Abstract
Introduction
We used a computer simulation of HIV progression and transmission to evaluate the cost-effectiveness of a scale-up of three strategies to seek out and test individuals with undiagnosed HIV in New York City (NYC).
Setting
Hypothetical NYC population
Methods
We incorporated the observed effects and costs of the three “seek and test” strategies in a computer simulation of HIV in NYC, comparing a scenario in which the strategies were scaled up with a one-year implementation or a long-term implementation with a counterfactual scenario with no scale-up. The simulation combined a deterministic compartmental model of HIV transmission with a stochastic microsimulation of HIV progression, calibrated to NYC epidemiological data from 2003 to 2015. The three approaches were respondent driven sampling (RDS) with anonymous HIV testing (“RDS-A”), RDS with a two-session confidential HIV testing approach (“RDS-C”), and venue-based sampling (“VBS”).
Results
RDS-A was the most cost-effective strategy tested. When implemented for only one year and then stopped thereafter, using a societal perspective, the cost per quality-adjusted life-year (QALY) gained versus no intervention was $812/QALY, $18,110/QALY, and $20,362/QALY for RDS-A, RDS-C, and VBS, respectively. When interventions were implemented long-term, the cost per QALY gained versus no intervention was cost-saving, $31,773/QALY, and $35,148/QALY for RDS-A, RDS-C, and VBS, respectively. When compared to RDS-A the incremental cost effectiveness ratios (ICERs) for both VBS and RDS-C were dominated.
Conclusion
The expansion of the RDS-A strategy would substantially reduce HIV-related deaths and new HIV infections in NYC, and would be either cost-saving or have favorable cost-effectiveness.
Keywords: cost effectiveness, undiagnosed HIV, respondent driven sampling, venue based sampling, heterosexuals, health disparities, HIV testing
Introduction
Individuals with an undiagnosed HIV infection are linked to nearly a third of all HIV transmission events in the United States (US).1 Indeed, the aim of ending HIV transmission in the US cannot be achieved without improved methods to identify these individuals with undiagnosed HIV. In the effort to uncover these undiagnosed cases, the Centers for Disease Control and Prevention (CDC) has recommended persons at high risk for HIV infection receive diagnostic HIV testing at least annually.2 Yet approximately 13% of persons living with HIV (PLWH) are unaware of their status3 and identifying these undiagnosed HIV cases, the first step to engagement along the HIV care continuum, remains a significant challenge.4
Insufficient HIV diagnostic testing, leading to elevated rates of undiagnosed HIV infection, disproportionately affects heterosexual populations at high-risk for HIV (HHR) compared to other risk groups.3 Consistent with the CDC National HIV Behavioral Surveillance (NHBS) studies, we define HHR as those who are heterosexually active and socially networked within geographic areas with both excess HIV burden and socioeconomic disadvantage, referred to as “high-risk areas.”5, 6 Overall, less than half of heterosexuals have tested for HIV in their lives (43.5%), compared to more than two-thirds (69%) of men who have sex with men (MSM).7–9 Among the subpopulation of HHR, most (> 90%) have been tested for HIV at least once in the lifetimes, but regular, annual testing is uncommon.10 This is a concern because HIV prevalence is substantially higher among HHR, who are predominantly African American/Black and Hispanic, than among heterosexuals in the general population (2.0% vs. 0.1).11
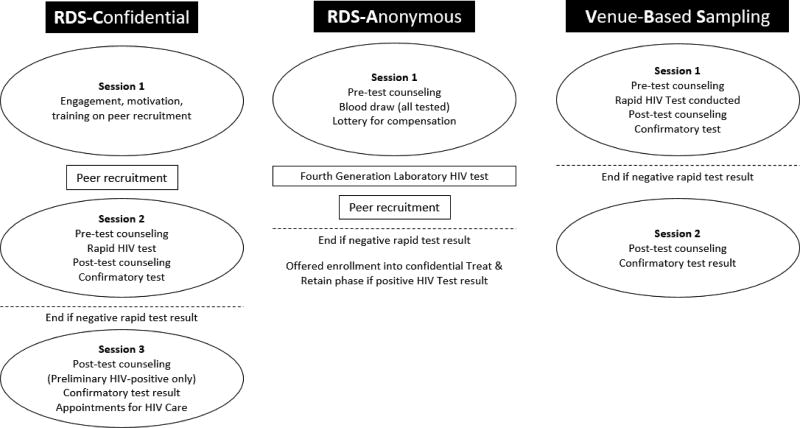
Barriers at individual/attitudinal-, social-, and structural-levels of influence impede access to and uptake of regular, annual HIV testing among HHR, including insufficient knowledge of HIV, substance use, fear of stigma, distrust of medical settings, social norms that deter HIV testing, and poor access to settings where high-quality HIV testing is offered.12, 13 Accordingly, in 2010, the National Institute on Drug Abuse (NIDA) at the National Institutes of Health called for research on new approaches to seek out persons with undiagnosed HIV, provide them with HIV counseling and testing, and link those found to be HIV infected into medical care, with high retention, which are referred to as “Seek, Test, Treat, and Retain” (STTR) studies.14 Under this initiative, we recently evaluated a set of innovative seek and test approaches in a high-risk area (due to elevated rates of heterosexual HIV prevalence and poverty) in the borough of Brooklyn in New York City (NYC). This past study, which focused on African American/Black and Hispanic HHR to provide culturally based intervention approaches, tested three different seek and test strategies designed to be acceptable and easy to access for this high-risk population including: 1) participant recruitment through respondent-driven sampling (RDS) and confidential HIV testing in two sessions [referred to as RDS-Confidential (RDS-C)]; 2) participant recruitment through RDS and anonymous HIV testing in one session, where the participant’s name was not recorded [RDS-Anonymous (RDS-A)]; and 3) participant recruitment through venue-based sampling (VBS) and confidential HIV testing in a single session (Figure 1).15
Figure 1. Schematic of study activities.
