Abstract
Background
Associations have been observed between an aggregate viral load measure, the community viral load (CVL) and new HIV diagnoses. The CVL aggregates viral loads within chosen geographic areas, restricting inferences about HIV acquisition risk to these areas. We develop a more precise metric, the Network Viral Load (NVL) to measure the composite viral load within a risk network of an HIV negative individual.
Methods
We examined the relationship between NVL and HIV infection among Young Men who have Sex with Men (YMSM) in Chicago, United States. Networks were generated using Respondent Driven Sampling. NVL was defined as the prevalence of viremic individuals in one’s risk network, characterized as those with a viral load ≥20k copies/mL. Permutation tests were conducted to account for dependency.
Results
After controlling for total connections, age, substance use during sex, syphilis diagnosis (previous 12 months), and frequency of condomless anal sex (previous 6 months), we found a positive association between NVL and HIV infection. Compared to a network with all HIV-seronegative members, the odds of HIV infection with a NVL of <10% viremia were 1.85 (95% C.I. 1.18–2.92) times higher and a NVL of ≥10% viremia were 2.73 (95% C.I. 1.54–4.85) times higher.
Conclusion
We found a positive association between NVL and HIV seroprevalence. While limited in its ability to infer causality, NVL could have substantial public health implications for persons most at risk for HIV infection given that this novel metric avoids overreliance on individual level behavior or broad community indices.
Keywords: HIV, Viral Load, MSM, Youth, Social Networks
BACKGROUND
HIV prevention interventions, such as pre-exposure prophylaxis (PrEP) and treatment as prevention (TasP) offer potential for great reductions in HIV transmissions1–4. In recent years, the number of new HIV diagnoses in the United States (U.S.), for example, has remained stable at around 50,000 cases per year,5 indicating the need for more effective HIV prevention programs to achieve further reductions in new HIV diagnoses.
TasP has been shown to reduce the number of phylogenetically linked HIV transmissions by 96%1. The TasP approach is effective because it lowers HIV viral load, which limits onwards transmission.6,7 Some public health departments have utilized this association on a population level for the targeting of HIV prevention resources: The San Francisco Department of Public Health, for example, calculated an aggregate viral load measure for different geographic areas within the city, the “community viral load” (CVL), and considered associations between CVL and new HIV diagnoses by geographic area.8 Decreasing CVL was found to be associated with a decline in the number new HIV diagnoses over a five year period.8 While these findings are promising, the CVL approach has several limitations,9 most notably that the CVL assumes that individuals transmit HIV primarily to other individuals in the same geographic area as them. With the advent of hook-up apps and easier mobility within and across cities,10 HIV transmission has increased potential to occur in more heterogeneous contexts and networks. A recent phylogenetic analysis in Chicago, for example, demonstrates that the HIV virus of Black YMSM in Chicago was phylogenetically similar throughout geographically diverse areas where HIV infected YMSM reside. This indicates that HIV is transmitted across communities, and that the CVL may therefore not be capturing these cross-community exposures.11
Miller et al.9 and Herbeck and Tanser12 also note that the aggregate CVL measure is limited in that it only captures those in HIV care, it does not consider the prevalence of the underlying population, and it ignores the composition of one’s sexual network.9 They suggest instead calculating a “population viral load” that estimates the viral load of an “entire population” in a community (i.e. HIV-seronegative and HIV-seropositive) and establishing thresholds for increased transmission potential.9 This population viral load measure, however, is still geographically bounded as it does not account for risk experienced outside one’s geographic area or within their geographic area with those from without.
A more precise metric would account for the composite viral loads of the risk networks of an HIV negative individual, regardless of the geographic location of either the individual or the network member. Here, we develop this new metric, the Network Viral Load (NVL)13 that accounts for the composite viral loads of a risk network sample of an HIV negative individual. We test its association with HIV infection among a population-based cohort of YMSM.
METHODS
Study Population
Data come from the baseline sample of uConnect, a longitudinal study of YMSM ages 16–29 who reside in Chicago, conducted over an 18-month period from 2013–2016.14–17 Respondent Driven Sampling (RDS) was used for recruitment. RDS seeds were selected from a distribution of social spaces that YMSM occupy (both physical spaces and virtual spaces such as Facebook). Eligibility criteria included: 1) self-identification as African American or Black, 2) born male, 3) between 16 and 29 years of age (inclusive), 4) report of oral or anal sex with a male within the past 24 months 5), willing and able to provide informed consent at the time of the study visit, 6) Primary residence in South Chicago, the most populous contiguous Black community in the U.S.14 The city of Chicago is broken down into 77 community areas, and south Chicago is a 95-mi2 area that houses 34 of these community areas. 18 Respondents were given six vouchers to recruit others they know who they have frequent contact with who fit the eligibility criteria. Each respondent was given $60 for participation and $20 for each successful recruit enrolled into the study. Respondents were administered a behavioral questionnaire and tested for HIV and HIV RNA. The Institutional Review Board at the University of Chicago and the National Opinion Research Center at the University of Chicago approved all procedures.
