Abstract
Introduction
This survey was conducted to evaluate carpal tunnel syndrome (CTS) symptom severity, functional status and outcome of CTS therapies in patients with inherited neuropathies.
Methods
Validated questionnaires were used to compare symptom severity and functional status in patients with and without a diagnosis of CTS and a diagnosis of an inherited neuropathy.
Results
309 patients with inherited neuropathies participated in this study. The CTS symptom severity score (SSS),was found to be the most useful tool in assessing CTS severity in patients with inherited neuropathy. Splint therapy and surgery were associated with significant improvement in carpal tunnel symptoms as measured through the SSS.
Discussion
This study provides an insight into the assessment of CTS symptom severity and patient reported outcomes to CTS therapy in individuals with inherited neuropathies. The SSS appears useful for evaluation of CTS symptoms and of patient reported outcomes following CTS interventions in individuals with inherited neuropathies.
Keywords: Charcot-Marie-Tooth disease, Inherited Neuropathy, Hereditary Neuropathy with liability to Pressure Palsies, Boston Carpal Tunnel Questionnaire, Carpal Tunnel Syndrome, CMT, HNPP
Introduction
Charcot-Marie-Tooth (CMT) disease, or inherited peripheral neuropathy, has an estimated prevalence of 41/100,000 (1 in 2500).1 Individuals with CMT generally have progressive weakness beginning in the distal limb muscles. Many patients with CMT develop loss of hand strength and functional limitations in the upper extremities.2–5 Upper limb and hand dysfunction is therefore a major issue for patients with inherited neuropathy.6
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy, affecting approximately 3–6 % of adults in the general population.7 The prevalence of CTS is not precisely known in most forms of CMT. Diagnosing CTS in the setting of a neuropathy can be problematic, owing to the overlap of symptoms in the hands in both conditions, and because the electrophysiological criteria used to diagnose CTS are less reliable in the face of axonal loss and demyelination produced by a generalized neuropathy. Data is lacking regarding outcomes of interventions for CTS in inherited neuropathies to inform development of standards of care for the upper extremity in different forms of CMT and hereditary neuropathy with liability to pressure palsies (HNPP). The role of carpal tunnel release in patients with inherited neuropathy is currently unknown.8 This study was designed to evaluate CTS symptom severity and therapy outcomes in patients with inherited neuropathy.
Materials and Methods
Study participants
This study utilized the web-based Inherited Neuropathies Consortium (INC) Rare Diseases Clinical Research Network (RDCRN (1U54NS0657)) Contact Registry. This is a patient-based registry that allows patients with all forms of CMT to self-register. All registry participants over the age of 18 were asked to participate. Other known CMT is defined as individuals who are diagnosed with CMT, but are not classified as CMT1A, CMT1B, CMT2A, CMTX, CMT4 or HNPP (for example CMT1C, CMT1D, or CMT3). Unknown CMT is defined as individuals who have CMT but do not know the specific form. Carpal tunnel symptom severity score (SSS) has a range of 11–55, with higher numbers indicating more severe symptoms.
Standard protocol approvals, registrations, and patient consents
The National Institute of Health-RDCRN and University of South Florida institutional review board (IRB) approved all aspects of this study. As directed by the institutional review board, all registry and survey participants received and reviewed a detailed information letter and provided consent before their involvement with this research.
Survey construction
The survey instrument was constructed based on the CMT symptom score (CMTSS) for the evaluation of CMT disease severity 9,10 and two validated questionnaires for the evaluation of outcomes related to carpal tunnel syndrome; the Boston Carpal Tunnel Questionnaire (BCTQ) 11 and the Disability of Arm, Shoulder and Hand (DASH) questionnaire.12 DASH, however, is not specific to CTS and has also been used to assess upper limb function in CMT; 13 as such, it was included to assess the overlap in reported symptoms in patients with CMT and CTS. Data collected from participants with a diagnosis or history of therapies for CTS was compared to data from participants without a diagnosis or prior treatment for CTS. The survey consisted of 109 items, including 9 items related to demography, 16 items to assess CMTSS, 9 items to assess CTS history, 11 items related to SSS from the BCTQ and 8 items related to a functional status score (FSS), from the BCTQ as it pertains to pre- and post-any CTS therapies. The survey also included 7 items related to CTS therapies and 30 items related to the DASH.
