stability. Significant moderators of the training were observed, suggesting that youth with stronger starting balances and less severe stereotyped and ritualistic behaviors exhibited more robust training gains. Participants and their family members reported that the training was beneficial and enjoyable, suggesting that this training was positively viewed by the stakeholders.
Our findings suggest that visual-based biofeedback training improves balance in ASD. These findings are consistent with previous research that postural stability in ASD can be improved through various means (Ajzenman et al. 2013; Cheldavi et al. 2014; Chen and Tsai 2016; Smoot Reinert et al. 2015). However, this work expands beyond previous findings by examining whether these training-related gains accounted for postural sway improvements in tasks outside the game that were not explicitly trained. Importantly, we found that balance improvements from the in-lab game related to post-training postural sway, above-and-beyond pre-training postural sway, and these effects were observed under all three sensory conditions. These results suggest that more robust training-related balance improvements in the game generalized to better post-training postural stability. However, performance on the Wii Fit games did not translate to better post-training postural stability, suggesting that the in-lab game was most likely the driving force behind the balance changes observed in this study. That said, it is possible that the Wii Fit games had auxiliary effects on the training, especially since previous research has found Wii gaming to benefit postural stability in children with cerebral palsy (Tarakci et al. 2013; 2016). While these results point to the efficacy of this training as a whole, future randomized controlled studies comparing this training to an active control group are needed. Additionally, future research is needed to further examine the behavioral and neural mechanisms of this balance training in order to streamline and optimize the training for this population.
While significant balance improvements were observed at the group level, there was a great deal of variability within the ASD group in starting balance ability and the rate of balance changes over time. This variability is perhaps unsurprising in that we specifically designed the game to accommodate individuals with a wide variety of balance abilities. We examined individual difference in training-related balance improvements in relation to individual characteristics to determine which individuals might be ideal candidates for this training in the future. We found that individuals with better balance at the start of the study (as measured by our standardized motor assessment) and individuals with less severe stereotyped and ritualistic behaviors demonstrated the most robust training-related gains. Speculatively, those with better balance at the start of the game might have perceived themselves as better at the game, thus enhancing motivation to play it. Overall repetitive behaviors demonstrated a medium-sized correlation with balance-training progress, but stereotyped and ritualistic behaviors seemed to be driving this effect. In the measures we used, stereotyped behaviors included whole-body rocking or swaying which might have interfered with being able to maintain the static poses during our biofeedback-based balance training. Ritualistic behaviors, on the other hand, included items that examined insistence on following rigid routines during play or leisure. Anecdotally, some participants in the study insisted on specific ways of playing the Wii games that we needed to account for during study sessions. Insistence on routine might have taken time away from or interfered with the participant’s training. Previous research has shown that repetitive behaviors are associated with postural stability in ASD (Radonovich et al. 2013; Travers et al. 2013). The present findings introduce the possibility that more severe repetitive behaviors may impede improvements in balance training, which in part might account for the link between repetitive behaviors and balance in ASD. Alternatively, it is possible that co-occurring features in ASD, such as sensory features, may be driving these effects, as sensory features and repetitive behaviors have been found to be associated in ASD (Boyd et al., 2011). Indeed, the list of moderators that we examined in the present study were not exhaustive. Dyspraxia, attention deficit disorder, sensory features, and anxiety are among potential moderators of postural stability that will be important to examine in future research.
In terms of other potential moderators, age had a positive, medium-sized correlation with training-related gains, although this relation was not significant after accounting for multiple comparisons. The positive direction of this relation is interesting because it suggests that adolescents were more likely to make postural stability gains, even though postural stability has been shown to plateau early in adolescents with ASD (Minshew et al. 2004). Although the present study did not examine how long postural stability improvements are maintained after the training, a potential implication of larger training-related gains in adolescents is that this training might be able to avert the plateau in postural stability during adolescence in this population, which is a key avenue for future research.
Surprisingly, BMI, verbal IQ, performance IQ, overall motor performance, and social responsivity did not moderate training-related gains, despite having substantial variability among these variables within the present sample. The lack of IQ-training relations suggested that neither verbal nor performance IQ predicted who made the most substantial training gains, indicating that we achieved our goal of making this game accessible to individuals across a wide IQ range. However, all participants in the present study were verbal and did not have a co-occurring diagnosis of intellectual disability. Therefore, future research should expand this training to be more representative of the ASD population as a whole by including minimally verbal individuals. In all, these results suggest that this training may be beneficial for individuals with a wide range of motor and cognitive abilities.