Laboratory Testing
HIV infection was determined by three assays applied to samples eluted from dry blood spot samples: ARCHITECT HIV Ag/Ab Combo; Multispot HIV-1/HIV-2 Bio-Rad; and Realtime HIV-1 RNA, Abbot. In cases where test data were missing at the study visit, available HIV viral load and serostatus surveillance data were used from the Health Department by matching on name and demographic information. We obtained a Release of Information from each respondent to obtain these data.
Network Construction
HIV risk-related networks were constructed from the RDS recruitment network as previously defined by Tsang and colleagues, consisting of RDS referrals and referees.19 This “risk environment network” definition exploits the RDS referral structure in a novel way by situating the referrals and referees as part of a respondent’s HIV risk environment network.20–23 The risk network as we define it represents a sample of network members from their immediate risk environment. Risk environments have been previously defined as the composition of risk factors external to the individual, such as community level norms and practices.24 This definition is particularly relevant to YMSM by virtue of their tendency to recruit and be recruited by individuals who share close social connections. Likewise, the networks of YMSM are dynamic, with social connections becoming sexual connections and vice versa at a high frequency over time.17 Given the high HIV prevalence in this population, the significant overlap between the sexual and social networks of Black MSM25 and the notable influence that social networks have on HIV preventive behaviors and transmission patterns,25,26 this definition of one’s HIV-related network could be useful for effective HIV prevention efforts.
Analytic Plan
Measures
The outcome of interest was HIV serostatus, defined by laboratory testing during the study visit or data obtained though the Health Department. The primary independent variable of interest was a respondent’s NVL, NVL was defined as the prevalence rate of viremic individuals in one’s immediate (first degree) risk network. Viremia was characterized as those with a viral load of ≥20k copies/mL.6 The NVL was then summarized into the following categories: all network members were HIV seronegative, at least one network member was HIV infected and <10% of the network was viremic, and at least one network member was HIV infected and ≥10% of the network was viremic (Figure 1). These categories reflect relative cutoffs that designate risk of transmissibility6 and the distribution of the NVL in the sample.
Figure 1.
Example sociogram depicting Network Viral Load calculation
Other covariates include 1.) Non-injection drug use or alcohol use during sex (drugs included marijuana, MDMA, volatile nitrates, cocaine, heroin, psychoactive drugs, methamphetamines, and prescription pain killers). Sex-drug use was a dichotomous measure indicating use of any of the aforementioned substances; 2.) Frequency of condomless anal sex in the past 6 months (defined as the sum of the number of times the respondent reported sex with each sexual partner they reported inconsistent condom use with in the past 6 months; 3.) Self-reported syphilis diagnosis in the past 12 months (any/none); 4.) Degree (total number of sex partners and confidants reported by the respondent combined); 5.) Other demographics and social characteristics. Injection drug use was not assessed due to its low prevalence in the sample. Analyses excluded respondents in the last RDS wave because their networks were incomplete, and were therefore unable to recruit others as a result of the sample design.19
Analysis
Multivariate logistic regression was used to assess the relationship between HIV serostatus and NVL at baseline. Variables significant at the p≤ 0.1 level in bivariate analysis were considered in the multivariable model. All variables retained in the final model were significant at the p≤.05 level.
Two additional exploratory analyses were conducted to try and ascertain causality. They first assessed the association between NVL and HIV seroconversion over the 18-month study period. Seroconversion was defined as having a HIV negative lab result at baseline and having an HIV seropositive lab result at either of the two follow-up visits. Lastly, we calculated mean CVL using Enhanced HIV/AIDS Reporting System data (state mandated laboratory data) from the Health Department. We assessed associations between CVL and respondent HIV serostatus using logistic regression. The second, conducted by Morgan,27 assessed the overlap between the NVL network and phylogenetic data. Genotypes were collected at baseline and through the Chicago Department of Public Health HIV surveillance data on participants identified as HIV-positive. Potential molecular ties were identified via pairwise genetic distance analysis of HIV-1 pol sequences with links inferred between individuals whose viral sequences were ≤1.5% genetically distant. Putative molecular clusters were defined as ≥1 connection to another individual.