Our previous results15 demonstrated that the use of RDS, a peer-referral approach, was more efficacious than VBS for uncovering HHR with undiagnosed HIV. We found RDS-A (2.8%, 95% CI 1.1% - 4.3%) and RDS-C (1.0%, 95% CI 1.1% - 1.9%) yielded significantly higher rates of newly diagnosed HIV than VBS (0.5%, 95% CI 0.2% - 0.8%), not accounting for RDS weighting associated with social network size.15 These peer-based strategies, therefore, may have a vital role to play in efforts to eliminate HIV transmission, but they are costly. Therefore, assessing their cost-effectiveness is a necessary prerequisite for considering scale-up. The objective of the present study was to use a computer simulation of HIV progression and transmission to evaluate and compare the cost-effectiveness of a scale-up of these three seek and test strategies in NYC.
Methods
A previously validated simulation of HIV progression and transmission16, 17 was modified to incorporate the observed effects and costs of the three seek and test interventions. Using the simulation, the impact and cost-effectiveness of a scale-up of the seek and test interventions in NYC were estimated.
Model Overview
The simulation integrates information from an individual-based stochastic Monte Carlo microsimulation of HIV progression with a deterministic compartmental model of HIV transmission.16, 17 The simulation is composed of two models. The first model is a natural history model that follows a cohort of HIV-infected patients and predicts time until HIV antiretroviral therapy (ART) failure, accumulation of resistance mutations, and patient survival. Patients progress to AIDS and AIDS-related deaths at varying rates depending on whether they adhere to ART regimens and/or develop resistance to ART, based on HIV viral load suppression and CD4 trajectory. This progression model provides data to inform the second model, a transmission model. The model was developed using C/C++. This process is described in detail in Section 2.1 of the Appendix.
The transmission of HIV through the NYC population is predicted by a compartmental model. Segments of a hypothetical population can become HIV infected, have their infection detected, and access treatment, which can modify their infectivity. Segments of this population can also modify their infectivity by exhibiting risk behaviors including multiple sexual partnerships, neglecting to use condoms, having sexually transmitted infections (STIs), and using illicit substances. The model includes both sexual transmission of HIV and transmission through syringe and injection-related paraphernalia-sharing during injection drug use (IDU). HIV transmission was modeled using a binomial process and assumed assortative mixing in the population. The probability of transmission between partners was adjusted to account for infected partner's gender, disease state, and treatment status.
Transmission model compartments are stratified by age, sexual activity level, presence of unhealthy alcohol use, IDU, HIV status, and if infected, HIV viral load, CD4, and ART resistance patterns. The design of the simulation, as well as its calibration and validation, is described in more detail in the Appendix and elsewhere.16, 17 We used the calibrated simulation to evaluate the impact and value of the three seek and test interventions in NYC.
The seek and test interventions’ effects were represented in the transmission model pathway by accelerating transitions from “infected but undetected” compartments to “infected and detected” compartments. We conservatively assumed that intervention effects only persisted while the respective intervention program was continued. The simulation was calibrated to NYC epidemiological data with the goal of replicating trends in NYC HIV prevalence, incidence, deaths, and persons with HIV from 2003 to 2015. The analyses performed were assumed to have started in 2015 with an estimated 2015 HIV prevalence, deaths, and incidence being represented in the model. The model inputs and the intervention effects are described in more detail in Table 1.
Table 1.
