Abstract
We found that the anticoagulant plasma protease, activated protein C (APC), stimulates the energy sensor kinase, AMPK, in the stressed heart by activating protease-activated receptor 1 (PAR1) on cardiomyocytes. Wild-type (WT) and AMPK-kinase dead (KD) transgenic mice were subjected to transverse aortic constriction (TAC) surgery. The results demonstrated that while no phenotypic differences can be observed between WT and AMPK-KD mice under normal physiological conditions, AMPK-KD mice exhibit significantly larger hearts after 4 weeks of TAC surgery. Analysis by echocardiography suggested that the impairment in the cardiac function of AMPK-KD hearts is significantly greater than that of WT hearts. Immunohistochemical staining revealed increased macrophage infiltration and ROS generation in AMPK-KD hearts after 4 weeks of TAC surgery. Immunoblotting results demonstrated that the redox markers, pShc66, 4-hydroxynonenal and ERK, were all up-regulated at a higher extent in AMPK-KD hearts after 4 weeks of TAC surgery. Administration of APC-WT and the signaling selective APC-2Cys mutant, but not the anticoagulant selective APC-E170A mutant, significantly attenuated pressure overload-induced hypertrophy and fibrosis. Macrophage infiltration and pShc66 activation caused by pressure overload were also inhibited by APC and APC-2Cys but not by APC-E170A. Therefore, the cardiac AMPK protects against pressure overload-induced hypertrophy and the signaling selective APC-2Cys may have therapeutic potential for treating hypertension-related hypertrophy without increasing the risk of bleeding.
Keywords: APC, PAR1, Cytoprotective, AMPK, Hypertrophy
1. Introduction
Nearly 1 in 3 Americans has hypertension [1]. Hypertension is a major risk factor for death and disability from the heart and vascular diseases [2]. Hypertensive heart disease refers to a number of disorders that include heart failure, left ventricular hypertrophy (LVH), and other cardiac conditions which may have clinical symptoms such as arrhythmias and symptomatic heart failure [3]. LVH is a compensatory mechanism in response to high blood pressure. However, there is considerable individual variability in the progression of LVH and geometric pattern (concentric and eccentric hypertrophy). There is compelling evidence pointing toward operative genetic influences being important contributors to different outcomes of hypertensive heart disease [3].
The cardiac hypertrophy caused by pressure overload is characterized by increased AMP and decreased ATP levels [4]. The increased ratio of AMP to ATP (AMP/ATP) can result in the activation of adenosine monophosphate-activated protein kinase (AMPK), which is the major energy sensor in the heart [5,6]. The AMPK activity and protein expression levels are both shown to be increased by pressure overload-induced hypertrophy. The up-regulation of AMPK not only can decrease fatty acid oxidation, but also increase glycolysis and glucose uptake in the hypertrophied heart, which are considered as compensating metabolic adaptive mechanisms essential for maintaining energy homeostasis [7]. Furthermore, AMPK can reduce the endoplasmic reticulum (ER) stress and also inhibit nuclear factor κB (NF-κB) activation in vascular endothelial cells, indicating that AMPK plays an important role in regulating inflammatory pathways [8,9]. However, the mechanism of the anti-inflammatory effect of AMPK has not been investigated in the pressure overload-induced hypertrophy model.
Inflammation plays a pivotal role in ventricular remodeling and cardiac damage [10]. Recent studies have identified the activation of a variety of inflammatory cells and molecules in the failing heart, such as T cells and inflammatory cytokines (interleukin-6, tumor necrosis factor-alpha) [11,12]. It follows, therefore, that immunosuppressive and anti-inflammatory strategies could constitute new therapeutic approaches to ameliorate hypertensive heart disease [11,13]. Activated protein C (APC) is a vitamin-K dependent plasma serine protease which inhibits blood clotting. APC also exhibits anti-inflammatory and anti-apoptotic activities and up-regulates expression of cytoprotective genes. APC has been demonstrated to protect the heart against ischemia-reperfusion damage through the activation of AMPK and the down-regulation of NF-κB and inflammatory cytokines [14]. Furthermore, using an angiotensin II infusion model, APC has been demonstrated to protect the heart from myocardial fibrosis development [15]. However, the function of APC in the pressure overload-induced cardiac hypertrophy model has not been investigated.
Thus, this study was designed to address this question and to examine the role of AMPK in the pressure overload-induced hypertrophic heart. We used both wild-type (WT) and AMPK kinase dead (AMPK-KD) mice to perform the trans-aortic constriction (TAC) surgery to investigate these questions [16].
2. Material and Methods
2.1 Experimental animals
We used wild-type (WT) C57BL/6J and AMPK kinase dead mice (AMPK KD, expressing a KD α2 K45R mutation driven in the heart and skeletal muscles by the muscle creatine kinase promoter) [17]. All mice were 4–6 months old and of a C57BL/6 background. All animal protocols in this study were approved by the Institutional Animal Care and Use Committee of the University at State University of New York at Buffalo and the University of Mississippi Medical Center.
2.2 Transverse-aortic constriction (TAC) procedure
The mice were anesthetized with 1.5% isoflurane. TAC was performed as previously described with some emendations [18]. Briefly, the animals were intubated and ventilated under a dissecting microscope, with a small-animal respirator (Harvard Apparatus, Holliston, MA) at a rate of 135 breaths/min and a tidal volume of 120 ml/100g body weight. A blunted 7-0 suture was wrapped underneath the aortic arch between the brachiocephalic trunk and the left common carotid artery. A shortened and blunted 27-gauge needle was then tied onto the aortic arch with the 7-0 suture. Once tightly secured, the needle was removed from the knot, leaving the aorta permanently constricted. The chest cavity was sutured back together in layers with a 5-0 absorbable suture. The mice were slowly weaned off the ventilator, kept warm, and returned to their cages. Medications for pain relief were administered post-surgery.