All regression analyses were conducted using Stata version 14.28 Permutation analyses were conducted in R version 3.3.1.29
Sensitivity Analysis
Assumptions of independence between observations assumed by the logistic regression30 are violated by network data. A series of permutation tests were conducted to verify the associations found in the multiple logistic regression model. Each permutation retained the original network structure, but randomly assigned the viral loads and HIV serostatuses throughout the network. This null hypothesis was that one’ s own HIV serostatus is unrelated to the viral loads of the individuals in one’s egocentric network. Five thousand permutations were performed to obtain an estimate of the permutation distribution for the odds ratios, and the likelihood of the observed odds ratios were evaluated relative to this distribution, yielding a 2-sided permutation p-value.19
We repeated the NVL analysis using lower viral load thresholds of 3,500 copies/mL, and 10,000 copies/mL as well as replacing the NVL with the proportion of respondents’ networks that are HIV infected to determine if there is any added benefit of assessing NVL over network HIV infection alone, a method which we have conducted previously on a network sample of people who inject drugs (PWID).19 HIV-seropositive network environment proportions were coded as 0%, 1%–49%, 50%–≤75% and ≥75%. Categories were selected based upon the distribution of the HIV-seropositive network proportions in the sample.
RESULTS
Our sample included 65 seeds who generated a baseline sample of 618 respondents. Referred network members were mostly individuals known to the index, and in 77% of the cases included close confidants, sex partners, or family members (largely family of choice rather than biological family). Only productive seeds (n=38), defined as seeds who recruited at least one participant, were included in the analyses. Excluded from the analyses (including their presence in networks) were 89 respondents in the final RDS wave, because they were unable to recruit by design, and thus the size of their networks was restricted in a way that the networks of other respondents were not. The total sample size after these exclusions was 502; of these, we had laboratory data on 91%, yielding a total sample size of 457.
The mean age of the participants was 23 (range 16–29), 100% were Black/African-American, 61 (13%) had less than a high school degree, 331 (66%) and 138 (27%) identified as gay and bisexual respectively, 149 (33%) were unemployed, 235 (51%) had health care coverage, 20 (4%) had ever used PrEP, 129 (26%) were unstably housed in the past 12 months, and 233 (46%) had ever experienced criminal justice involvement (Table 1). The HIV prevalence in the sample was 39% (n=182).
Table 1.
Demographics, behavioral characteristics, and HIV serostatus uConnect (n=457)
| n (%) | |
|---|---|
| Age at interview (years) | |
| 16–18 | 33 (7) |
| 19–20 | 83 (18) |
| 21–24 | 211 (46) |
| 25+ | 130 (28) |
| Education | |
| < High school Degree | 61 (13) |
| High school graduate or equivalent | 112 (25) |
| Some college or higher | 284 (62) |
| Unemployed (previous year) | 149 (33) |
| Health care coverage* | 235 (51) |
| Ever used PrEP | 20 (4) |
| Unstably housed (previous year)* | 129 (26) |
| Number of Residences (previous year) | |
| 1 | 252 (55) |
| 2 | 124 (28) |
| 3+ | 80 (18) |
| Criminal justice involvement in lifetimea | 233 (46) |
| Drug Use (previous year) | |
| Marijuana | 388 (77) |
| Volatile Nitrates | 33 (7) |
| MDMA | 43 (9) |
| Psychoactive drugs | 3 (<1) |
| Methamphetamine | 3 (<1) |
| Cocaine/Crack | 23 (5) |
| Heroin | 3 (<1) |
| Sex drug use (previous 6 months) | 118 (24) |
| Sexual orientation | |
| Gay | 331 (66) |
| Straight | 20 (4) |
| Bisexual | 138 (27) |
| Other | 12 (2) |
| Number of male sex partners (previous 6 months) (median, IQR)b* | 2 (1,3) |
| Number of condomless male sex partners (previous 6 months) (median, IQR)b | 1 (0,1) |
| Frequency of anal intercourse (previous 6 months) (median, IQR)b | 11 (3,15) |
| Frequency of condomless anal intercourse (previous 6 months) (median, IQR)b | 2 (0,11) |
| Number of social & sexual connections “Degree” (median, IQR)b | 5 (3,7) |
| HIV Characteristics | |
| HIV Serostatus | |
| Positive | 182 (39) |
| Use of ARVs (Current)c | |
| Yes | 88 (48) |
| Percentage of risk network members HIV+ (median, IQR) | 25% (0%,100%) |
| Network Viral Load | |
| All HIV-seronegative network | 212 (46) |
| <10% of network viremic* | 169 (37) |
| 10%–100% of network viremic | 76 (17) |
O.R.=Odds Ratio, CI=Confidence Interval
Includes jail/parole
Obtained from network elicitation
% among HIV+ aware
Missing data (n): Healthcare coverage (11), unstably housed (2), detained (1), # males sex partners (20)
The mean risk-related network size was 1.9 (range 1–7). The distribution of NVL in the sample was as follows: 212 (46%) had entirely HIV-seronegative networks, 169 (37%) had a NVL of <10% viremic, 76 (17%) had a NVL of ≥10% viremic. HIV-seropositive network proportions were as follows: 212 (46%) had 0% HIV-seropositive network partners, 45 (10%) had 1%–49% HIV-seropositive network partners, 68 (15%) had 50% to ≤75% HIV-seropositive network partners, and 132 (29%) had ≥75% HIV-seropositive network partners. Respondents had an average of 2 male or transgender anal sex partners in the past 6 months (range 1–6) and reported having condomless sex with an average of 1 partner in the past 6 months (range 0–6).