Key Input parameters
| Parameter or input | Value | Reference |
|---|---|---|
| Sexual risk characteristics | ||
| Proportion of population who are abstinent | 21.0% | 33 |
| Probability of monogamous relationship (if sexually active) | ||
| Men who have sex with women (MSW) | 78.2% | 34 |
| Men who have sex with men (MSM) | 55.8% | 34 |
| Women who have sex with men (WSM) | 91.1% | 34 |
| Women who have sex with women (WSW) | 48.9% | 34 |
| Probability of multiple partnerships (if sexually active) | ||
| MSW | 21.8% | 34 |
| MSM | 44.2% | 34 |
| WSM | 8.9% | 34 |
| WSW | 51.1% | 34 |
| Proportion of men who are MSM | 5.6% | 34 |
| Proportion of men who are MSW | 94.4% | 34 |
| Proportion of women who are WSW | 2.4% | 34 |
| Proportion of women who are WSM | 97.6% | 34 |
| Injection Drug Use Characteristics | ||
| Proportion of population that injects drugs | 1.43% | 35 |
| Proportion of injection drug users (IDUs) who have unsafe injection practices | 32% | 36 |
| Proportion of IDUs who are male | 70% | 36 |
| Sexual and IDU transmission | ||
| Transmission risk per sex act | ||
| Male-to-male | 0.00167 | 37 |
| Female-to-male | 0.00042 | 37 |
| Male-to-female | 0.00081 | 37 |
| Transmission risk per unsafe needle sharing act | 0.003 | 38 |
| Relative risk of transmission dependent on viral load | 0.16 – 9.03 | 39 |
| Sex acts (per partnership) per year | 89 | 40 |
| Shared injections per year | 70 | Assumption |
| HIV risk behaviors and biological/behavioral modifiers of transmission | ||
| Prevalence of untreated sexually transmitted infection | 6.9% | 41, 42 |
| Prevalence of unhealthy alcohol use | 5% | 43 |
| Prevalence of consistent condom usage | 35% | 34 |
| HIV disease related | ||
| Probability of annual HIV test | 31% | 34 |
| Probability of linkage to care | 75% | Unpublished NYC DOMH data |
| Probability of initiating ART if in care | 87% | Unpublished NYC DOMH data |
| ART compliance | 62% | 44 |
| Demographics | ||
| Age-related mortality rate | 0.0068 (6.8/1000 pop) | 45 |
| Fertility rate | 0.0156 (15.6/1000 pop/year) | 45 |
| Interventions | ||
| VBS hit rate | 0.496% | BCAP Trial |
| RDS-C hit rate | 0.931% | BCAP Trial |
| RDS-A hit rate | 2.81% | BCAP Trial |
| Costs | ||
| VBS per person enrolled | $368 | BCAP Trial |
| RDS-C per person enrolled | $648 | BCAP Trial |
| RDS-A per person enrolled | $531 | BCAP Trial |
| Cost of care for individuals with CD4<100 | $58,320 | 46 |
| Cost of care for individuals with CD4>100 | $30,312 | 46 |
ART: antiretroviral therapy
To examine hypothetical effects on HIV with scaling of the seek and test programs, we varied “reach,” which we defined as the proportion of the population with an unknown HIV status enrolled in the program. To compare the costs and impact of each intervention for a hypothetical implementation of just one year, the reach of the interventions was set to 27.5 persons enrolled per 1,000 population, correlating to the level at which the most effective intervention (RDS-A) identified 50% of the previously unknown HIV-positive population in one year. A one year implementation was selected to investigate the impact of a potential programmatic choice for a one-off short term implementation. To evaluate the costs and impact of the intervention implemented long-term over a 20-year horizon the program reach was set to 4.5 persons enrolled per 1,000 population, correlating to the level at which the most effective intervention (RDS-A) identified 50% of the previously unknown HIV-positive population after 20 years. The benchmark of 50% was chosen arbitrarily.
Costs and effects were discounted at 3%, our time horizon was 20 years, and costs were assessed from a societal perspective, which included the cost of treatment, as well as, a programmatic perspective, that did not include treatment costs, using 2015 $US. Other than specifying a finite time horizon, all other aspects of the cost-effectiveness analysis were conducted in line with recommendations by the Panel on Cost-Effectiveness in Health and Medicine.18 We chose a 20 year rather than infinite time horizon because we found that it was the longest time horizon viewed as credible by stakeholders (e.g. public health decision makers) in general. The simulated population consisted of HIV-infected and uninfected New Yorkers from 2015 through 2035, with the intervention assumed to begin in the year 2015. More complete details of model specification, initial population structure and parameterization are located in the appendix and have been published elsewhere.16, 17
Seek and Test Interventions
As noted above, the seek and test study included three different interventions designed to be acceptable and easy to access for this high-risk population including: 1) respondent driven sampling (RDS) with anonymous HIV testing (“RDS-A”), 2) RDS with a two-session confidential HIV testing approach (“RDS-C”), 3) and venue-based sampling (“VBS”) (Figure 1).15 All three seek and test interventions included components to refer those testing HIV-negative to prevention services, and link newly diagnosed individuals to HIV primary care (i.e., a “treat & retain” study phase). Complete methods are described elsewhere.19–21 The primary outcome that was incorporated as an input into the HIV model was the number of newly diagnosed HIV infections per number enrolled (“hit rate”) and the cost per patient of each intervention (Table 1).