2.3 Transthoracic echocardiography
Echocardiography was performed as described previously [19]. Briefly, mice were anesthetized with 1.5% isoflurane by inhalation. End-diastolic (d) and end-systolic (s) LV internal diameters (LVID), interventricular septum thickness (IVS), and posterior wall thickness (PW) [20] were measured by using two-dimensional guided M-Mode echocardiography with a Vevo 770 Imaging System (VisualSonics, Toronto, Canada) at the papillary muscle level. Fractional shortening was calculated as (LVIDd-LVIDs)/LVIDd*100 and relative wall thickness (RWT) was defined by the ratio of PW plus IVS thickness to LVIDd [21]. Once measurements were completed, animals were euthanized with a 100 mg/kg sodium pentobarbital intraperitoneal injection (Sigma, St. Louis, MO). The heart was rapidly excised, weighed, rinsed with ice-cold PBS, and then sectioned. The hearts were then either snap-frozen in liquid nitrogen for biochemical analysis or fixed for histological staining.
2.4 Immunoblotting
Immunoblotting was performed as previously described [22]. The heart tissue samples were homogenized in ice-cold lysis buffer. The Bradford method (Bio-Rad Laboratories, Hercules, CA, USA) was performed for measuring the lysate protein concentration. The homogenates were then resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. For re-probing, membranes were stripped using restore western blot stripping buffer (Thermo Fisher Scientific). Rabbit antibodies p-AMPKα (Thr172), AMPKα, p-acetyl-CoA carboxylase (ACC) (Ser79), ACC, p-ULK1 (Ser555), ULK1, p-ERK1/2 (Thr202/Tyr204), ERK, p62, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling (Danvers, MA, USA). Rabbit 4-hydroxynonenal antibody was obtained from Abcam (Cambridge, MA, USA). Mouse p-pShc66 (Ser36) was obtained from Calbiochem (Billerica, MA, USA). The results were quantified with the ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
2.5 Evaluation of fibrosis, hypertrophy, and inflammation
Paraffin tissue sections (5 μm) were stained with hematoxylin and eosin as previously described [22]. Masson trichrome stain (Sigma, St. Louis, MO) was used for detection of fibrosis. Fluorescein conjugated wheat germ agglutinin conjugate stain (Alexa Fluor-488, Invitrogen, Carlsbad, CA) was used to evaluate the size of cardiomyocytes and the nucleus was stained using 4′,6-diamidino-2-phenylindole (DAPI). The presence of inflammatory macrophages was detected by an anti-rabbit polyclonal galectin-3 protein antibody, also known as Mac-2 antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA), using a previously published protocol with some emendations [23]. Briefly, paraffin sections were deparaffinized, followed by antigen unmasking, and blocking with 3% hydrogen peroxide and blocking solution separately. The slice was incubated in the Mac-2 antibody at a final concentration of 1 μg/ml at 4 °C overnight. The next day, the secondary antibody was added followed by developing with DAB and counterstaining with hematoxylin. Images were captured using a Zeiss AxioImager fluorescence microscope (Carl Zeiss, Oberkochen, Germany) and analyzed with MATLAB software (MathWorks, Natick, MA, USA).
2.6 mRNA analysis by real-time PCR
The heart RNA was purified using TRIzol® reagent (Invitrogen, Eugene, OR, USA) [14]. cDNA was synthesized using the ThermoScriptTM RT-PCR system (Invitrogen, Eugene, OR, USA). A Q-PCR machine (Bio Rad CFX96 Touch PCR) and SYBR Green Supermix (Bio Rad, USA) were used. For each target gene, a standard curve was constructed and the starting quantity of mRNA was calculated using the Bio-Rad real-time PCR detection system software. Pro-inflammatory cytokine primers were designed based on the literature [24]. For β-actin, the forward primer was: AGAGGGAAATCGTGCGTGAC; and the reverse primer was: CAATAGTGATGACCTGGCCGT; for atrial natriuretic peptide (ANP), the forward primer was: ATCACCAAGGGCTTCTTCCT, and the reverse primer was: TGTTGGACACCGCACTGTA. All transcripts were analyzed in duplicates and normalized to β-actin. The delta Ct (DDCt) method was used to analyze the results.
2.7 Cellular GSH/GSSG measurement
The ratio of reduced glutathione (GSH)/oxidized glutathione (GSSG) in the heart tissue was measured with a glutathione detection kit (Enzo Life Sciences, Farmingdale, NY, USA) and a plate reader in accordance with the manufacturer’s instruction.
2.8 Statistical analysis
Data was expressed as the mean ± standard errors [25]. For experiments comparing two groups, a two-tailed unpaired Student’s t-test was used; for experiments making comparisons between at least 3 groups, two-way ANOVA was used with appropriate post-test analysis. GraphPad Prism 5.0 Software (GraphPad Software, La Jolla, CA) was used to analyze the data. A P value of < 0.05 was considered statistically significant.
