Abstract
Isolated MYC rearrangement without other recurrent genetic abnormalities is rare in B lymphoblastic leukemia/lymphoma (B-ALL/LBL), with most cases reported in pediatric patients. We report three adult cases with lymphoblasts showing a precursor B cell immunophenotype, and isolated MYC/IGH translocation. All three cases occurred in male patients with initial presentation of diffuse lymphadenopathy. Cases and 1 and 2 had B-ALL with significantly increased lymphoblasts in peripheral blood and bone marrow. Case 3, a patient with human immunodeficiency virus infection, had the diagnosis of B-LBL made on a retroperitoneal lymph node biopsy and had no peripheral blood or bone marrow involvement. The leukemic and lymphoma cells in all three cases demonstrated Burkitt lymphoma-like morphology with deeply basophilic cytoplasm and numerous cytoplasmic vacuoles. However, all three had immature immunophenotypes including expression of TdT, absence of BCL6, and dim-to-negative CD45. CD20 was largely negative in 2 of 3 cases. All three had confirmed MYC/IGH translocation, but lacked rearrangements of BCL2 or BCL6. EBV was negative by EBER in situ hybridization. Treatment protocols varied, including both high risk ALL-type (protocol 8707) and high grade lymphoma regimens (Hyper-CVAD), but no patient achieved continuous complete remission. These cases appear to represent a distinct biological phenomenon, in which a MYC translocation may be acquired at an immature stage of differentiation, thus manifesting features of both B-ALL/LBL and Burkitt lymphoma.
Keywords: B lymphoblastic leukemia/lymphoma, MYC rearrangement, Burkitt lymphoma, terminal transferase, gene rearrangement
INTRODUCTION
B lymphoblastic leukemia/lymphoma (B-ALL/LBL) is a hematologic malignancy characterized by proliferation of neoplastic B-lymphoblasts in the bone marrow, peripheral blood, and lymphoid organs. It is the predominant form of ALL/LBL and accounts for 80–85% of all cases 1. In the past few decades, the survival rate of B-ALL/LBL has improved dramatically and is approximately 90% in the pediatric population 2. However, the 5-year overall survival (OS) remains at 30%–40% in adults and elderly patients despite our current knowledge of the molecular and cytogenetic underpinnings of B-ALL/LBL 3. Therefore, a better understanding of the biology of B-ALL/LBL is needed to determine additional factors related to these poor outcomes.
MYC (c-MYC) is a transcription factor that plays multifunctional roles in cell cycle regulation, apoptosis, and cellular transformation to high grade malignancies. In the pathogenesis of lymphoid neoplasms, the MYC gene on chromosome 8q24 is translocated to an immunoglobulin heavy chain gene on chromosome 14q32, or less commonly, the kappa and lambda light chain genes on chromosomes 2p12 and 22q11, leading to overexpression of c-MYC and induction of cellular proliferation 4. MYC rearrangement was first discovered in Burkitt lymphoma and is commonly seen in other aggressive mature B cell lymphomas such as diffuse large B cell lymphoma (DLBCL) and plasmablastic lymphoma (PBL) 5, 6. MYC rearrangement also has been described in B-ALL/LBL with concurrent BCL2 rearrangement. However, these cases are most often secondary, representing progression from follicular lymphoma 7–10. MYC rearrangement is rarely observed in B-ALL/LBL in the absence of the t(14;18) IGH/BCL2 rearrangement.
In this report we present three unusual adult cases of B-ALL/LBL with Burkitt lymphoma-like morphology. Lymphoblasts in all three cases harbored t(8;14)(q24;q32) IGH/MYC translocation. The initial differential diagnosis included Burkitt leukemia/lymphoma, B ALL/LBL, and high grade B cell lymphoma, NOS, based on the morphology and MYC translocation 6. However, all cases displayed an immature B-cell immunophenotype, leading to a final diagnosis of MYC+B-ALL/LBL.
MATERIALS AND METHODS
Clinical Features
Case 1
A 70-year-old man presented with dyspnea on exertion, worsening back pain and acute renal failure. The patient had a past medical history of hypertension, diabetes, hyperlipidemia, pulmonary embolism and chronic back pain. Imaging studies revealed massive splenomegaly, anterior pericardial, upper abdominal, and retroperitoneal lymphadenopathy. A complete blood count (CBC) showed a white cell count of 14.3 × 109/L, Hemoglobin 11.0 g/dL, MCV 80 fL, and platelet count of 38 × 109/L. The patient was also found to have elevated LDH (2309 U/L, reference range 122–222 U/L), uric acid (11.3 mg/dl, reference range 3.7–8.0 mg/dL), and Beta hydroxybutyrate (1.3 mmol/L, reference range <0.4 mmol/L). Peripheral blood and bone marrow demonstrated 20% and 80% blasts, respectively. Flow cytometry and immunohistochemical stains confirmed the diagnosis of B-ALL. The patient received Hyper-CVAD (cyclophosphamide, vincristine, Adriamycin, and dexamethasone), and intrathecal methotrexate. Unfortunately, the patient developed kidney failure and expired while undergoing therapy.