Cost-effectiveness analysis
We conducted simulations where the seek and test interventions were activated, and calculated the health benefits, costs, and cost-effectiveness ratios of each over the 20-year time horizon. These simulations were compared to a reference case where no additional interventions were implemented. Outcomes measured include total quality-adjusted life years (QALYs) gained, cost per QALY gained, incremental cost effectiveness ratios (ICERs), number and proportion of new HIV infections averted, incremental cost per infection averted, infections detected and cost per infection detected. As a sensitivity analysis, we varied both intervention efficacy and cost independently across plausible ranges and evaluated their impact on cost-effectiveness. We then simulated the seek and test interventions and calculated the ICERs of the interventions. ICERs measure the additive benefit of each strategy compared with its next best alternative, and interpret this benefit together with its additive cost. A cost per QALY gained value less than $100k was considered cost-effective because it approximates the opportunity cost of achieving health benefit in the US health system and a cost per QALY gained value less than $20k was considered very cost effective because it approximates the opportunity cost of highly effective HIV-specific ART.22
Results
Effectiveness
One-Year Implementation
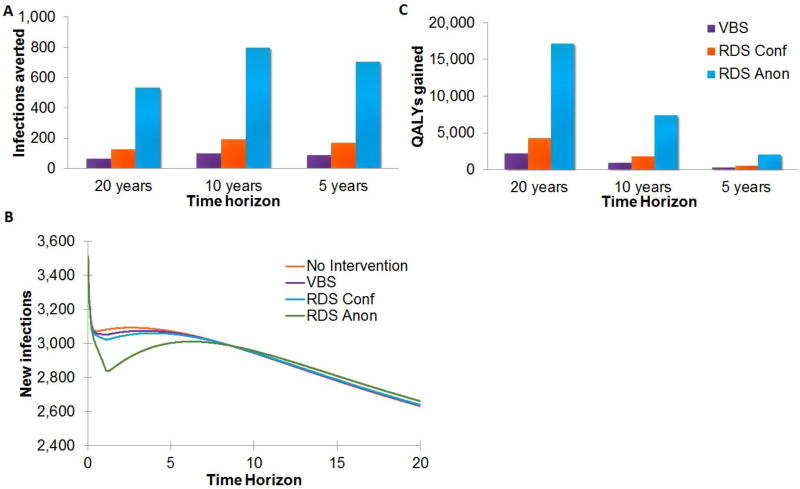
When implemented for only one year and then stopped thereafter, the RDS-A, RDS-C, and VBS interventions reduced the number of new HIV infections over 20 years by 533, 126, and 64 infections, respectively, versus the base case of 58,402 new infections in NYC (Figure 2a,b). With the RDS-A, RDS-C, and VBS interventions the number of QALYs gained was 17,191, 4,288, and 2,206, respectively (Figure 2c). With the RDS-A, RDS-C, and VBS interventions the number of HIV-related deaths over 20 years was reduced by 1,626, 404, and 208, respectively versus a base case of 14,324 deaths.
Figure 2. Impact of a 1 year implementation (a) infections averted, (b) new infections, (c) QALYS gained.
Note: The decrease in infections averted at the 20-year time horizon can be attributed to the generation of new infections by the patients on treatment that are living longer and have a greater number of opportunities to spread HIV. In the base case scenario, however, these individuals do not have the long term potential to infect others due to the high probability of death.
Long-term Implementation
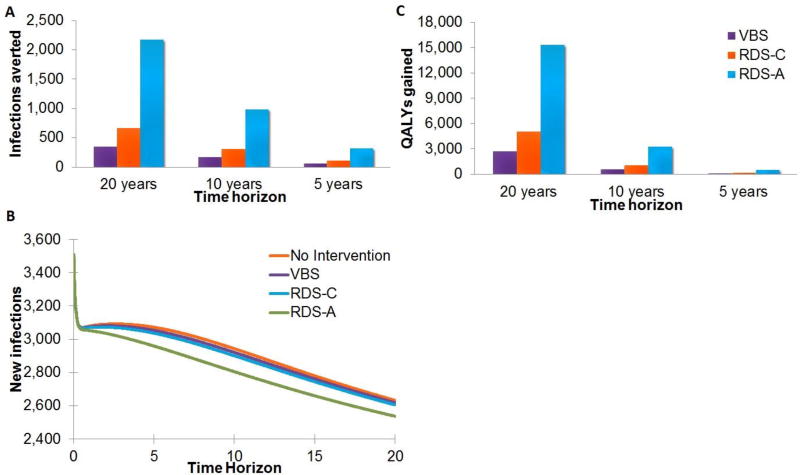
When implemented long-term, the RDS-A, RDS-C, and VBS interventions reduced the number of new HIV infections over 20 years by 2,166, 657, and 344 infections, respectively, versus base case of 58,402 HIV-infected persons in NYC (Figure 3a,b). With the RDS-A, RDS-C, and VBS interventions the number of QALYs gained was 15,291, 5,040, and 2,683, respectively (Figure 3c). With the RDS-A, RDS-C, and VBS interventions the number of HIV-related deaths over 20 years was reduced by 2,769, 916, and 488, respectively versus a base case of 14,324 deaths.
Figure 3. Impact of a long-term implementation (a) infections averted, (b) new infections, (c) QALYS gained.
Cost effectiveness
The cost per person enrolled in each strategy was $531, $648, and $368 for RDS-A, RDS-C, and VBS, respectively. From a programmatic perspective, the cost per newly detected HIV-positive person was $18,882, $69,612, and $74,206 for RDS-A, RDS-C, and VBS, respectively.
One-Year Implementation
When implemented for only one year and then stopped thereafter, and using a programmatic perspective, RDS-A, RDS-C, and VBS added discounted costs of $74,556,402, $91,019,837, and $51,693,795, respectively; corresponding to costs per infection averted of $139,808, $724,678, and $805,058, respectively. The cost per QALY gained versus no intervention was $4,337/QALY, $21,225/QALY, and $23,431/QALY for RDS-A, RDS-C, and VBS, respectively. When the three alternatives were considered together, the ICER for RDS-A was $1,526 compared to VBS, and RDS-C was dominated. (Table 2)
Table 2.