Another advantage of the current balance training protocol compared to previous research is that it was designed as a video game to meet the interests of the ASD population it aims to serve (Mazurek et al. 2012). To confirm that this training was motivating, we asked participants and one of their family members about their perceptions of the training. The responses indicated that participants and their family members generally found training to be beneficial and enjoyable, suggesting this training is a fun and motivating method of targeting balance challenges in individuals with ASD. The level of enjoyment expressed was in some ways surprising because the in-lab training game is physically demanding, requiring participants to consecutively hold one-footed poses for lengthy amounts of time. In line with this, participants did seem to express more enjoyment of the Wii games than the in-lab game. Participants offered excellent suggestions for enhancing the in-lab video game, including improving the graphics of the in-lab game and making it more enticing (i.e., adding variety to the poses). These recommendations may reflect certain elements of the videogame experience that were motivating to our sample and that can be capitalized on in future videogame-based interventions for this population. Finally, from these responses it became apparent that the participants valued having research staff facilitate these games, suggesting that this training is unlikely to be as effective or as enjoyable as an at-home, stand-alone training.
Conclusions
Our data suggest that a biofeedback-based balance training was able to elicit balance improvements in youth with ASD and that these training-related improvements generalized to balance improvements outside of the context of the training. Participants and their family members indicated that the training was beneficial and enjoyable, indicating that individuals with ASD may be motivated to use this intervention as a means of improving balance. While there was variability in balance-training gains across the sample, our data suggest that starting balance and stereotyped and ritualistic behaviors may help account for some of this variability. In all, this type of training may be able to address the postural stability challenges that are commonly reported in ASD.
Supplementary Material
Acknowledgments
Funding: This study was funded by the Brain and Behavior Research Foundation’s NARSAD Young Investigator Award, the Hartwell Foundation’s Individual Biomedical Award, the University of Wisconsin System’s WiSys Technology Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P30 HD003352, U54 HD090256, and T32 HD007489).
Footnotes
Conflict of Interest: Brittany G. Travers declares that she has no conflict of interest. Andrea Mason declares that she has no conflict of interest. Leigh Ann Mrotek declares that she has no conflict of interest. Anthony Ellertson is a founding partner of Prentice Technologies. Douglas C. Dean declares that he has no conflict of interest. Courtney Engel declares that she has no conflict of interest. Andres Gomez declares that he has no conflict of interest. Kristine McLaughlin declares that she has no conflict of interest.
Research ethics: This project was approved the University of Wisconsin-Madison Education and Social/Behavioral Science Institutional Review Board (protocol #2014-1248) and Health Sciences Institutional Review Board (protocol #1014-1499)
Compliance with Ethical Standards: No study team member have conflicts of interest to declare.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Ajzenman HF, Standeven JW, Shurtleff TL. Effect of hippotherapy on motor control, adaptive behaviors, and participation in children with autism spectrum disorder: a pilot study. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2013;67(6):653–663. doi: 10.5014/ajot.2013.008383. [DOI] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Bartlett HL, Ting LH, Bingham JT. Accuracy of force and center of pressure measures of the Wii Balance Board. Gait & Posture. 2014;39(1):224–228. doi: 10.1016/j.gaitpost.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of autism and developmental disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, Miller H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks RH, Bruininks BB. Bruininks-Oseretsky test of motor proficiency. 2. Minneapolis, MN: Pearson Assessment; 2005. [Google Scholar]
- Chang CH, Wade MG, Stoffregen TA, Hsu CY, Pan CY. Visual tasks and postural sway in children with and without autism spectrum disorders. Research in Developmental Disabilities. 2010;31(6):1536–1542. doi: 10.1016/j.ridd.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Cheldavi H, Shakerian S, Boshehri SNS, Zarghami M. The effects of balance training intervention on postural control of children with autism spectrum disorder: Role of sensory information. Research in Autism Spectrum Disorders. 2014;8(1):8–14. [Google Scholar]