Case 2
A 56-year-old previously healthy man presented with two weeks of increasing fatigue and intermittent headaches. Imaging studies demonstrated mediastinal and axillary lymphadenopathy and hepatosplenomegaly. CBC showed a white blood cell count of 129.0 × 109/L, Hemoglobin 10.8 g/dL, MCV 91 fL, and platelet count of 13 × 109/L. The patient was also found to have an elevated LDH 7033 U/L, creatinine (2.6 mg/dL, reference range 0.71–1.22 mg/dL), and markedly increased aspartate transaminase (233U/L, reference range 17–42 U/L). Both peripheral blood and bone marrow showed over 90% blasts. Flow cytometry and immunohistochemical stains confirmed the diagnosis of B-ALL. After diagnosis, the patient received intensified and shortened cyclical chemotherapy (protocol 8707) 11 with initial remission and minimal residual disease, but with progression two months after treatment. The patient refused further intervention and presented with recurrent leukocytosis seven months after initial diagnosis. Protocol 8707 resumed and patient achieved remission with pancytopenia. The patient was transferred to another hospital and was lost to follow up.
Case 3
A 44-year-old man presented with right flank and back pain for one month without fever or significant weight loss. He had a history of Human Immunodeficiency Virus (HIV) infection for six years without prior treatment. Imaging studies showed bulky mesenteric and retroperitoneal lymphadenopathy abutting the right ureter, and a few small right axillary and mediastinal lymph nodes. CBC showed white cell count of 6.9 × 109/L, Hemoglobin 12.9 g/dL, MCV 88 fL, and platelet count of 275 × 109/L. No circulating blasts were seen. The patient had elevated LDH 509 U/L while liver and kidney function tests were within the normal range. His CD4 T cell count was 316/μL with an HIV viral load of 24,000 copies/mL. A diagnosis of B-LBL was made on the retroperitoneal lymph node biopsy. Bone marrow biopsy revealed 15–20% polytypic plasma cells with no evidence of involvement by leukemia/lymphoma. Cerebrospinal fluid also showed no evidence of malignancy. After diagnosis, patient received Hyper-CVAD and decreased lymphadenopathy, but with persistent disease. The patient was deemed to be in partial remission and is being prepared for a stem cell transplant.
Immunohistochemistry & EBER in situ hybridization
Immunohistochemical stains for BCL-2, BCL-6, CD10, CD19, CD20, CD34, CD79a, CD99, CD117, C-MYC, Cyclin D1, MPO, MUM-1, PAX-5, TdT, and Ki-67 (DAKO, Santa Clara, CA; LEICA Microsystems, Buffalo Grove, IL; Abcam, Cambridge, MA) were performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections following routine procedures. EBER in situ hybridizattion was performed on FFPE sections as previously described 12.
Flow cytometry
Bone marrow aspirate and fresh tissue specimens were processed and incubated with antibodies for 15–20 minutes, washed, and resuspended with phosphate-buffered saline (PBS). The sample was then analyzed by flow cytometry immunophenotyping. Fluorochrome-conjugated monoclonal antibodies against the following antigens were used: CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD11b, CD11c, CD13, CD15, CD16, CD19, CD20, CD22, CD23, CD33, CD34, CD38, CD45, CD56, CD64, CD79a, CD117, cMPO, cCD3, HLA-DR, sKappa, sLambda, TdT, (Becton Dickinson, San Diego, CA, DAKO, Santa Clara, CA). Cell viability was distinguished by staining with 7-amino-actinomycin D or Propidium Iodide in case 2. All fresh samples and cell lines were gated according to their light scatter characteristics. Data were analyzed using BD FACS Canto II flow cytometer (Becton Dickinson, San Jose, CA).
Fluorescence in situ hybridization (FISH)
FISH analysis was performed on bone marrow aspirate and lymph node specimens according to standard protocols by Mayo Clinic Laboratories (Rochester, MN) in case 1, Integrated Oncology (Phoenix, AZ) in case 2, and NeoGenomics Laboratories (Fort Myers, FL) in case 3. At least 200 interphase nuclei were analyzed for each probe. FISH probes used in this study included C-MYC dual-color, break-apart rearrangement probe; MYC/IGH dual-color, dual fusion translocation probe; MYC/IGK dual-color, dual-fusion translocation probe; MYC/IGL dual-color, dual-fusion translocation probe; and CEN8/MYC/IGH three-color, dual-fusion translocation probe. For investigation of the BCL2 and BCL6 genes, dual-color break-apart rearrangement probes were used, targeting 18q21 and 3q27 respectively.