After controlling for age, substance use during sex, frequency of anal sex in the past 6 months, degree, and syphilis diagnosis in the past 12 months, we found increased odds of HIV infection with increased NVL (table 2). The odds of HIV infection with a NVL of <10% viremia were 1.85 times that of a network with all HIV-seronegative members (OR 1.85; 95% C.I. 1.18–2.92), the odds with a NVL of ≥10% viremia were 2.75 times that of a HIV-seronegative network (OR 2.75; 95% C.I. 1.54–4.85). A test for trend of the NVL was significant at p=0.001, indicating increased odds of HIV infection with increasing levels of NVL. Increasing age was also associated with increased odds of HIV infection (OR 1.10; 1.03–1.18) as well as being diagnosed with syphilis in the past 12 months (OR 4.26; 2.38–7.61). Drug use during sex was a strong confounder of the relationship between NVL and HIV infection. No effect modification was found between NVL and any of the covariates in the model.
Table 2.
Multivariate Logistic Regression: Factors Associated with HIV serostatus: uConnect Study (n=425)
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.10 | 1.03–1.18 | 0.006 |
| Syphilis Diagnosis (previous year) | 4.26 | 2.38–7.61 | <0.001 |
| Frequency of condomless anal intercourse (previous 6 months) | 1.02 | 0.99–1.05 | 0.08 |
| Number of social & sexual connections (Degree) | 0.98 | 0.95–1.02 | 0.37 |
| Sex Drug Use | 1.48 | 0.91–2.41 | 0.11 |
| Average Network Viral Load (Reference group: entire network HIV−) | 1.00 | ||
| <10% of network viremic* | 1.85 | 1.18–2.92 | 0.008 |
| 10%–100% of network viremic | 2.73 | 1.54–4.85 | 0.001 |
OR=Odds Ratio, CI=Confidence Interval
Defined as VL of ≥20k copies/mL
The permutation test results reveal that observed odds ratios of 1.85, and 2.73 comparing <10% Viremia NVL to and ≥10% NVL to the reference group of a completely HIV-seronegative NVL are both located on the extreme tails of the permutation distributions, yielding estimated p-values of <0·002 (8/5000) and 0.014 (70/5000) respectively (Figure 2). This finding indicates that the observed associations were unlikely under the null hypothesis.
Figure 2.

Comparison of observed NVL results and 5000 randomly generated networks (reference line indicates observed Odds Ratio)
Of the 275 respondents who were HIV negative at baseline, 270 (98%) had follow-up data. Of those, 28 (10%) seroconverted to HIV-seropositive over the 18 months of follow-up. While this rate is high, it is not substantial enough to be adequately powered for multivariate analysis. Nevertheless, descriptive results using lower NVL thresholds show signs of a potential association between NVL and HIV seroconversion. A slightly lower proportion of those who seroconverted had entirely HIV-seronegative networks at baseline (50%) compared to those who remained negative over the course of the study (56%). Additionally, a slightly higher proportion of those who seroconverted had a NVL of ≥10% viremia (14%) compared to those who remained negative (11%). These differences were not statistically significant (p=0.65).