Twenty Year Time Horizon Intervention Incremental Cost-Effectiveness
| Total Costs (discounted USD) |
Incremental Cost |
Total Discounted QALYs |
Incremental Effect (QALYs) |
$/QALY versus no intervention |
ICER ($/QALY) |
|
|---|---|---|---|---|---|---|
| 1 Yr. Implementation | ||||||
| Program Perspective | ||||||
| No Intervention | 0 | - | 104,051,017 | - | - | - |
| VBS | 51,693,795 | $51,693,795 | 104,053,224 | 2,206 | $23,431 | $23,431 |
| RDS-A | 74,556,402 | $74,556,402 | 104,068,209 | 17,191 | $4,337 | $1,526 |
| RDS-C | 91,019,837 | $91,019,837 | 104,055,306 | 4,288 | $21,225 | Dominated |
| Societal Perspective | ||||||
| No Intervention | 44,670,703,504 | - | 104,051,017 | - | - | - |
| RDS-A | 44,684,670,725 | $13,967,220 | 104,068,209 | 17,191 | $812 | $812 |
| VBS | 44,715,626,205 | $44,922,701 | 104,053,224 | 2,206 | $20,362 | Dominated |
| RDS-C | 44,748,362,898 | $77,659,394 | 104,055,306 | 4,288 | $18,110 | Dominated |
| Long-term Implementation | ||||||
| Program Perspective | ||||||
| No Intervention | 0 | - | 104,051,017 | - | - | - |
| VBS | 135,543,430 | $135,543,430 | 104,053,700 | 2,683 | $50,523 | $50,523 |
| RDS-A | 195,522,868 | $195,522,868 | 104,066,308 | 15,291 | $12,787 | $4,757 |
| RDS-C | 238,661,689 | $238,661,689 | 104,056,057 | 5,040 | $47,356 | Dominated |
| Societal Perspective | ||||||
| No Intervention | 44,670,703,504 | - | 104,051,017 | - | - | - |
| RDS-A | 44,610,518,121 | -$60,185,383 | 104,066,308 | 15,291 | -$3,936 | Cost-saving |
| VBS | 44,764,998,002 | $94,294,498 | 104,053,700 | 2,683 | $35,148 | Dominated |
| RDS-C | 44,830,830,915 | $160,127,410 | 104,056,057 | 5,040 | $31,773 | Dominated |
When implemented for only one year and then stopped thereafter, but using a societal perspective, over 20 years the one-year implementation of RDS-A, RDS-C, and VBS added discounted costs of $44,684,670,725, $44,748,362,898, and $44,715,626,205, respectively; corresponding to costs per infection averted of $26,191, $61,8306, and $699,608, respectively. The cost per QALY gained versus no intervention was $812/QALY, $18,110/QALY, and $20,362/QALY for RDS-A, RDS-C, and VBS, respectively. When the three alternatives were considered together, compared to RDS-A the ICERs for both VBS and RDS-C were dominated. (Table 2)
Long-term Implementation
When implemented long-term but using a programmatic perspective RDS-A, RDS-C, and VBS resulted in a total discounted cost of $195,522,868, $238,661,689, and $135,543,430, respectively; corresponding to costs per infection of $90,275, $363,252, and $393,662, respectively. The cost per QALY gained versus no intervention was $12,787/QALY, $47,356/QALY, $50,523/QALY for RDS-A, RDS-C, and VBS, respectively. When the three alternatives were considered together, the ICER for RDS-A was $4,757 compared to VBS, and RDS-C was dominated. (Table 2)
When implemented long-term but using a societal perspective, RDS-A, RDS-C, and VBS resulted in a total discounted cost of $44,610,518,121, $44,830,830,915, and $44,764,998,002, respectively; corresponding to costs per infection averted of cost-saving, $243,720, and $273,862, respectively. The cost per QALY gained versus no intervention was cost-saving, $31,773/QALY, and $35,148/QALY for RDS-A, RDS-C, and VBS, respectively. When compared to RDS-A the ICERs for both VBS and RDS-C were dominated. (Table 2)
Sensitivity Analyses
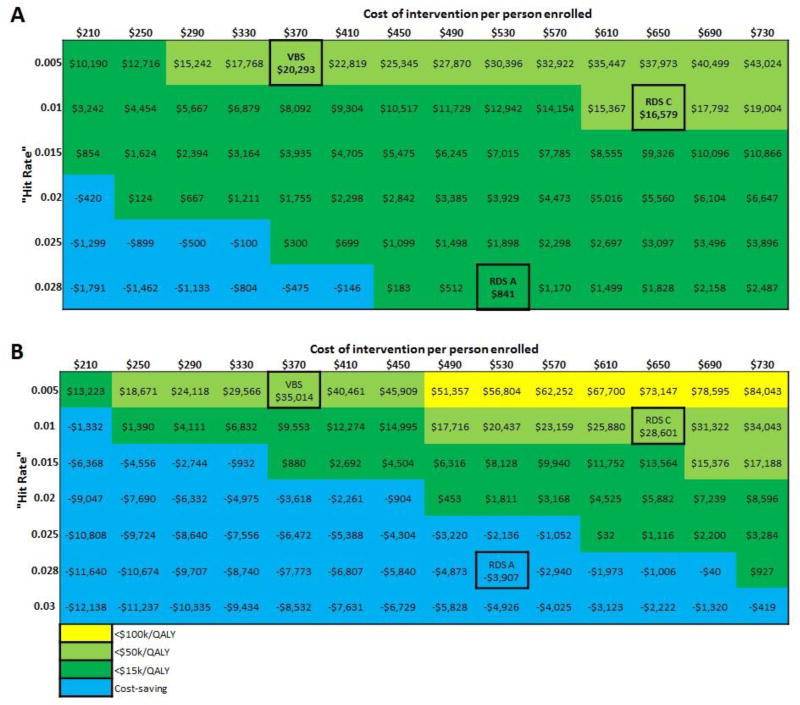
In sensitivity analyses of both a one-year and long-term implementation, all three interventions maintained favorable value across all costs and “hit rate” values assessed (Figure 4). In a one-year implementation, for the RDS-A strategy to become cost-saving, the cost of the program must be decreased by approximately 22.6% from $530 to $410. In long-term implementation, for the RDS-A strategy to remain cost-saving the strategy’s “hit rate” must be decreased by no more than 28.6% from 0.028 to 0.02 nor can the cost be increased by more than 37.7% from $530 to $730. To be cost-saving the RDS-C and VBS strategies would require significant reductions in programmatic costs. (Figure 4)
Figure 4. Sensitivity analyses for (a) one-year implementation and (b) long-term implementation from a societal perspective.