Cytogenetic analysis
Conventional cytogenetic analysis was performed on metaphase cells prepared from bone marrow aspirate specimens by Mayo Clinic Laboratories (Rochester, MN) in case 1, and UCSF Medical Center in case 2 using standard techniques. Giemsa-banded metaphases were analyzed, and the results were reported using the International System for Human Cytogenetic Nomenclature.
RESULTS
Microscopic findings
In case 1, peripheral blood and bone marrow demonstrated 20% and 80% blasts, respectively. In case 2, both peripheral blood and bone marrow showed over 90% blasts. Circulating blasts in both cases were intermediate in size, demonstrated round to ovoid nuclei, fine chromatin, and deeply basophilic cytoplasm with numerous vacuoles, resembling Burkitt lymphoma (Fig. 1). Bone marrow aspirate and biopsy revealed a diffuse infiltrate of blasts with similar morphology. Mitotic figures and tingible body macrophages were evident. In case 3, H&E sections of the core biopsy of retroperitoneal lymph node revealed an infiltrate of atypical lymphoid cells in a fibrotic backgroundl (Fig. 2). The cells were intermediate-to-large in size with irregular nuclei, fine chromatin, small basophilic nucleoli and a variably high nuclear-to-cytoplasmic ratio. Mitotic figures were easily identified. Scattered small mature lymphocytes were intermixed. The atypical lymphoid cells on touch preps showed Burkitt lymphoma-like morphology with frequent cytoplasmic vacuoles.
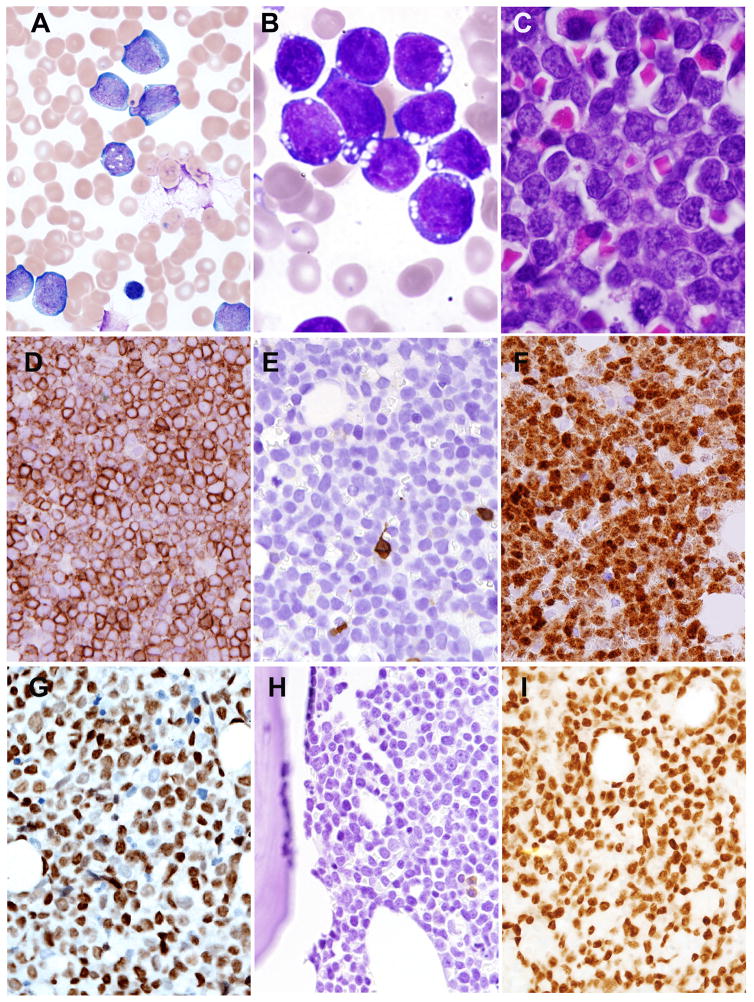
Fig. 1. Bone marrow and peripheral blood findings of MYC+ B-ALL/LBL.