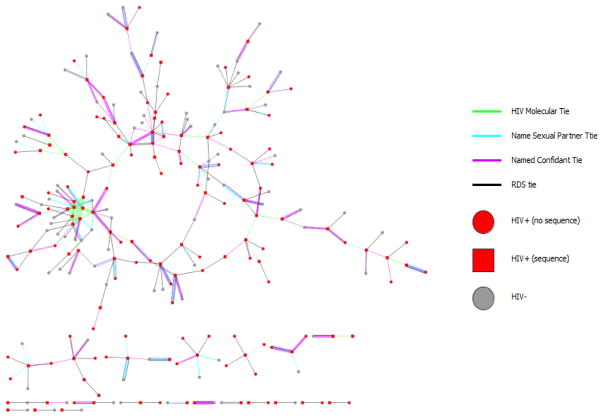
Of participants identified as HIV-positive, we obtained viral genetic sequences on 32%. Of these, 31 (40.7%) were tied to ≥1 other sequence with a total of 55 ties between all individuals. We did not find any overlap between the phylogentic network and the RDS network. There was overlap in members between the RDS network and the sexual and social networks, however. Approximately 44.6% of the RDS ties were also listed as social ties, and 30% of RDS ties were also listed as sexual ties (Figure 3).27
Figure 3.
Network diagrams for all individuals for whom we obtained an HIV genetic sequence in the molecular, RDS, sexual, and confidant networks.
The city of Chicago is organized into 77 community areas.31 We calculated mean CVLs for each community area in Chicago using Health Department data and assessed whether it was associated with HIV serostatus using logistic regression. We found insignificant results in a bivariate analysis between CVL and respondent HIV serostatus (OR 0.99; 95% C.I. 0.99–1.00) as well as in a multivariate analysis including the covariates from the NVL multivariate model (OR 0.99; 95% C.I. 0.99–1.00).
DISCUSSION
The current analysis establishes the relationship between a new metric, Network Viral Load (NVL), and HIV serostatus. We found that the strength of the association between NVL and HIV serostatus was positively associated with the magnitude of the NVL and that the association was significant after adjusting for other common drivers of HIV infection including age, non-injection drug use or alcohol use during sex, frequency of condomless anal sex, syphilis diagnosis and degree social connectivity.
The U.S. CDC recommends monitoring of CVL as a mechanism for measuring progress towards the National HIV/AIDS Strategy goals.32 However, shortly after this recommendation Miller et al.9 and Herbeck and Tanser12 emphasized the limitations of an aggregate viral load measure, highlighting the importance of considering the composition of an individual’s sexual network and the prevalence of the underlying population.9 The NVL is a response to these limitations as it is a more precise metric for addressing HIV transmission potential.
There is at least one other network index that has been explored previously. An analysis by Little et al. of recently HIV infected adults and adolescents in San Diego examined multiple factors, such as a transmission network score (an individual’s degree in a phylogenetic transmission network), viral load, and number of sex partners.33 The study found that the numeric transmission network score, viral load, and number of sex partners (all measured at baseline) predicted risk of HIV transmission during a 12-month period.33 The NVL model, in contrast, considers a variation of these factors, but focuses on the risk of acquisition among HIV seronegative individuals rather than the transmission capacity of those recently infected as done by Little and colleagues.
The utility of the NVL is enhanced because health departments can easily create it by combining partner services and electronic laboratory surveillance data. The NVL is also different from other approaches in that it focuses on the risk of acquisition of an HIV negative individual, which may have important implications for precision PrEP delivery. Furthermore, the NVL may also be useful for identifying HIV infected index individuals who have uncontrolled viremia given their propinquity to others (both social and sexual partners) who also have high viral loads. We examined the association between viral load of the index and NVL and found a correlation between the respondents’ viral loads and the viral load of their networks (r=0.16, p=0.001). The NVL metric could also be used for mathematical modeling to measure the additive impact of reducing the NVL by varying percentages. Providers could then use these results as added incentive to control their patients’ viermia because they would be better informed of impact that each controlled patient has on HIV transmission throughout different risk populations.
Our study is limited in its ability to infer causality between NVL and HIV acquisition due to the cross-sectional nature of the study design. However, our longitudinal seroconversion results indicate that baseline NVL may be associated with incident HIV infection in larger samples. Studies of YMSM in the U.S. with longer follow-up time which allows for a greater number of seroconversions is warranted. The metrics should also be tested in international settings among populations with higher incidence rates in order to assess their effectiveness. We did not find any overlap between phylogentic clusters and any other network type with the small number of samples we were able to sequence. This finding of limited overlap is similar to a growing body of research that demonstrates limited overlap between phylogenetic and other social/sexual/risk networks.34–39 The lack of overlap has been attributed to missing network or phylogenetic data (as in our case, we have selected only a sample of the network for this analysis) that is common, as well as temporal differences between phylogenetic sample collection and self-reported transmission network.
The current analysis provides proof of principle and does not account for the dynamic nature of the networks or the timeframe of the viral-load measurements. The network data collected can be viewed as a sample of one’s overall network, similar to how a study sample represents a larger population. The NVL is an approximation of a respondent’s average network composition at a given point in time and our assumption as in most dynamic systems is that the network ties that are represented are in flux with some forming and some dissolving. The permutation analysis randomly assigning network members to different networks demonstrates that there is no association between NVL and an index’s HIV serostatus when randomly assigned.