Note: “Hite rate” represents number of newly diagnosed HIV infections per number enrolled; VBS = venue-based sampling; RDS-C = respondent driven sampling, confidential; RDS-A = respondent driven sampling, anonymous; QALY = quality adjusted life year
Conclusion
We provide estimates of the impact and cost-effectiveness of the scale-up of three seek and test strategies for HHR conducted in a large urban high-risk area. Our analyses suggested that, from a societal perspective, a scale-up of RDS-A using a long-term implementation was likely to be cost-saving and dominated the two other seek and test strategies. Even when the strategies were limited to one year and discontinued thereafter, RDS-A remained cost-effective and dominated the other strategies investigated. Our findings were robust over a range of assumptions regarding cost and effectiveness. While other RDS-based strategies have been used in numerous research studies and intervention delivery settings for other high-risk populations, such as MSM and persons who inject drugs,23 and the CDC’s NHBS projects have used RDS for recruiting HHR populations nationally,24, 25 RDS and VBS seek and test strategies for HIV testing in HHR have not previously been evaluated for cost-effectiveness.
Our findings suggest that RDS-A, a single-session and low-threshold approach designed to be acceptable and easy to access for this high-risk population, is a cost-effective seek and test strategy for identifying HIV-infected persons who were otherwise undetected, overcoming many of the challenges to engaging HHR in HIV testing. In fact, past research has shown high-quality HIV testing experiences may foster more timely engagement in HIV care, in contrast to negative, or even coercive, experiences that may impede acceptance of the new HIV status and trigger medical distrust and fear.26 Descriptive exploratory findings from the “treat and retain” phase that followed the study’s seek and test phase, albeit with imprecise estimates given small sample sizes, showed majority of those newly diagnosed with HIV in RDS-A elected to enroll in a confidential treat and retain phase designed to foster linkage to HIV care in a timely fashion, and of these, the majority (> 80%) had achieved undetectable HIV viral load levels within six months.27 Similarly, the majority of participants found newly diagnosed with HIV in RDS-C engaged in HIV care and achieved undetectable HIV viral load levels (> 60%) by the final follow-up period, approximately a year after diagnosis.28 In sum, our cost-effectiveness analyses provide the strongest support for the implementation and scale-up of a RDS-A seek and test strategy in NYC and, we estimate, comparable urban environments, followed by RDS-C.
While all three seek and test strategies investigated were cost-effective compared to no intervention, the RDS-A strategy proved to be the most cost-effective RDS strategy tested, because not only was it more effective at finding undiagnosed cases of HIV in HHR,21 but it also incurred a lower programmatic cost than RDS-C. Further, while both RDS strategies performed better than VBS at identifying individuals with high rates of serious risk factors such as incarceration, unemployment, and homelessness, the RDS-A strategy outperformed RDS-C in this regard. We speculate that RDS-A out-performed RDS-C in part because it successfully engaged participants with high rates of multiple risk factors, including those with current or past substance use problems, who typically experience serious barriers to HIV testing and services. This, combined with the provision of HIV testing at the first contact, made the RDS-A intervention easy to access, and also, we speculate, helped reduce the fear of potential HIV stigma through anonymous testing.21 The same mechanism that may have reduced perceived stigma among participants; namely, anonymous testing in the first session, also acted to reduce the required programmatic investment per person enrolled. By decreasing personnel resources consumed by providing testing in a single session, the overall programmatic costs of RDS-A-based testing were reduced by roughly 18%. Thus single-session/anonymous strategy peer-referral approaches for uncovering HHR with undiagnosed HIV can reduce costs and have a vital role to play in efforts to eliminate HIV transmission.
In scenarios in which seek and test implementation lasted for only one year and was discontinued thereafter, the number of infections averted peaked approximately 8 years after program implementation for all three strategies. This finding can be attributed to the generation of new infections by the patients on ART that would have otherwise not occurred due to death from AIDS without the intervention. Although treatment reduces the infectivity of an individual, it does not completely eliminate the risk of HIV transmission;29 consequently, on treatment these patients are living longer and have a greater number of opportunities to spread HIV. In the base case scenario, however, these individuals do not have the long-term potential to infect others due to the high probability of death.