A: Blasts in peripheral blood smear (Wright-Giemsa stain, 1000× (case 2) were intermediate-in-size, with ovoid-to-irregular nuclei, fine chromatin, occasional prominent nucleoli, and scant-to-moderate deeply basophilic cytoplasm. B: Bone marrow aspirate smear, case 1 (Wright-Giemsa stain, 1000×) showed cells with high nuclear:cytoplasmic ratio, deeply basophilic cytoplasm, and cytoplasmic vacuoles. C: Bone marrow core biopsy (H&E 1000×, (case 1) showed a diffuse infiltrate of blasts with finely dispersed chromatin and small nucleoli. Immunohistochemical findings (Case 1) showed positive staining for CD19 (D), but absence of CD20 (E). TDT showed strong nuclear staining (F). The blasts show nuclear staining for MYC (Case 2) (G). BCL-6 was negative (H), but PAX5 was positive (I).
Fig 2. Lymph node biopsy in MYC+ B-ALL/LBL.
A. A core biopsy of retroperitoneal lymph node (H&E, 40×) (case 3) showed diffuse sclerosis with a dense atypical lymphoid infiltrate. B. The infiltrate (H&E, 400×) was composed of intermediate-to-large atypical lymphoid cells with irregular nuclei, fine chromatin, small nucleoli, and variably high nuclear-to-cytoplasmic ratio. Scattered small lymphocytes were intermixed. The tumor cells were positive for PAX5 (C) and showed strong nuclear staining for TDT (D).
Immunohistochemical stains were performed on bone marrow and lymph node biopsies. In case 1, the blasts were positive for CD19, PAX-5, CD79a, CD10, TdT (diffuse and strong), MUM-1, and C-MYC, and were negative for CD20, CD34, BCL-2, BCL-6, CyclinD1, CD99, MPO, and CD117. Ki-67 showed a proliferative rate of approximately 90%. In case 2, the blasts were positive for PAX-5, CD79a, CD10, TdT (10%), C-MYC, and were negative for CD20, CD34, BCL-2, BCL-6, and CyclinD1. Ki-67 showed a very high proliferative rate of >90%. In case 3, the atypical cells were positive for PAX-5, TdT (diffuse and strong), CD20, CD10, CD99, and were negative for CD34, BCL-2, BCL-6, MUM-1, and CD117. Ki-67 showed a proliferative rate of >90%. CD3 highlighted interspersed background small T lymphocytes. EBER in situ hybridization was negative in all three cases.
Flow cytometry
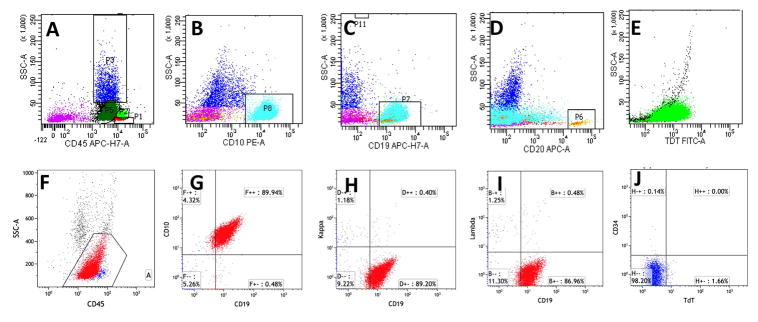
In case 1, flow cytometric analysis of bone marrow showed 75% of events were an atypical population in the CD45 dim gate (Fig. 3A) with expression of CD10 (Fig. 3B), CD19 (Fig. 3C), dim-to absent CD20 (Fig. 3D), TdT (Fig. 3E), and Lambda light chain restriction, and with no expression of CD34. In case 2, flow cytometry analysis of bone marrow demonstrated 87% of events were abnormal B-cells with slightly dim CD45 and low side scatter (Fig. 3F) with expression of CD10, CD19 (Fig. 3G), weak CD20 (15% of gated events), CD22, weak CD33, bright CD38, and cCD79a, without co-expression of surface Kappa (Fig. 3H) or Lambda light chains (Fig. 3I). Possible minor subsets expressed dim CD34 or TdT (<5% of gated events, Fig. 3J). These abnormal B-cells were negative for T-cell markers, myeloid markers, MPO, and cCD3. In case 3, flow cytometry analysis was limited by low cellularity, while the CD45 negative cells were positive for CD19, CD20, and CD10 and were negative for surface light chains.
Fig. 3. Flow cytometry analysis of bone marrow.
In case 1, the atypical lymphoid cells showed dim CD45 and lower side scatter (A, dark green), with expression of CD10 (B, cyan), CD19 (C, cyan), dim-to-absent expression of CD20 (D, cyan), and TdT (E, green); In case 2, the atypical lymphoid cells with dim CD45 and low side scatter gate (F) showed expression of CD19 (G), without expression of kappa (H) and lambda (I) light chains. Only few cells expressed dim CD34 or TdT (<5% of gated events, J).