3. Results
3.1 Cardiac AMPK deficiency exacerbates TAC-induced heart hypertrophy
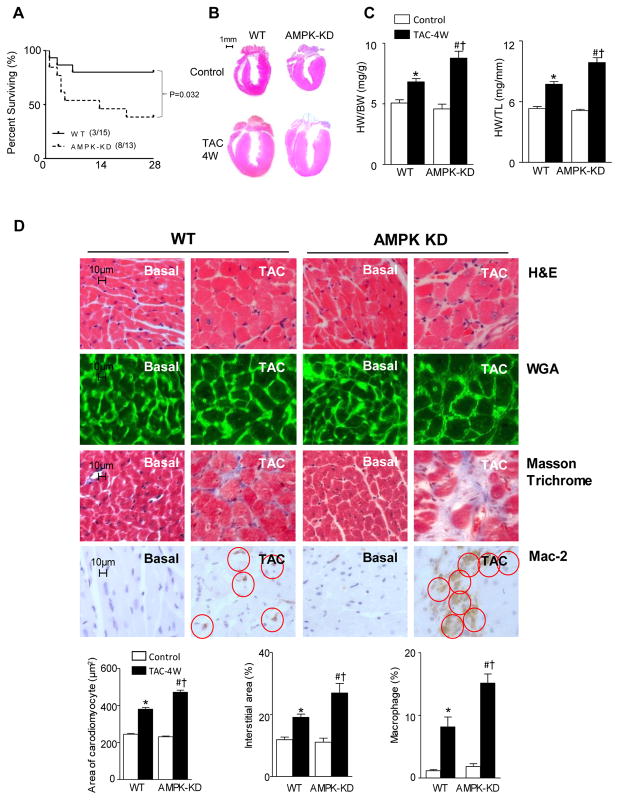
Both WT and AMPK-KD mice were subjected to similar transverse aortic constriction (TAC) surgery. The mortality of AMPK-KD mice was significantly higher than that of WT mice during TAC-induced heart failure (Figure 1A). Under basal conditions, there was no difference in heart size (Figure 1B), heart weight/body-weight, and heart weight/tibia length between WT mice and AMPK-KD mice (Figure 1C). However, four weeks after TAC surgery, the heart size and heart weight (normalized to both body weight and tibial length) increased significantly in both WT and AMPK-KD mice (Figure 1B and 1C). In addition, both the heart size and heart weight were significantly augmented in AMPK-KD versus WT mice (Figure 1B and 1C). Hematoxylin-eosin (HE) and wheat germ agglutinin conjugate (WGA) staining showed the transverse cross-sectional area of cardiomyocytes was not different between WT and AMPK-KD mice under basal conditions (Figure 1D) while WGA staining revealed increased cardiomyocyte transverse cross-sectional area in both WT and AMPK-KD TAC groups (Figure 1D). Moreover, in comparison to WT hearts, significantly larger areas of single cardiomyocytes were observed in AMPK-KD hearts after four weeks of TAC surgery (Figure 1D). In order to determine whether or not fibrosis occurred and to evaluate the macrophage infiltration, Masson Trichrome staining and immunohistochemistry staining with Mac-2 antibody were performed, respectively. The results demonstrated that there is no significant difference in the occurrence of fibrosis and inflammation between WT and AMPK-KD hearts under basal conditions. However, while four weeks of TAC similarly induced fibrosis and inflammation in both WT and AMPK-KD hearts, the effects nevertheless were significantly worse in AMPK-KD hearts (Figure 1D). These findings suggested that the deficiency of AMPK activity exacerbates cardiac anomalies in pressure overload-induced hypertrophy, which may lead to heart failure and enhance mortality.
Figure 1.
AMPK deficiency exacerbates TAC induced heart hypertrophy. (A) The survival rate of WT and AMPK-KD mice after TAC surgery. (B) The representative heart slice. (C) The heart weight divided by the body weight or the left tibia length of WT and AMPK-KD mice under control and 4 weeks TAC conditions. Values are means ± SE, n=5–7 per group, *p<0.05 vs. WT control, #p<0.05 vs. AMPK-KD control, †p<0.05 vs. WT TAC. (D) Immunohistochemistry staining. Hematoxylin and eosin stain (H & E), wheat germ agglutinin (WGA) conjugates staining, Masson Trichrome staining, and immunohistochemical staining with Mac-2 antibody of WT and AMPK-KD mice under control and 4 weeks TAC conditions. Values are means ± SE, n=4–5 per group, *p<0.05 vs. WT control, #p<0.05 vs. AMPK-KD control, †p<0.05 vs. WT TAC.
3.2 AMPK activity is critical for the heart function during the pressure overload
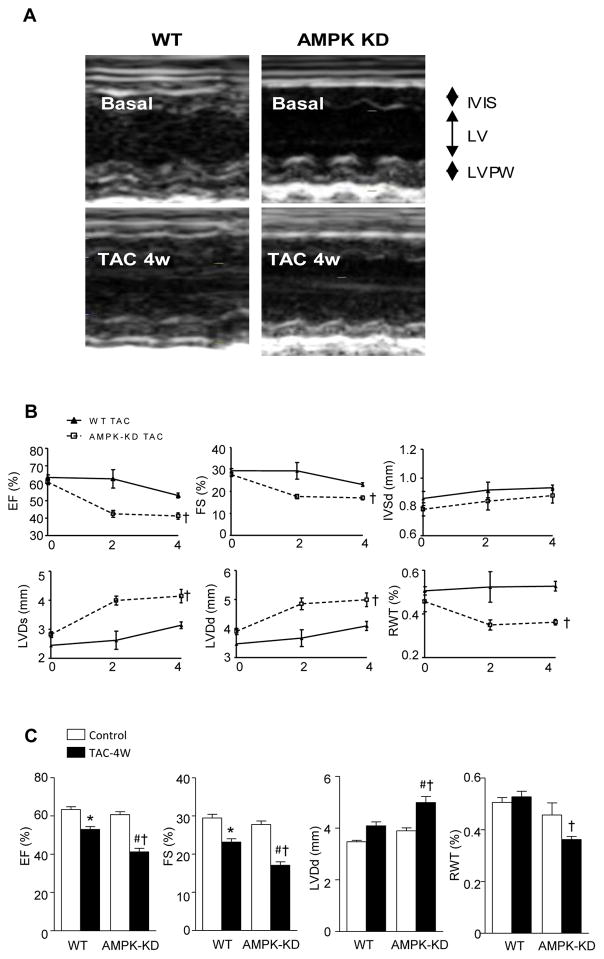
Echocardiography was used to measure cardiac functions of WT and AMPK-KD hearts under basal and four weeks of TAC surgery conditions (Figure 2A). There was no significant difference in the ejection fraction (EF), fractional shortening (FS), end-diastolic interventricular septum thickness (IVsd), end-diastolic (d) and end-systolic (s) LV internal diameters (LVDd and LVDs), or relative wall thickness (RWT) between WT and AMPK-KD mice under normal physiological conditions, indicating that AMPK activity had no impact on the heart structure and function under basal conditions (Figure 2B and 2C). Two weeks and four weeks after TAC surgery, IVSd, LVDs, LVDd, and RWT of WT mice were increased with slightly impaired EF and FS (Figure 2B and 2C). This concentric hypertrophy of the heart is regarded as an adaptive and beneficial event initially, as it serves to normalize wall stress and compensates the heart [26]. Intriguingly, two weeks and four weeks after TAC surgery, the EF and FS of AMPK-KD mice were decreased significantly when compared to that of WT mice (Figure 2B and 2C), indicating the exacerbated heart pump function in the AMPK-KD mice. The decreased RWT accompanied by increased LV diameter of the AMPK-KD mice suggested the eccentric hypertrophy. These observations indicated that the AMPK-KD heart had impaired adaptive remodeling which may have caused detrimental heart function and pressure overload-induced hypertrophy. It appears that AMPK may also play an important role in patterning of the LV geometry (Figure 1 and 2).