Another potential concern is whether or not NVL is a better indicator of HIV risk than assessing the proportion of HIV+ individuals in one’s network as we have measured previously,19 or if other viral load cut-off should be used for the definition of the NVL. After controlling for all the variables in the main multivariate analysis, using a cut-off of 3,500 copies/mL revealed that the odds of HIV infection with a NVL of <10% viremia were 2.01 times that of a network with all HIV-seronegative members (OR 2.01; 95% C.I. 1.20–3.35), and the odds with a NVL of ≥10% viremia were 2.59 times that of a HIV-seronegative network (OR 2.59; 95% C.I. 1.55–4.33). Using a cut-off of 10,000 copies/mL in the same model, the odds of HIV infection with a NVL of <10% viremia were 1.97 times that of a network with all HIV-seronegative members (OR 1.97; 95% C.I. 1.23–3.16), the odds with a NVL of ≥10% viremia were 2.30 times that of a HIV-seronegative network (OR 2.30; 95% C.I. 1.36–3.89). This indicates that any level of virus in the network >3,500 copies/mL may be of concern.
The sensitivity analysis replacing NVL with HIV network proportions in the multivariate logistic regression model showed that when compared to an entirely HIV seronegative network, only networks consisting of ≥ 50% HIV infected members are associated with HIV infection. The NVL is a more precise measure of circulating virus in the risk environment than the proportion of HIV seropositive individuals who may or may not be virally suppressed.
The NVL association with HIV serostatus could likely be strengthened if all sexual connections and HIV viral loads of those partners are included. However, sex network data is rarely complete.40 It is difficult to determine how the incomplete data will bias the analyses. If participants do not report network partners who are likely to be HIV positive with uncontrolled viremia then our analysis would be a conservative estimate of the association between network viral load and HIV status. However, if respondents neglected to report HIV negative respondents then the current analysis would be overestimating one’s network viral load. We assessed a model with an interaction between NVL and one’s frequency of anal sex but found null results, perhaps due to the possibility that anal sex occurred with individuals who were unobserved in these analytic networks. While the networks in our analysis may not be complete, they may be a more realistic representation of the network that would be obtained through partner services, which is the standard practice. In partner services, less than 50% of contacts are typically ever identified.40 Targeting YMSM through strictly defined sex networks has proven unsuccessful in Chicago and in North Carolina in the U.S., given stigma and the fact that an increasing percentage of sex partners are being found online.10
The NVL has potential to improve public health practice in the U.S. Current state laboratory reporting laws combined with existing contact tracing practices and the increase in uptake of electronic lab reporting could allow for a NVL calculation of individuals at risk for HIV infection. Currently, 36 states plus the District of Columbia require laboratory reporting of all levels of CD4 and viral loads of all HIV seropositive individuals, and this number is expected to increase.41 Viral load information is becoming increasingly available. There has been a recent scale-up of point-of-care viral load testing,42 and viral load information is becoming more accessible as a result of the CDC providing supplemental HIV surveillance funding to support the implementation and maintenance of electronic lab reporting for all HIV related test results.43 Concurrently, partner services is already the standard of care for syphilis and HIV control efforts by local Public Health Services, which helps identify large numbers of individuals who are of negative or unknown HIV serostatus. Combining these two pre-existing public health data sources after inquiring about a patient’s social and sexual connections would allow for the calculation of NVL, which could then be used for a public health approach towards HIV prevention interventions where interventions such as PrEP are focused upon HIV seronegative individuals who have a high NVL. Guidelines for PrEP44 for example are still based upon individual level behaviors which may be inadequate (half of seroconverters in the uConnect cohort were not eligible for PreP according to CDC guidelines, data not shown). A paradigm shift in how we use PrEP that includes characteristics of the network, which could include NVL, is needed. Future studies should test the association between NVL and HIV seroconversion to further validate the metric and advance efforts towards HIV elimination.
Acknowledgments
We would like to thank the uConnect study participants for the time they contributed to this study. We would also like to thank staff for the collection of the data as well as Stuart Michaels, Phil Schumm, Lindsay E. Young and Nicola Lancki for their contributions. This work received funding from the National Institutes of Health grants R01 DA039934, R01 DA033875, T32 HS000084, T32 AI007384 as well as the University of Chicago, Biological Sciences Division, Office of Diversity & Inclusion.
Sources of support: This work received funding from the National Institutes of Health grants R01 DA039934, R01 DA033875, T32 HS000084, T32 AI007384 as well as the University of Chicago, Biological Sciences Division, Office of Diversity & Inclusion.