FISH
FISH studies of all three cases revealed t(8;14)(q24;q32) IGH/MYC translocation. There was no evidence of t(14;18)(q32;21) IGH/BCL2 or BCL6 rearrangement. In case 1, Approximately 8% of nuclei had three to four copies of the IGL gene region (at 22q11.2). This result indicated additional copies of chromosome 22 or a 22q duplication. In case 3, there were additional copies of the BCL6 gene or chromosome 3, suggesting gains of BCL6 gene or chromosome 3.
Cytogenetic findings
In case 1, 7 of the 20 metaphases were normal, while 8 metaphases had a 5q deletion and 5 metaphases had loss of the Y chromosome. To determine if the metaphases with 5q deletion were associated with the B-cell lymphoma clone, FISH for MYC and IGH was performed on several metaphases with the 5q deletion. These results indicated a normal result for MYC and IGH in the 5q deletion metaphases. The combined chromosome and metaphase FISH results strongly suggested that the metaphases with the 5q deletion represent a neoplastic process unrelated to the B-ALL/LBL and were concerning for an evolving/underlying myeloid neoplasm. It was noted that megakaryocytes were clustered in the marrow with small hypolobated forms. This was consistent with the cytogenetics finding of 5q- deletion and a diagnosis of myelodysplastic syndrome 5q- 13. In case 2, chromosomal study was limited with reduced capability of detecting clonal abnormalities. Only 4 analyzable metaphase cells were obtained from this specimen and 3 of them had an apparently normal male karyotype. The remaining cell showed a loss of chromosome 14, which could not be further evaluated in this study. A full cytogenetic karyotype was not obtained in case 3.
DISCUSSION
In the WHO classification, Burkitt lymphoma is defined as a mature B-cell lymphoma, and lacks the precursor B cell immunophenotype of B-ALL/LBL. While it occasionally presents with bone marrow or peripheral blood involvement, as seen in the L3 subtype of ALL in the FAB classification (1990) 14, these cases share the mature B-cell phenotype of Burkitt lymphoma. Although implicated in many mature B cell lymphomas, MYC rearrangement has been reported only rarely in B-ALL/LBL. In a study of 5280 cases of pediatric ALL, only five with isolated MYC rearrangement were identified, fewer than 0.1% 15. MYC rearrangement has been reported rarely in lymphoblastic malignancies occurring as progression of underlying follicular lymphoma. These tumors manifest a double-hit, with rearrangements of both the MYC and BCL2 genes, and can present either in bone marrow or peripheral lymphoid tissues 8–10. De novo lymphoblastic malignancies with an isolated MYC translocation have been only rarely identified 7.
Since 1980, only 13 cases of B-ALL/LBL with isolated MYC rearrangement were reported 15–23. Like other B-ALL/LBLs, the majority of the cases occurred in pediatric patients and only three cases were reported in adults 17, 24, 25 (Table 1), including two cases of B-ALL and one case of B-LBL without bone marrow involvement 25. The majority of the cases demonstrated Burkitt-like morphology. In this study, we report three additional adult cases with a precursor B cell immunophenotype, Burkitt leukemia/lymphoma-like morphology, and isolated MYC rearrangement. Similar to previously reported adult cases, two of our cases were B-ALL and one was B-LBL without bone marrow and peripheral blood involvement.
Table 1.