Figure 2.
Cardiac AMPK deficiency exacerbates heart function after 4 weeks of TAC. (A) The representative picture of M-mode echocardiography. (B) The EF, FS, IVSd, LVDs, LVDd, and RWT at basal condition, 2 weeks and 4 weeks after TAC surgery. (C) The quantification of EF, FS, LVDd, and RWT after 4 weeks of TAC surgery. Values are means ± SE, n=4–6 per group, *p<0.05 vs. WT control, #p<0.05 vs. AMPK-KD control, †p<0.05 vs. WT TAC.
3.3 AMPK deficiency impairs autophagy and redox balance by pressure overload
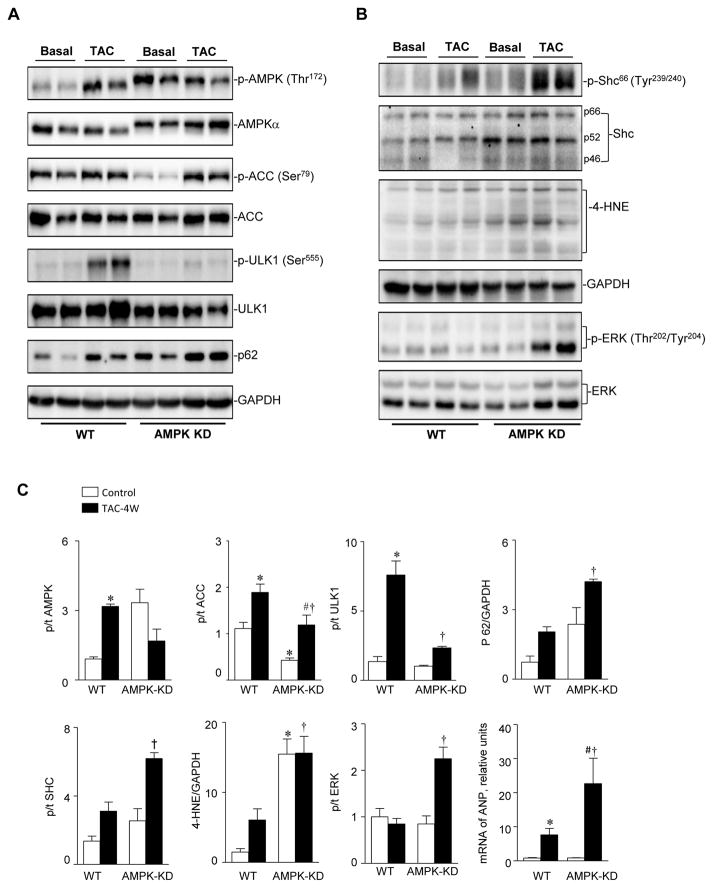
Immunoblotting results showed that the phosphorylation of both the AMPKα catalytic subunit at Thr172 and the downstream acetyl-CoA carboxylase (ACC) were significantly increased in response to four weeks of TAC-induced pressure overload in WT hearts (Figure 3A). AMPK activation also triggered the phosphorylation of serine/threonine-protein kinase ULK1 (Figure 3A). ACC plays an important role in maintaining metabolic homeostasis [25,27], and ULK1 is a critical component of the autophagy regulatory pathway in mammalian cells [28]. Our previous results revealed that AMPK-KD mice have an elevated expression level of mutant c-myc-tagged K45R α2, which efficiently competes with the WT AMPKα isoforms [16]. The immunoblotting results showed that AMPKα in the AMPK-KD heart migrated more slowly due to the c-myc tag and exhibited a slightly increased expression level for total AMPK protein in AMPK-KD mice (Figure 3A). After four weeks of TAC-induced pressure overload of AMPK-KD mice, the downstream signaling pathways, p-ACC and p-ULK1 were both blunted due to a non-functional overexpression of AMPKα2 in the mutant mice (Figure 3A). These findings indicated that AMPK-KD mice had dampened AMPK-ACC and AMPK-ULK1 signaling axis as well as enhanced p62 signaling in the pressure overload-induced hypertrophy model. These results indicate that the dysregulation of metabolism and autophagy in AMPK-KD mice may contribute to exacerbated function and remodeling during TAC-induced pressure overload.
Figure 3.
AMPK-KD mice have impaired levels of p-ACC and p-ULK1 during pressure overload. (A) Immunoblotting of p-AMPK, AMPKα, p-ACC, ACC, p-ULK1, ULK1 and p62 to show the protein expression levels and phosphorylation modification levels. (B) Immunoblotting of p-Shc66, Shc, 4-HNE, p-ERK, and ERK to show protein expression levels and oxidation or phosphorylation modification levels. (C) The quantification of expression levels and modification levels of proteins. Values are means ± SE, n=4–5 per group *p<0.05 vs. WT control, #p<0.05 vs. AMPK-KD control, †p<0.05 vs. WT TAC. p/t in y-axis represents the ratio of the phosphorylated to total level of indicated molecules.