Similarly, the RDS-A strategy can be seen to accrue a greater number of QALYs gained in a one year implementation as compared to a long-term intervention. The greater number of QALYs gained is a result of placing a greater number of people on ART at an earlier time, therefore allowing those on treatment to generate an increased rate of QALYs for longer. The difference between the long-term implementation and the one-year implementation, however, decreases with time as more individuals are placed on treatment in the long-term implementation, and less effective interventions such as RDS-C and VBS, which have a greater number of QALYs gained at shorter time horizons, can be seen to have fewer QALYs gained at 20 years than a long-term implementation.
The waning impact of a single wave of seek and test emphasizes the need for a long-term implementation. By achieving cost-saving at 5-years, scaled up implementation of RDS-A has the potential to have great health impact (prevent 0.9% of infections and 11.4% of deaths) while being cost-saving in the short-term. Long-term implementation, while not necessarily cost-saving in the short-term, are cost-effective, have the potential to prevent 3.7% of infections and 19.3% of deaths, and are cost-saving in the long term.
Our analysis has a number of limitations. First, our model did not incorporate the potential limitations of RDS as a sampling strategy, including potentially diminishing returns as a smaller proportion of HIV-positive cases remain undetected. This may underestimate the cost per infection identified for the two RDS strategies, as over time more people may need to be enrolled in order to identify a consistent number of infections. However, we limited our scale-up assumption to 50% to minimize the bias of diminishing marginal returns. Second, the model cannot distinguish between the identification of new infections and HIV-positive individuals that have been previously tested and diagnosed. Those previously diagnosed but mislabeled as newly diagnosed may lead to overestimation of cost-effectiveness, as some will have made progress on the continuum of care prior to the program. Also, those previously diagnosed but not linked to care, are more likely to have characteristics that are associated with being less likely to receive ART and stay engaged in care than a newly diagnosed individual.30, 31 Third, the analysis included the same intervention costs for all individuals enrolled in the study, including those that did not ultimately receive HIV testing. This, however, likely overestimates the total cost of implementation as individuals not receiving HIV testing would not incur the full intervention cost. Fourth, this study was unable to distinguish whether different populations were reached by the different strategies. Therefore, further study may be needed to determine whether unique populations are reached by each strategy and to assess whether combination strategies may be appropriate. Finally, the seek and test interventions were carried out in a population that has a relatively high prevalence of HIV and HHR compared to other populations in the US. Consequently our results may not be applicable to all populations. However, the HIV prevalence rate is similar to high-risk neighborhoods in 11 of the 15 most populous cities in the US, as well as, in numerous other smaller cities.32, 33 Further testing is needed to investigate the RDS-A strategy’s effectiveness in populations outside of densely populated high risk urban areas.
The expansion of the RDS-A seek and test strategy would substantially reduce HIV-related deaths and avert new HIV infections in NYC, and would be either cost-saving or have favorable cost-effectiveness. Further testing is needed to investigate the strategy’s effectiveness in urban populations outside of NYC and outside of densely populated high risk urban areas.
Supplementary Material
Acknowledgments
The study was supported by the National Institute on Drug Abuse (R01DA032083) and the Center for Drug Use and HIV Research (CDUHR; P30 DA011041; Sherry Deren, PhD and Holly Hagan PhD, Co-Principal Investigators). We wish to thank our Program Officer at NIDA, Dr. Richard Jenkins, for support and guidance throughout the study, and our research participants. The BCAP Collaborative Research Team that carried out the study also includes Noelle R. Leonard, Ph.D., Mindy Belkin, MA, Talaya McCright-Gill, MA, Belkis Martinez, MA, Angela Banfield, MPH, Elizabeth Silverman, LMSW, Kerri O’Meally; Amy Braksmajer, PhD; Robert Quiles; Amani Sampson; Jenny Panzo, RN; Lisa Sanfillipo, RN, David Perlman, MD, Ann Kurth, PhD, Holly Hagan, PhD, Sam Jenness, PhD, Quentin Swain, Bridget Cross, MSW, Kathy Ha, MSW, Dawa Sherpa, BA, and Ether Ampofo, BS.