Clinical and pathological features of adult B-ALL/LBL with isolated MYC translocation
| Prior and current reports | Age | Sex | Initial presentation | Treatment and Course | FISH(%) Cytogenetics |
BCL2 R BCL6 R |
CD10 | CD19 | CD20 | BCL-6 | CD34 | TdT | sIg | EBER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Komrokji R, et al | 45 | M | B symptoms, splenomegaly | Hyper-CVAD alternating with high dose MTX/ARA-C | t(8,14)(q24,q32), +i(1)(q10) | NT | + | + | + | NT | − | − | − | NT |
| Shiratori S, et al | 64 | F | Choroid and skin involvement by B-LBL | Achieved CR after R-CHOP × 6; relapsed on skin 2 years later, achieved CR after radiation therapy; a further 2 years later, skin and choriod relapse as in initial stage | t(8;14)(q24;q32); positive IGH and TCR gene rearrangement | − | + | + | + | − | NT | + | NT | NT |
| Slavutsky I, et al | 20 | F | B symptoms, hepatomegaly, large retroperitoneal lymphadenopathy, and nephromegaly | n/a | t(8;22)(q24;q 11); hyperploid | NT | + | + | NT | NT | NT | NT | − | − |
| Li Y, et al | ||||||||||||||
| Case 1 | 70 | M | Diffuse lymphadnopathy, splenomegaly, PB and BM involvement | Hyper-CVAD and intrathecal methotrexate. Expired during the second cycle of chemotherapy | MYC/IGH (55%); 5q deleion (7/20) metaphases | − | + | + | − | − | − | + | + | − |
| Case 2 | 56 | M | Diffuse lymphadenopathy, hepatosplenomegaly, PB and BM involvment | High-risk ALL protocol (8707) with initial remission, but with progression two months after treatment. | MYC/IGH (60%) | − | + | + | − | − | − | −/+ | − | − |
| Case 3 | 44 | M | Bulky intrabdominal lymphadenopathy, negative BM | Hyper-CVAD with partial remission, pending stem cell transplant | MYC/IGH (63%) | − | + | NT | + | − | − | + | NT | − |
Abbreviations: NT, not tested; PB, peripheral blood; BM, bone marrow
In the two cases of adult B-ALL with isolated MYC rearrangement, the morphology of the blasts resembled that of Burkitt lymphoma, with deeply basophlic cytoplasm and cytoplasmic vacuoles. In the case of B-LBL, the touch prep showed similar cytological features as the B-ALL cases, while the nuclei were more pleomorphic on the core biopsy, probably due to the background sclerosis. Based on these morphological features, Burkitt leukemia/lymphoma was considered in the differential diagnosis. The distinction between Burkitt leukemia/lymphoma and B-ALL/LBL is important, as different treatment strategies would be applied, depending on the final diagnosis.
Among all of the reported adult B-ALL/LBL cases, case 1 in our report is the only one showing surface light chain immunoglobulin expression. Case 1 was classified as B-ALL/LBL based on the dim CD45 expression, strong and diffuse TdT expression and dim-to-absent CD20 in the lymphoblasts. Although unusual, coexpression of surface light chains and TdT has been reported in B-ALL previously 23, 26. In fact, the expression of TdT was variable in the reported pediatric and adult cases B-ALL/LBL with isolated MYC rearrangement, a finding recapitulated in our series. Case 2 was positive for TdT in only 10% of the cells, but had an immature phenotype by other criteria. CD34 has been reported negative in all adult cases, and was positive in only one of the pediatric cases 15. Futher evidence against a diagnosis of Burkitt lymphoma was absence of BCL-6 expression in all reported cases, including the current report. The variation in CD20, CD34, TdT, and surface immunoglobulin expression in these cases indicates the arrest of differentiation at varying stages of precursor B-cell maturation.
Compared to MYC+ B-ALL, B-LBL with isolated MYC rearrangement is extremely rare. To the best of our knowledge, there was only one case reported previously. Case 3 in this study may represent the second case, presenting as lymphadenopathy without bone marrow and peripheral blood involvement. Hepatomegaly or splenomegaly were part of the initial clinical presentation of the MYC+ B-ALL cases but were not seen in those presenting as lymphoma. The previously reported MYC+ B-LBL patient was treated with R-CHOP and our patient was treated with Hyper-CVAD. Both patients showed a good response to chemotherapy, suggesting that a treatment approach for Burkitt lymphoma rather than B-ALL/LBL may be warranted in these patients.
MYC rearrangement is most frequently associated with Burkitt lymphoma, yet it also has been reported in other mature and immature B-cell neoplasms. The rearrangement most commonly involves MYC at 8q24 and the IGH gene 14q32. A minority of the cases carry t(2;8)(p11;q24) or t(8;22)(q24;q11) chromosomal rearrangements. These three rearrangements share the juxtaposition of MYC proto-oncogene on chromosome 8 and either the heavy or light chain immunoglobulin genes 27. MYC rearrangement was also reported to involve a non-immunoglobulin chromosomal locus t(4;8)(q31.1;q24.1) in the B-lymphoblasts of a 4-year-old boy 19. This novel translocation was detected at the time of his second relapse, and the patient died 3 months later without achieving complete remission. Due to the rarity of these cases, MYC is not routinely tested in B-ALL/LBL. However, our study calls attention to this rare event, and additional FISH or cytogenetic studies would be indicated in cases with variant morphology, as reported here.
Isolated MYC rearrangement without other recurrent genetic abnormalities represents a rare entity in B-ALL/LBL, especially in adult patients.
These cases appear to represent a distinct biological phenomenon, in which a MYC translocation may be acquired at an immature stage of differentiation, in which the tumor cells still express TDT and lack a mature B-cell phenotype. The mechanism underlying the MYC translocation may differ in these novel cases, and requires further investigation. Differences in the site of the MYC break have been reported in different forms of Burkitt lymphoma, with differences seen in endemic and sporadic cases 28, 29. Thus, the maturational stage of the B-cell in which the translocation occurs is thought to influence the site of the MYC break. Although some pediatric B-ALL/LBL with isolated MYC rearrangement have been successfully treated, relapse is common in adult cases, which indicates a need for a better understanding of this rare entity in the adult population.