Increased formation of reactive oxygen species (ROS) is thought to be involved in heart failure, and pShc66 has been implicated as a major regulator of ROS production in all cardiac diseases [29]. Thus, the level of phosphorylated Shc was analyzed in both WT and AMPK-KD hearts. The results showed that there was little p-Shc66 in the WT heart at basal conditions with only slightly increased level after four weeks of TAC surgery (Figure 3B). However, AMPK-KD mice had enhanced p-Shc66 at basal conditions, and its level was significantly elevated at four weeks after TAC surgery (Figure 3B and 3C). These findings suggested that the lack of AMPK activity resulted in up-regulation of the cardiac p-Shc66 level, thereby leading to increased ROS generation as evidenced by increased 4-hydroxynoneal (4-HNE) in the pressure overload-induced hypertrophied heart (Figure 3B). In addition, the oxidative stress sensitive signaling pathway, extracellular regulated protein kinase (ERK), was also significantly phosphorylated in the AMPK-KD hearts after 4 weeks of TAC-induced pressure overload (Figure 3B and 3C). The accumulation of 4-HNE-protein adducts has been reported to be associated with heart failure [30]. 4-HNE results in a dose-dependent reduction in the efficiency of mitochondrial oxidative phosphorylation, which may contribute to the cellular damage and heart remodeling [31]. The elevated level of 4-HNE in the AMPK-KD heart may cause mitochondrial dysfunction and ROS damage, which can exacerbate heart function and remodeling in the pressure overload-induced hypertrophied heart. Furthermore, the level of atrial natriuretic peptide (ANP) was significantly increased in the TAC induced hypertrophic heart [32]. RT-PCR results showed AMPK-KD had no effect on the ANP mRNA at basal conditions. However, it augmented the ANP level within pressure overload-induced hypertrophy (Figure 3C).
3.4 AMPK agonist APC ameliorates cardiac dysfunction caused by pressure overload
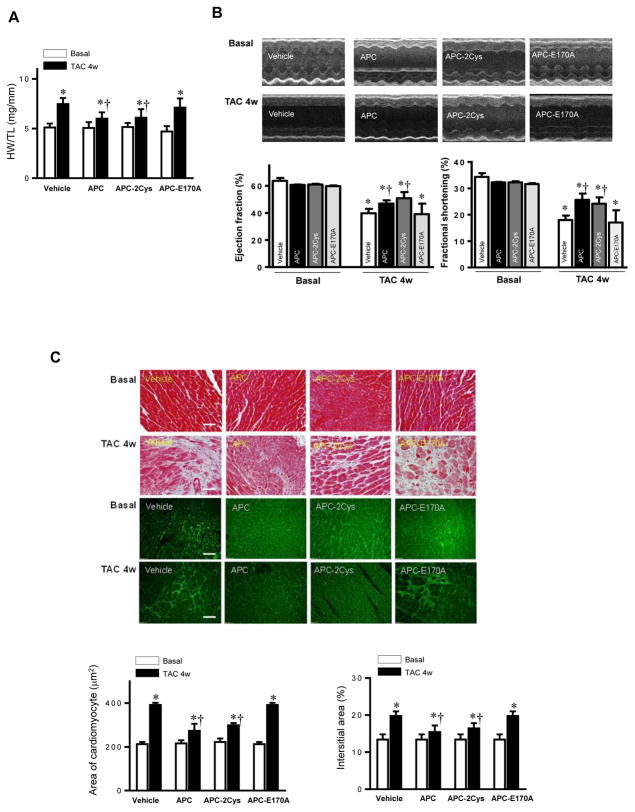
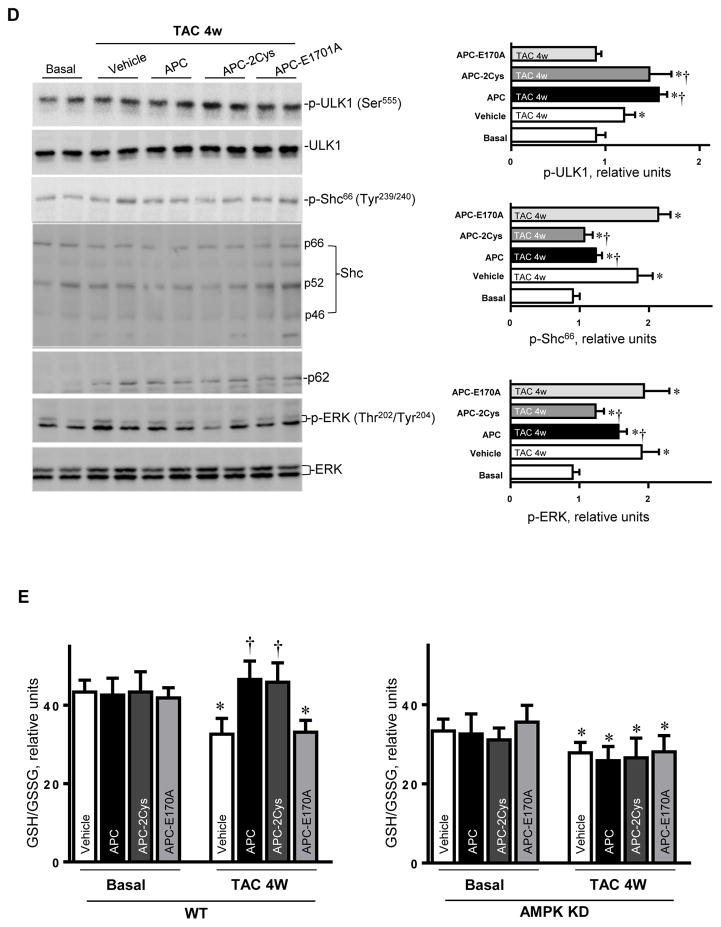
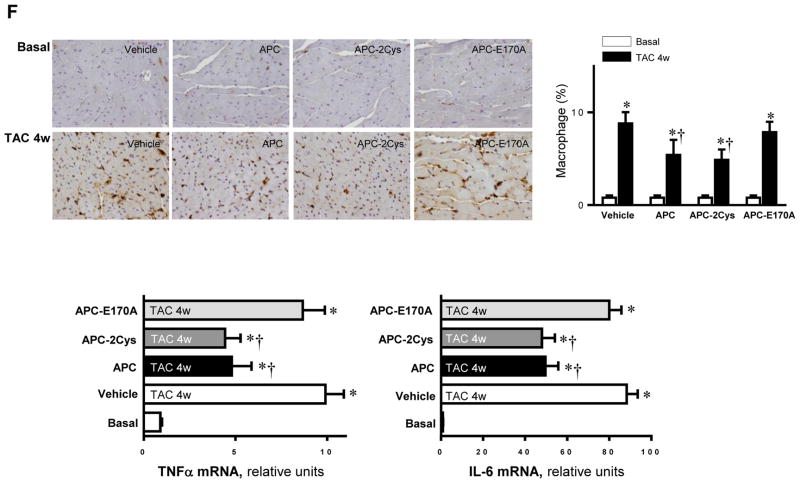
APC is an endogenous anticoagulant which is also known to possess potent anti-inflammatory and cytoprotective activities through EPCR-dependent activation of PAR1 [14]. The recombinant APC was approved by the FDA as a drug for treating patients with severe sepsis. One major drawback of APC is increased incidence of bleeding, which limits its use at elevated concentrations. Thus, there is an interest in developing APC variants with decreased anticoagulant but normal cytoprotective activity. We have prepared such variants, which are function-selective and possess either signaling activity (APC-2Cys) or anticoagulant activity (APC-E170A), but not both [14]. We previously demonstrated that APC exhibits cardioprotective activity in ischemia/reperfusion injury through stimulation of AMPK activity. To determine whether APC exerts a protective effect in pressure-overload induced hypertrophy, APC was administrated after TAC surgery. Both wild-type APC and APC-2Cys, but not APC-E170A, significantly reduced the increased heart weight (normalized to tibia length) by pressure-overload (Figure 4A). The echocardiography results demonstrated that both APC and APC-2Cys significantly ameliorated the cardiac dysfunction caused by pressure overload as shown by ejection fraction and fractional shortening, while the APC-E170A mutant did not show any beneficial effect