- Chen FC, Tsai CL. A light fingertip touch reduces postural sway in children with autism spectrum disorders. Gait & Posture. 2016;43:137–140. doi: 10.1016/j.gaitpost.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Clark RA, Bryant AL, Pua Y, McCrory P, Bennell K, Hunt M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait & Posture. 2010;31(3):307–310. doi: 10.1016/j.gaitpost.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Constantino J. The social responsiveness scale. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Constantino J, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dickinson K, Place M. A randomised control trial of the impact of a computer-based activity programme upon the fitness of children with autism. Autism Research and Treatment. 2014:419653. doi: 10.1155/2014/419653. doi: http://dx.doi.org/10.1155/2014/419653. [DOI] [PMC free article] [PubMed]
- Doumas M, McKenna R, Murphy B. Postural control deficits in Autism Spectrum Disorder: The role of sensory integration. Journal of Autism and Developmental Disorders. 2016;46(3):853–861. doi: 10.1007/s10803-015-2621-4. [DOI] [PubMed] [Google Scholar]
- Getchell N, Miccinello D, Blom M, Morris L, Szaroleta M. Comparing energy expenditure in adolescents with and without Autism while playing Nintendo(®) Wii(™) games. Games for Health Journal. 2012;1(1):58–61. doi: 10.1089/g4h.2011.0019. [DOI] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KSL, Aman MG. The repetitive behavior scale-revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lim YH, Partridge K, Girdler S, Morris SL. Standing postural control in individuals with autism spectrum disorder: Systematic review and meta-analysis. Journal of Autism and Developmental Disorders. 2017;47(7):2238–2253. doi: 10.1007/s10803-017-3144-y. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule–2nd edition (ADOS-2) Los Angeles, CA: Western Psychological Corporation; 2012. [Google Scholar]
- Mazurek MO, Shattuck PT, Wagner M, Cooper BP. Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(8):1757–1767. doi: 10.1007/s10803-011-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari AH, Ghanouni P, Shayestehfar M, Ziaee V, Moshayedi P. Effects of visual search vs. auditory tasks on postural control in children with autism spectrum disorder. Gait & Posture. 2014;39(1):229–234. doi: 10.1016/j.gaitpost.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.WNL.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2003;33(6):643–652. doi: 10.1023/B:JADD.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Monteiro-Junior RS, Ferreira AS, Puell VN, Lattari E, Machado S, Vaghetti CAO, Silva EB. Wii Balance Board: Reliability and clinical use in assessment of balance in healthy elderly women. CNS & Neurological Disorders-Drug Targets. 2015;14(9):1165–1170. doi: 10.2174/1871527315666151111120403. [DOI] [PubMed] [Google Scholar]
- Morris SL, Foster CJ, Parsons R, Falkmer M, Falkmer T, Rosalie SM. Differences in the use of vision and proprioception for postural control in autism spectrum disorder. Neuroscience. 2015;307:273–280. doi: 10.1016/j.neuroscience.2015.08.040. [DOI] [PubMed] [Google Scholar]
- Radonovich KJ, Fournier KA, Hass CJ. Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Frontiers in Integrative Neuroscience. 2013;7:28. doi: 10.3389/fnint.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/ [Google Scholar]
- Smoot Reinert S, Jackson K, Bigelow K. Using posturography to examine the immediate effects of vestibular therapy for children with Autism Spectrum Disorders: A feasibility study. Physical & Occupational Therapy in Pediatrics. 2015;35(4):365–380. doi: 10.3109/01942638.2014.975313. [DOI] [PubMed] [Google Scholar]
- Tarakci D, Ersoz Huseyinsinoglu B, Tarakci E, Razak Ozdincler A. Effects of Nintendo Wii-Fit(®) video games on balance in children with mild cerebral palsy. Pediatrics International: Official Journal of the Japan Pediatric Society. 2016;58(10):1042–1050. doi: 10.1111/ped.12942. [DOI] [PubMed] [Google Scholar]
- Tarakci D, Ozdincler AR, Tarakci E, Tutuncuoglu F, Ozmen M. Wii-based balance therapy to improve balance function of children with Cerebral Palsy: A pilot study. Journal of Physical Therapy Science. 2013;25(9):1123–1127. doi: 10.1589/jpts.25.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Powell PS, Klinger LG, Klinger MR. Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability. Journal of Autism and Developmental Disorders. 2013;43(7):1568–1583. doi: 10.1007/s10803-012-1702-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Hsiao-pin C. WASI-II: Wechsler abbreviated scale of intelligence. San Antonio, TX: Pearson; 2011. [Google Scholar]
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: Evidence for a deficit in proprioception. Journal of Developmental and Behavioral Pediatrics. 2001;22(2):92–101. doi: 10.1097/00004703-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. New York, NY: John Wiley & Sons; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.