Fig. 4. FISH analysis of bone marrow, Case 1.
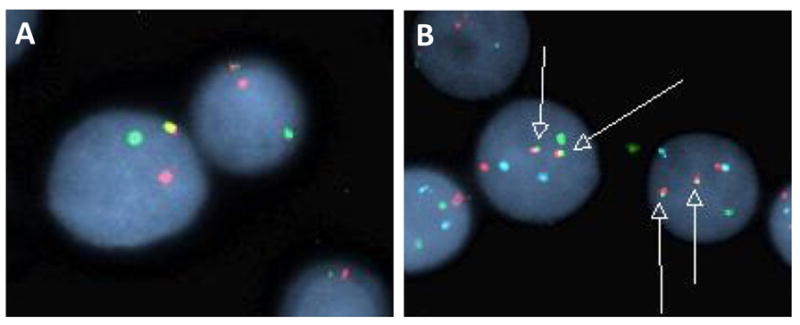
A. Breakapart probe with red fluorochrome labeling 5′MYC and the green fluorochrome labeling 3′MYC. Result shows split of red-green signals in tumor cells. B. Dual color double fusion probes with the red fluorochrome directly labeling MYC spanning the known breakpoints (8q24), the green fluorochrome directly labeling the known breakpoints of IGH (14q32), and an aqua fluorochrome directed at the alpha satellite centromeric region of chromosome 8 (D8Z2). Result indicates one separate red signal, one separate green signal and two orange/green fusion signals (arrows) indicating the MYC/IGH translocation.
Footnotes
The authors report no disclosures or conflicts of interest.
References
- 1.Steven H, Swerdlow EC, Harris Nancy L, Jaffe Elaine S. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. World Health Organization; 2008. [Google Scholar]
- 2.Antillon FG, Blanco JG, Valverde PD, et al. The treatment of childhood acute lymphoblastic leukemia in Guatemala: Biologic features, treatment hurdles, and results. Cancer. 2016 doi: 10.1002/cncr.30257. [DOI] [PubMed] [Google Scholar]
- 3.Jabbour E, O’Brien S, Konopleva M, et al. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121:2517–2528. doi: 10.1002/cncr.29383. [DOI] [PubMed] [Google Scholar]
- 4.Van Krieken JH, Kluin PM. The association of c-myc rearrangements with specific types of human non-Hodgkin’s lymphomas. Leuk Lymphoma. 1992;7:371–376. doi: 10.3109/10428199209049793. [DOI] [PubMed] [Google Scholar]
- 5.Cai Q, Medeiros LJ, Xu X, et al. MYC-driven aggressive B-cell lymphomas: biology, entity, differential diagnosis and clinical management. Oncotarget. 2015;6:38591–38616. doi: 10.18632/oncotarget.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loghavi S, Kutok JL, Jorgensen JL. B-acute lymphoblastic leukemia/lymphoblastic lymphoma. Am J Clin Pathol. 2015;144:393–410. doi: 10.1309/AJCPAN7BH5DNYWZB. [DOI] [PubMed] [Google Scholar]
- 8.Geyer JT, Subramaniyam S, Jiang Y, et al. Lymphoblastic transformation of follicular lymphoma: a clinicopathologic and molecular analysis of 7 patients. Hum Pathol. 2015;46:260–271. doi: 10.1016/j.humpath.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Kobrin C, Cha SC, Qin H, et al. Molecular analysis of light-chain switch and acute lymphoblastic leukemia transformation in two follicular lymphomas: implications for lymphomagenesis. Leuk Lymphoma. 2006;47:1523–1534. doi: 10.1080/10428190600612909. [DOI] [PubMed] [Google Scholar]
- 10.de Jong D, Voetdujk B, Baverstock G, et al. Activation of the c-myc oncogene in a precursor B-cell blast crisis of follicular lymphoma, presenting as composite lymphoma. N Engl J Med. 1988;318:1373–1378. doi: 10.1056/NEJM198805263182106. [DOI] [PubMed] [Google Scholar]
- 11.Linker C, Damon L, Ries C, et al. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2464–2471. doi: 10.1200/JCO.2002.07.116. [DOI] [PubMed] [Google Scholar]
- 12.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 14.Imamura N, Mtasiwa DM, Ota H, et al. FAB L3 type of B-cell acute lymphoblastic leukemia (B-ALL) without chromosome abnormalities. Am J Hematol. 1990;35:216–218. doi: 10.1002/ajh.2830350316. [DOI] [PubMed] [Google Scholar]
- 15.Navid F, Mosijczuk AD, Head DR, et al. Acute lymphoblastic leukemia with the (8;14)(q24;q32) translocation and FAB L3 morphology associated with a B-precursor immunophenotype: the Pediatric Oncology Group experience. Leukemia. 1999;13:135–141. doi: 10.1038/sj.leu.2401244. [DOI] [PubMed] [Google Scholar]