on the pressure overload-induced cardiac dysfunction (Figure 4B). The immunohistochemistry staining showed that both APC and APC-2Cys, but not APC-E170A reduced pressure overload-induced fibrosis and hypertrophy as demonstrated by Masson Trichrome staining and WGA staining (Figure 4C). Furthermore, APC and APC-2Cys treatments augmented pressure overload-induced autophagy and reduced cellular oxidative stress mediated by pressure overload. This conclusion is based on the analysis of the phosphorylation levels of the autophagy-related ULK1, cellular redox sensor Shc, p62 and ERK (Figure 4D). Moreover, the intracellular redox indicator (i.e. the ratio of GSH/GSSG) was decreased by pressure overload, however, treatment with APC or APC-2Cys resulted in an increase in the ratio of GSH/GSSG (Figure 4E). By contrast, neither APC derivative exhibited an effect on the reduced ratio of GSH/GSSG by pressure-overload in AMPK-KD hearts (Figure 4E). Immunohistochemistry with Mac-2 antibody showed macrophage infiltration under basal and TAC-induced pressure overload conditions and both APC and APC-2Cys, but not APC-E170A, down-regulated inflammatory cell infiltration (Figure 4F). The real-time PCR data clearly showed that both APC and APC-2Cys significantly inhibit mRNA levels of pro-inflammatory cytokines TNFα and interleukin 6, induced by pressure overload (Figure 4F).
Figure 4.
Activated protein C (APC) derivatives ameliorate heart dysfunction mediated by pressure overload. (A) The left tibia length of WT mice with or without APC derivatives treatment under basal and 4 weeks of TAC conditions. Values are means ± SE, n=4–5 per group, *p<0.05 vs. corresponding Basal, †p<0.05 vs. Vehicle TAC. (B) Echocardiography showed the beneficial effects of APC derivatives on cardiac functions under basal and TAC-induced pressure overload conditions. Values are means ± SE, n=4 per group, *p<0.05 vs. Basal; †p<0.05 vs. Vehicle TAC. (C) The Masson trichrome and WGA staining to demonstrate the effects of APC derivatives on the fibrosis and hypertrophy caused by TAC-induced pressure overload. Values are means ± SE, n=4–5 per group, *p<0.05 vs. corresponding Basal, †p<0.05 vs. Vehicle TAC. (D) Immunoblotting showed the modulating effects of APC derivatives on autophagy, ULK1 signaling and p62 expression level, oxidative stress Shc and ERK signaling pathways in response to TAC-induced pressure overload. Value are means ± SE, n=5–6 per group, *p<0.05 vs. Basal; †p<0.05 vs. Vehicle TAC. (E) The ratio GSH/GSSG showed effects of APC derivatives on the intracellular redox status of hearts in response to TAC-induced pressure overload. Values are means ± SE, n=4–6 per group, *p<0.05 vs. corresponding Basal; †p<0.05 vs. Vehicle TAC. (F) Immunohistochemistry with Mac-2 antibody to show the macrophage infiltration under basal and TAC-induced pressure overload conditions. Lower panel: The real-time RT-PCR demonstrated that APC derivatives attenuate mRNA levels of TNFα and IL-6 proinflammatory cytokines caused by TAC-induced pressure overload. Values are means ± SE, n=3–6 per group, *p<0.05 vs. Basal; †p<0.05 vs. Vehicle TAC.
These findings suggest that APC may attenuate pressure overload-induced hypertrophy and dysfunction by activating AMPK and suppressing pro-inflammatory cytokines. The results further suggest that the beneficial effects of APC on the hypertrophic heart are due to its cytoprotective signaling and not its anticoagulant function.