Footnotes
Competing interests: We have no competing interests to declare.
Presented in part: International AIDS Society Conference July 2016 and Conference on Retroviruses and Opportunistic Infections (CROI) February 2017.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New England Journal of Medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson PL, Glidden DVLA, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci Transl Med. 2012;4(15) doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall BDL, Friedman SR, Monteiro JFG, et al. Prevention And Treatment Produced Large Decreases In HIV Incidence In A Model Of People Who Inject Drugs. Health affairs (Project Hope) 2014;33(3):401–409. doi: 10.1377/hlthaff.2013.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevention CfDCa. [Accessed January 15, 2016];HIV in the United States: At A Glance. 2015 http://www.cdc.gov/hiv/statistics/overview/ataglance.html.
- 6.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England journal of medicine. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 7.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. doi: 10.1097/QAD.0b013e32832b7dca. 20090630 DCOM- 20091214 (1473-5571 (Electronic)) [DOI] [PubMed] [Google Scholar]
- 8.Das M, Chu PL, Santos G-M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS one. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller WC, Powers Ka, Smith Mk, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. doi: 10.1016/S1473-3099(12)70314-6. 20130426 DCOM- 20130620 (1474-4457 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurt CB, Beagle S, Leone Pa, Sugarbaker A, et al. Investigating a sexual network of black men who have sex with men: implications for transmission and prevention of HIV infection in the United States. doi: 10.1097/QAI.0b013e31827076a4. 20121109 DCOM- 20130123 (1944-7884 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan EOAM, Townsell S, Peace D, Benbow N, Schneider JA. Movement of HIV-1 Infection Through Social Networks of Younger Persons in Chicago, Illinois. Under Review. 2016 [Google Scholar]
- 12.Herbeck J, Tanser F. Community viral load as an index of HIV transmission potential. The Lancet HIV. 3(4):e152–e154. doi: 10.1016/S2352-3018(16)00036-9. [DOI] [PubMed] [Google Scholar]
- 13.Livak BSJ. Sunbelt XXXIV/INSNA. St. Pete Beach; Florida: Feb 18–22, 2014. The Network viral load: A novel HIV risk assessment strategy. [Google Scholar]
- 14.Khanna A, Michaels S, Skaathun B, Morgan E, Green K, YLSJ Preexposure Prophylaxis Awareness and Use in a Population-Based Sample of Young Black Men Who Have Sex With Men. JAMA Internal Medicine. 2016 Jan;176(1) doi: 10.1001/jamainternmed.2015.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voisin DR, Hotton AL, Schneider JA. The relationship between life stressors and drug and sexual behaviors among a population-based sample of young Black men who have sex with men in Chicago. doi: 10.1080/09540121.2016.1224303. 20160903 (1360-0451 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan E, Khanna AS, Skaathun B, et al. Marijuana Use Among Young Black Men Who Have Sex With Men and the HIV Care Continuum: Findings From the uConnect Cohort. doi: 10.1080/10826084.2016.1197265. 20160825 (1532-2491 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider JACB, Jonas A, Behler R, Lancki N, Skaathun B, Michaels S, Khanna AS, Young LE, Morgan E, Duvoisin R, Friedman S, Schumm P, Laumann EO. Network dynamics and HIV risk and prevention in a population-based cohort of Young Black Men Who have Sex with Men. Network Science. 2016 In Press. [Google Scholar]
- 18.Bureau USC. 2005–2009 American Community Survey 5-Year Estimates. 2011. [Google Scholar]
- 19.Tsang MA, Schneider Ja, Sypsa V, Schumm P, et al. Network Characteristics of People Who Inject Drugs Within a New HIV Epidemic Following Austerity in Athens, Greece. doi: 10.1097/QAI.0000000000000665. 20150627 DCOM- 20150911 (1944-7884 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman SAS. Social Networks, Risk-Potential Networks, Health, and Disease. Journal of Urban Health. 2001 Sep;78(3):411–418. doi: 10.1093/jurban/78.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strathdee SHTB, Bobrova N, Rhodes T, Booth R, Abdool R, Hankins CA. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes T. The ‘risk environment’: a framework for understanding and reducing drug-related harm. International Journal of Drug Policy. 2002;13:85–94. [Google Scholar]
- 23.Friedman SR, MD, Smyrnov P, Nikolopoulos GS, John A, Schneider JA, Livak BM, Gkikas, Slobodianyk L, Vasylyeva TI, Paraskevis D, Psichogiou M, Sypsa VM, MM, Hatzakis A. Socially-integrated transdisciplinary HIV prevention. doi: 10.1007/s10461-013-0643-5. 20140923 2014(1573-3254 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodes TSM. Transition and the HIV risk environment. BMJ. 2005 Jul 23;331:220–223. doi: 10.1136/bmj.331.7510.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tieu H-V, LT-Y, Hussen S, Cnnor M, Wang L, et al. Sexual Networks and HIV Risk among Black Men Who Have Sex with Men in 6 U.S. Cities. PloS one. 2015;10(8) doi: 10.1371/journal.pone.0134085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider JCB, Ostrow DMS, Schumm P, Laumann EOFS. Network Mixing and Network Influences Most Linked to HIV Infection and Risk Behavior in the HIV Epidemic Among Black Men Who Have Sex With Men. AJPH. 2012;103(1) doi: 10.2105/AJPH.2012.301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan E. Social, Molecular, and HIV Transmission Networks: A Sociomolecular Approach Toward HIV Prevention. Chicago, IL: Public Health Sciences, Division of the Biological Sciences and the Pritzger School of Medicine, University of Chicago; ; 2017. [Google Scholar]
- 28.Stata Statistical Software: Release 14 [computer program] College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 29.R [computer program].