- 16.Yasuhiko Kaneko JR, Check Irene, Variakojis Diana, Moohr John. The 14q+ chromosome in Pre-B-ALL. Blood. 1980;56:782–785. [PubMed] [Google Scholar]
- 17.Komrokji R, Lancet J, Felgar R, et al. Burkitt’s leukemia with precursor B-cell immunophenotype and atypical morphology (atypical Burkitt’s leukemia/lymphoma): case report and review of literature. Leukemia Research. 2003;27:561–566. doi: 10.1016/s0145-2126(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 18.Meznarich J, Miles R, Paxton CN, et al. Pediatric B-Cell Lymphoma With Lymphoblastic Morphology, TdT Expression, MYC Rearrangement, and Features Overlapping With Burkitt Lymphoma. Pediatr Blood Cancer. 2016;63:938–940. doi: 10.1002/pbc.25907. [DOI] [PubMed] [Google Scholar]
- 19.Seo JY, Lee SH, Kim HJ, et al. MYC rearrangement involving a novel non-immunoglobulin chromosomal locus in precursor B-cell acute lymphoblastic leukemia. Ann Lab Med. 2012;32:289–293. doi: 10.3343/alm.2012.32.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan R, Felisbino F, Stefanoff CG, et al. Burkitt lymphoma/leukaemia transformed from a precursor B cell: clinical and molecular aspects. Eur J Haematol. 2008;80:265–270. doi: 10.1111/j.1600-0609.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AA, Grant R, Shago M, et al. Occurrence of t(8;22)(q24.1;q11.2) involving the MYC locus in a case of pediatric acute lymphoblastic leukemia with a precursor B cell immunophenotype. J Pediatr Hematol Oncol. 2004;26:532–534. doi: 10.1097/01.mph.0000132736.31514.28. [DOI] [PubMed] [Google Scholar]
- 22.Kaplinsky C, Rechavi G. Acute lymphoblastic leukemia of Burkitt type (L3 ALL) with t(8;14) lacking surface and cytoplasmic immunoglobulins. Med Pediatr Oncol. 1998;31:36–38. doi: 10.1002/(sici)1096-911x(199807)31:1<36::aid-mpo9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Higa B, Alkan S, Barton K, et al. Precursor B-cell acute lymphoblastic leukaemia with FAB L3 (i.e., Burkitt’s leukaemia/lymphoma) morphology and co-expression of monoclonal surface light chains and Tdt: report of a unique case and review of the literature. Pathology. 2009;41:495–498. doi: 10.1080/00313020903040988. [DOI] [PubMed] [Google Scholar]
- 24.Slavutsky I, Andreoli G, Gutierrez M, et al. Variant (8;22) translocation in lymphoblastic lymphoma. Leuk Lymphoma. 1996;21:169–172. doi: 10.3109/10428199609067595. [DOI] [PubMed] [Google Scholar]
- 25.Shiratori S, Kondo T, Fujisawa S, et al. c-myc rearrangement in B-cell lymphoblastic lymphoma with the involvement of multiple extranodal lesions. Leuk Lymphoma. 2011;52:716–718. doi: 10.3109/10428194.2010.551158. [DOI] [PubMed] [Google Scholar]
- 26.Nelson BP, Treaba D, Goolsby C, et al. Surface immunoglobulin positive lymphoblastic leukemia in adults; a genetic spectrum. Leuk Lymphoma. 2006;47:1352–1359. doi: 10.1080/10428190500473238. [DOI] [PubMed] [Google Scholar]
- 27.Behm FCD. Immunophenotyping. In: Pui C-H, editor. Childhood leukemias. 1999. p. 111. [Google Scholar]
- 28.Bertrand P, Bastard C, Maingonnat C, et al. Mapping of MYC breakpoints in 8q24 rearrangements involving non-immunoglobulin partners in B-cell lymphomas. Leukemia. 2007;21:515–523. doi: 10.1038/sj.leu.2404529. [DOI] [PubMed] [Google Scholar]
- 29.Pelicci P, Knowles D, Magrath I, et al. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci USA. 1986;83:2984–2988. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]