4. Discussion
The results of this study suggest that no structural or functional differences exist between WT and AMPK-KD hearts under basal conditions. This is consistent with a previous study which reported that AMPK-KD mice had normal fractional shortening with no heart failure, cardiac hypertrophy or fibrosis [16]. In response to pressure overload, the left ventricular (LV) wall thickens as a compensatory mechanism to minimize wall stress. Subsequently, the heart undergoes failure along with dilation of the LV and declination of LV ejection fraction (EF) [3]. In our study, AMPK was activated after TAC in WT mice hearts, whereas AMPK-KD mice were unable to maintain LV function. This is in agreement with reports that AMPK activity increased in the rat heart with pressure overload-induced hypertrophy [4,7,33]. Previous research, which studied AMPKα2 gene deficient (KO) and WT mice subjected to 3 weeks of TAC, showed that AMPKα2 KO had significantly increased TAC-induced ventricular hypertrophy while having decreased ejection fraction [19]. The muscle specific AMPKβ1/β2 KO also lead to the dilated cardiomyopathy [34]. Interestingly, we found that AMPK-KD mice had different LV geometry in comparison to WT mice in response to TAC. This could be due to loss of both AMPKα1 and AMPKα2 activity in hearts of AMPK KD mice, while the global AMPKα2 KO mice still maintain the normal function of the AMPKα1 catalytic subunit [19,35]. LV mass can increase from either wall thickening or chamber dilation. Wall thickening usually occurs in response to pressure overload, while chamber dilation happens in response to volume overload [3]. Thus, the relative wall thickening was calculated in order to clarify concentric (wall thickening) and eccentric hypertrophy (chamber dilation). Our results showed that in response to 4 weeks of TAC, AMPK-KD mice developed eccentric hypertrophy whereas WT mice developed concentric hypertrophy. This data suggested that AMPK activity affects the pattern of hypertrophic response. Furthermore, a previous study showed that treatment with well-known AMPK activator, metformin, protected the heart against systolic overload-induced heart failure in rats (13). However, another study used AMPKα2 KO mice to reveal that metformin protected against systolic overload-induced heart failure independent of the AMPKα2 catalytic subunit [36]. Thus, AMPKα1 activation or other pharmacological actions of metformin could contribute the cardioprotection against heart failure induced by systolic overload. Taken together, these findings indicate that AMPK plays a protective role against pressure overload-induced hypertrophy. Thus, AMPK activators are potential therapeutic reagents to rescue heart dysfunction in hypertrophic hearts.
AMPK, as an energy sensor, is activated in response to stress mediated by hypoxia, oxidation, exercise, and drugs prescribed to control systemic energy balance and metabolism [5]. AMPK has been reported to exert multiple protective effects in vascular cells by inhibiting inflammation and oxidant production. However, the role of AMPK in pressure overload-induced hypertrophy remains unclear. Cell proliferation and protein synthesis related signaling pathways such as mTOR and eEF2 have been recognized as AMPK downstream targets involved hypertrophic cardiomyopathy [33,34]. There are studies also indicated that most of the cardiac damage occurring during the heart failure is due to exacerbated ROS generation [5,27,29]. It has been reported that AMPK activation can prevent hyperglycemia-induced NADH oxidase activation to reduce ROS production in the cardiomyocytes [37]. In addition, Shc66 has been considered to be a major regulator of ROS production in all cardiac diseases, including atherosclerosis and heart failure [29]. For instance, mice devoid of Shc66 displayed a significant reduction in both the occurrence of apoptosis and the degree of hypertrophy induced by angiotensin II [29]. In our study, we found an enhanced level of phosphorylation of Shc66 in AMPK-KD mice after TAC, which may lead to the formation of ROS overload that can cause cell death and contractile impairment.
Moreover, ROS overload can lead to excessive oxidation of polyunsaturated fatty acids and accumulation of reactive aldehydes, such as 4-HNE [30]. It has been demonstrated that 4-HNE levels are consistently elevated in both plasma and hearts of congestive heart failure patients and inversely correlated with left ventricular contractility. Increased 4-HNE resulted in a dose-dependent reduction in the efficiency of mitochondrial oxidative phosphorylation, which may contribute to the cellular damage and heart remodeling [31]. Our results showed an increased generation of 4-HNE in AMPK-KD mice, indicating that mitochondrial dysfunction and ROS generation could occur in the AMPK-KD heart during pressure overload induced by TAC surgery. In addition, the impaired responsive reaction of the AMPK-ULK1 pathway in AMPK-KD mice suggests that the autophagy dysfunction may also contribute to the decreased LV function.
APC is a vitamin-K dependent plasma anticoagulant which also possesses anti-inflammatory and cytoprotective properties [14,38]. Because of its anticoagulant and anti-inflammatory properties, recombinant APC was used for treating severe sepsis in clinical patients [14]. Our data showed for the first time that APC can attenuate pressure overload-induced heart hypertrophy and improve heart function by regulating ACC, ULK1, and Shc66 pathways. Moreover, APC decreased ROS burden and inflammatory responses in pressure overload-induced hypertrophic heart. The beneficial effects of APC on hypertrophic hearts were found to be due to its cytoprotective signaling and not its anticoagulant function. It is interesting to note that a similar protective signaling mechanism for APC was reported in an experimental model of diabetic nephropathy in which it was demonstrated that APC inhibits ROS-mediated up-regulation of Shc66 expression in podocytes by a PAR1-dependent mechanism [39].
The prognosis for patients with heart failure remains dim with the disease taking a substantial toll on patients, the society, and the economy. As a result, there is an urgent need for discovering new therapeutic strategies. Further studies with APC may be warranted to explore the possibility of harnessing the cardioprotective effect of APC for reducing the burden of hypertensive heart disease and heart failure.
Acknowledgments
These studies were supported by American Diabetes Association Grant 1-17-IBS-296, NIH R01HL101917, R01AG043895, P01HL051971 and P20GM104357.
Footnotes
Disclosure of Conflict of Interests
None.