- 30.Scott M. Applied Logistic Regression Analysis. Thousand Oaks, CA: Sage University; 1995. [Google Scholar]
- 31.Hunter A. Symbolic communities : the persistence and change of Chicago’s local communities. Chicago: University of Chicago Press; 1974. [Google Scholar]
- 32.Prevention CfDCa; Services HaH, editor. Guidance on Community Viral Load: A Family of Measures, Definitions, and Method for Calculation. Atlanta, GA: 2011. [Google Scholar]
- 33.Little SJ, Kosakovsky Pond SL, Anderson CM, et al. Using HIV Networks to Inform Real Time Prevention Interventions. PloS one. Jun 05; doi: 10.1371/journal.pone.0098443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostaki EN, GK, Pavlitina E, Williams L, Magiorkinis G, Schneider JA, Skaathun B, Morgan E, Psichogiou M, Sypsa V, Smyrnov P, Korobchuk A, Malliori M, Hatzakis A, Friedman SR, Paraskevis D. Molecular analysis of HIV-1 infected individuals in a network-based intervention (TRIP): Phylogenetics identify HIV-1 infected individuals with social links. 2017 doi: 10.1093/infdis/jiy239. (Under Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wertheim JOKPS, Forgione LA, Mehta SR, Murrell B, Shah S, et al. Social and Genetic Networks of HIV-1 Transmission in New York City. PLoS Pathog. 2017 doi: 10.1371/journal.ppat.1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith DMMS, Tweeten S, Drumright L, Pacold ME, Kosakovsky Pond SL, et al. A public health model for the molecular surveillance of HIV transmission in San Diego, California. AIDS. 2009;23(2):225–232. doi: 10.1097/QAD.0b013e32831d2a81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell MSMJ, Hughes JP, Celum C, Wong KG, Raugi DN, et al. Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PloS one. 2011;6(3) doi: 10.1371/journal.pone.0016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dennis AMMW, de Maria Hernandez F, Guardado ME, Nieto AI, Lorenzana de Rivera I, et al. Social network-based recruitment successfully reveals HIV-1 transmission networks among high-risk individuals in El Salvador. Journal of acquired immune deficiency syndromes. 1999;63(1):135–141. doi: 10.1097/QAI.0b013e318288b246. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilon RLL, Kim J, Vallee D, De Rubeis E, Jolly AM, et al. Transmission patterns of HIV and hepatitis C virus among networks of people who inject drugs. PloS one. 2011;7(7) doi: 10.1371/journal.pone.0022245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golden MR, Hogben M, Handsfield Hh, St Lawrence JS, Potterat JJ, Potterat Jj, Holmes KK. Partner notification for HIV and STD in the United States: low coverage for gonorrhea, chlamydial infection, and HIV. doi: 10.1097/00007435-200306000-00004. 20030603 DCOM- 20031006 (0148-5717 (Print)) [DOI] [PubMed] [Google Scholar]
- 41.CDC. State Laboratory Reporting Laws: Viral Load and CD4 Requirements. 2014. [Google Scholar]
- 42.Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. Jan 06; doi: 10.1093/cid/ciw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC. Using Viral Load Data to Monitor HIV Burden and Treatment Outcomes in the United States. 2012. [Google Scholar]
- 44.Prevention CfDCa; Services HaH, editor. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014 Clinical Practice Guideline. Atlanta, GA: US Department of Health & Human Services; 2014. [Google Scholar]