Funding: NIH R01DA032083, CDUHR P30DA011041
References
- 1.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4(R) cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore RD, Keruly JC. CD4R cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 3.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4R count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Lelyveld SF, Gras L, Kesselring A, Zhang S, De Wolf F, Wensing AM, et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS. 2012;26:465–474. doi: 10.1097/QAD.0b013e32834f32f8. [DOI] [PubMed] [Google Scholar]
- 6.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitchen CMR. Nonparametric variable selection using machine learning algorithms in high dimensional (largep, smalln) biomedical applications. In: Laskovski A, editor. Biomedical engineering: trends in electronics, communication and software. 2011. p. AQ6. [Google Scholar]
- 9.Diaz-Uriarte R, Alvarez de Andes S. Gene selection and classification of microarray data using random forests. BMC Bioinformatics. 2006;7:3. doi: 10.1186/1471-2105-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobl C, Boulesteix A, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strobl C, Boulesteix A, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Sinclair E, Jain V, Huang Y, Epling L, Van Natta M, et al. Low proportions of CD28-CD8R T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis. 2014;210:374–382. doi: 10.1093/infdis/jiu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatano H, Jain V, Hunt PW, Lee T, Sinclair E, Do T, et al. Cellbased measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4R T cells. J Infect Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conn D, Ramirez C. Random and fuzzy forests applied to feature selection in biomedical research. In: Alvarez R, editor. Computational social science: discovery and prediction. Oxford, UK: Cambridge University Press; 2015. (In Press) [Google Scholar]
- 15.Conn DAQ7, Ngun T, Gang L, Ramirez C. Fuzzy Forests: Extending Random Forests for Correlated, High-Dimensional, Data. UCLA Biostatistics Working Paper Series. 2015 http://www.escholarship.org/uc/item/55h4h0w7.
- 16.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 17.Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL. IGFBP2 is a biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104:5563–5568. doi: 10.1073/pnas.0609139104. Immunophenotypes in aviremic HIVR adults Ramirez et al. 9 CE: Swati, AIDS-D-15-0-0127; Total nos of Pages: 11; AIDS-D-15-00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Y, Yang Q, Cuenco K, Cupples L, DeStefano A, Lunetta KL. Two-stage approach for identifying single-nucleotide polymorphisms associated with rheumatoid arthritis using random forests and Bayesian networks. BMC Proc. 2007;1:S56. doi: 10.1186/1753-6561-1-s1-s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Y, Yu Y, Adrienne Cupples L, Farrer L, Lunetta K. Performance of random forest when SNPs are in linkage disequilibrium. BMC Bioinformatics. 2009;10:78. doi: 10.1186/1471-2105-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicodemus K, Wang W, Shugart Y. Stability of variable importance scores and rankings using statistical learning tools on single nucleotide polymorphisms (SNPs) and risk factors involved in gene-gene and gene-environment interaction. BMC Proc. 2007;1:S58. doi: 10.1186/1753-6561-1-s1-s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1128. Epub 2005 Aug 2012. [DOI] [PubMed] [Google Scholar]
- 22.Deeks S, Kitchen C, Liu L, Guo H, Gasson R, Narvaez A, et al. Immune activation set point during HIV infection predicts subsequent T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 23.Deeks S, Martin JN, Sinclair E, Harris J, Neilands TB, Maecker HT, et al. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug resistant human immunodeficiency virus type 1. J Infect Dis. 2004;189:312–321. doi: 10.1086/380098. [DOI] [PubMed] [Google Scholar]
- 24.Deeks SG. Virologic outcomes with protease inhibitor therapy in an urban AIDS clinic: relationship between baseline characteristics and response to both initial and salvage therapy. AIDS. 1999;13:F34–F44. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 25.Deeks SG, Barbour JD, Grant RM, Martin JN. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. AIDS. 2002;16:201–207. doi: 10.1097/00002030-200201250-00009. [DOI] [PubMed] [Google Scholar]
- 26.Deeks SG, Barbour JD, Martin JN, Swanson MS, Grant R. Sustained CD4R T-cell response after virologic failure of protease-based regimens in patients with HIV infection. J Infect Dis. 2000;181:946–953. doi: 10.1086/315334. [DOI] [PubMed] [Google Scholar]
- 27.Hatano H, Delwart E, Norris PJ, Tzong-Hae L, Dunn-Williams J, Hunt P, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt P, Brenchley J, Sinclair E, McCune JM, Roland M, PageShafer K, et al. Relationship between T cell activation and CD4R T cell count in HIV seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicodemus K, Malley J, Strobl C, Ziegler A. The behaviour of random forest permutation-based variable importance measures under predictor correlation. BMC Bioinformatics. 2010;11:110. doi: 10.1186/1471-2105-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference frameork. J Comput Graph Stat. 2006;15:651–674. [Google Scholar]
- 31.Ishwaran H. Variable importance in binary regression trees and forests. Electron J Stat. 2007;1:519–537. [Google Scholar]
- 32.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, PageShafer K, et al. Relationship between T cell activation and CD4R T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt PW, Landay AL, Sinclair E, Martinson JA, Hatano H, Emu B, et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One. 2011;6:e15924. doi: 10.1371/journal.pone.0015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8R T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, et al. Phenotypic, functional, and kinetic parameters associated with apparent t-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 2005;79:14169–14178. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, PageShafer K, et al. Relationship between t cell activation and CD4(R) T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vingert B, Benati D, Lambotte O, de Truchis P, Slama L, Jeannin P, et al. HIV controllers maintain a population of highly efficient Th1 effector cells in contrast to patients treated in the long term. J Virol. 2012;86:10661–10674. doi: 10.1128/JVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowell T, Gebo K, Blankson J, Korthuis P, Yehia B, Rutstein R, et al. Elite controllers are hospitalized more often than persons with mediclaly controlled HIV. J Infect Dis. 2015;211:1692–1702. doi: 10.1093/infdis/jiu809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira L, Valdez H, McCune JM, Koup R, Badley A, Hellerstein MK, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 43.Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4R T cells. J Infect Dis. 2012 doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower cd4R t cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 45.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.