References
- 1.Arnett DK. Transforming cardiovascular health through genes and environment: presidential address at the American Heart Association 2012 Scientific Sessions. Circulation. 2013;127:2066–2070. doi: 10.1161/CIR.0b013e318295b369. [DOI] [PubMed] [Google Scholar]
- 2.The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 4.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 5.Morrison A, Li J. PPAR-gamma and AMPK--advantageous targets for myocardial ischemia/reperfusion therapy. Biochem Pharmacol. 2011;82:195–200. doi: 10.1016/j.bcp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Tong C, Yan X, Yeung E, Gandavadi S, Hare AA, Du X, Chen Y, Xiong H, Ma C, Leng L, Young LH, Jorgensen WL, Li J, Bucala R. Limiting cardiac ischemic injury by pharmacological augmentation of macrophage migration inhibitory factor-AMP-activated protein kinase signal transduction. Circulation. 2013;128:225–236. doi: 10.1161/CIRCULATIONAHA.112.000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 10.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 12.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–182. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 13.Zhang CX, Pan SN, Meng RS, Peng CQ, Xiong ZJ, Chen BL, Chen GQ, Yao FJ, Chen YL, Ma YD, Dong YG. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin Exp Pharmacol Physiol. 2011;38:55–62. doi: 10.1111/j.1440-1681.2010.05461.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost. 2011;9:1308–1317. doi: 10.1111/j.1538-7836.2011.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sopel MJ, Rosin NL, Falkenham AG, Bezuhly M, Esmon CT, Lee TD, Liwski RS, Legare JF. Treatment with activated protein C (aPC) is protective during the development of myocardial fibrosis: an angiotensin II infusion model in mice. PLoS One. 2012;7:e45663. doi: 10.1371/journal.pone.0045663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, Horie T, Nagao K, Kuwabara Y, Baba O, Nishi H, Sowa N, Narazaki M, Matsuda T, Takemura G, Wada H, Hasegawa K, Kimura T, Ono K. Cardiac-specific inhibition of kinase activity in calcium/calmodulin-dependent protein kinase kinase-beta leads to accelerated left ventricular remodeling and heart failure after transverse aortic constriction in mice. PLoS One. 2014;9:e108201. doi: 10.1371/journal.pone.0108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, Xu W, Wiczer B, Bernlohr DA, Bache RJ, Chen Y. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 21.Negri F, Sala C, Re A, Mancia G, Cuspidi C. Left ventricular geometry and diastolic function in the hypertensive heart: impact of age. Blood Press. 2013;22:1–8. doi: 10.3109/08037051.2012.707307. [DOI] [PubMed] [Google Scholar]
- 22.Morrison A, Chen L, Wang J, Zhang M, Yang H, Ma Y, Budanov A, Lee JH, Karin M, Li J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015;29:408–417. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 2013;27:4332–4342. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velten M, Duerr GD, Pessies T, Schild J, Lohner R, Mersmann J, Dewald O, Zacharowski K, Klaschik S, Hilbert T, Hoeft A, Baumgarten G, Meyer R, Boehm O, Knuefermann P. Priming with synthetic oligonucleotides attenuates pressure overload-induced inflammation and cardiac hypertrophy in mice. Cardiovasc Res. 2012;96:422–432. doi: 10.1093/cvr/cvs280. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Wang J, Gao J, Yang H, Wang Y, Manithody C, Li J, Rezaie AR. Antithrombin up-regulates AMP-activated protein kinase signalling during myocardial ischaemia/reperfusion injury. Thromb Haemost. 2015;113:338–349. doi: 10.1160/TH14-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frohlich ED, Susic D. Pressure overload. Heart Fail Clin. 2012;8:21–32. doi: 10.1016/j.hfc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Li J. Metabolic shifts during aging and pathology. Compr Physiol. 2015;5:667–686. doi: 10.1002/cphy.c140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 30.Mali VR, Palaniyandi SS. Regulation and therapeutic strategies of 4-hydroxy-2-nonenal metabolism in heart disease. Free Radic Res. 2014;48:251–263. doi: 10.3109/10715762.2013.864761. [DOI] [PubMed] [Google Scholar]
- 31.Gomes KM, Campos JC, Bechara LR, Queliconi B, Lima VM, Disatnik MH, Magno P, Chen CH, Brum PC, Kowaltowski AJ, Mochly-Rosen D, Ferreira JC. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forssmann WG, Richter R, Meyer M. The endocrine heart and natriuretic peptides: histochemistry, cell biology, and functional aspects of the renal urodilatin system. Histochem Cell Biol. 1998;110:335–357. doi: 10.1007/s004180050295. [DOI] [PubMed] [Google Scholar]
- 33.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung MM, Zordoky BN, Bujak AL, Lally JS, Fung D, Young ME, Horman S, Miller EJ, Light PE, Kemp BE, Steinberg GR, Dyck JR. AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovasc Res. 2015;107:235–245. doi: 10.1093/cvr/cvv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Lu Z, Fassett J, Zhang P, Hu X, Liu X, Kwak D, Li J, Zhu G, Tao Y, Hou M, Wang H, Guo H, Viollet B, McFalls EO, Bache RJ, Chen Y. Metformin protects against systolic overload-induced heart failure independent of AMP-activated protein kinase alpha2. Hypertension. 2014;63:723–728. doi: 10.1161/HYPERTENSIONAHA.113.02619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balteau M, Van Steenbergen A, Timmermans AD, Dessy C, Behets-Wydemans G, Tajeddine N, Castanares-Zapatero D, Gilon P, Vanoverschelde JL, Horman S, Hue L, Bertrand L, Beauloye C. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am J Physiol Heart Circ Physiol. 2014;307:H1120–1133. doi: 10.1152/ajpheart.00210.2014. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Li J. Activated protein C: a potential cardioprotective factor against ischemic injury during ischemia/reperfusion. Am J Transl Res. 2009;1:381–392. [PMC free article] [PubMed] [Google Scholar]
- 39.Bock F, Shahzad K, Wang H, Stoyanov S, Wolter J, Dong W, Pelicci PG, Kashif M, Ranjan S, Schmidt S, Ritzel R, Schwenger V, Reymann KG, Esmon CT, Madhusudhan T, Nawroth PP, Isermann B. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci U S A. 2013;110:648–653. doi: 10.1073/pnas.1218667110. [DOI] [PMC free article] [PubMed] [Google Scholar]