The CMTSS is the symptom component of the CMT neuropathy score (CMTNS).14 The BCTQ was developed specifically for carpal tunnel syndrome and consists of 11 SSS items and 8 FSS questions.11 BCTQ has established reliability,11 high internal consistency11 and convergent validity.15,16 Responsiveness of the BCTQ after carpal tunnel surgery has been demonstrated in randomized clinical trial settings.17–19 The DASH questionnaire is a 30 item scale which assesses symptoms and physical function.12 The validity of DASH has been demonstrated through correlation with BCTQ.15,20 BCTQ was found to be more responsive than DASH for carpal tunnel syndrome.21
Statistical analysis
Descriptive statistics was used in the analysis of all demographic data. Responses were categorized by age, gender, CMT type, CMTSS, CTS history and therapies, SSS (BCTQ), FSS (BCTQ), and DASH scores. The BCTQ, SSS, FSS, and DASH scores were analyzed with Spearman’s rank correlation with CMTSS and age. Fisher’s exact tests were employed to compare the proportion of participants with CTS among the different CMT subgroups and when evaluating SSS distribution in respondents with CTS and those with no history of CTS. The Mann-Whitney U test was used to compare the mean scores between the participants with and without a CTS history. Wilcoxon signed-rank tests were used to compare the distributions of mean scores across various CTS therapies among the participants with a CTS history. A two-tailed p value < 0.05 was considered significant.
Results
Of 331 participants who consented to this online study, 309 completed the study (completion rate 93%) with a mean age of 54 ± 14 years. Patients with CMT and a previous diagnosis of CTS comprised 31% of the respondents. The diagnosis of CTS was made by physicians according to all respondents with CTS (neurologists 49%, hand surgeons 23%, other specialists 15% and primary physicians 13%). Of the respondents with a diagnosis of CTS, 77% reported having nerve conduction studies (NCS), whilst 17% did not think they had NCS and 6% were not sure if they had NCS. There was no statistical difference in the mean age and gender ratio between respondents with CTS and those with no history of CTS. More females than males participated in the study (Table 1).
Table 1.
Demographic, genetic and questionnaire score characteristics of CMT patients with and without a diagnosis of carpal tunnel syndrome.
| Characteristics | Cohort | w CTS | w/o CTS | p-value |
|---|---|---|---|---|
| CMT, n (%) | 309 (100) | 97 (31) | 212 (69) | < 0.000001δ |
| Age, mean ± SD [yrs] | 54 ± 14 | 54 ± 12 | 55 ± 14 | 0.4† |
| Female to Male ratio | 1.6 | 4.1 | 1.2 | 1.0* |
| CMTSS, mean ± SD | 7 ± 3 | 7 ± 3 | 7 ± 3 | 0.1† |
| BCTQ, mean ± SD | 45 ± 16 | 55 ± 14 | 40 ± 15 | <0.0000001† |
| SSS, mean ± SD | 25 ± 10 | 33 ± 9 | 22 ± 8 | <0.0000001† |
| FSS, mean ± SD | 19 ± 7 | 22 ± 6 | 18 ± 7 | <0.000003† |
| DASH, mean ± SD | 71 ± 27 | 77 ± 26 | 68 ± 27 | <0.003† |
| CMT1A/PMP22dup, n (%) | 152 (49) | 44 (45) | 108 (51) | 0.4* |
| CMT1B/MPZ, n (%) | 17 (6) | 6 (6) | 11 (5) | 0.9* |
| CMT2A/MFN2, n (%) | 31 (10) | 5 (5) | 26 (12) | 1.0* |
| CMTX1/GJB1, n (%) | 20 (6) | 9 (9) | 11 (5) | 0.3* |
| HNPP/PMP22del, n (%) | 12 (4) | 9 (9) | 3 (1) | <0.004* |
| CMT4, n (%) | 6 (2) | 1 (1 ) | 5 (2) | 1.0* |
| Other known CMT, n (%) | 21 (7) | 8 (8) | 13 (6) | 0.6* |
| Unknown CMT, n (%) | 49 (16) | 14 (14) | 35 (17) | 1.0* |
| Diabetes | 28 (9) | 11 (11) | 17 (8) | 0.4* |
| Thyroid disease | 51 (17) | 21 (22) | 30 (14) | 0.1* |
| Comorbidities¶ | 53 (17) | 27 (28) | 26 (12) | <0.002* |
binomial test (5% estimated prevalence);
Fisher’s exact test;
Mann-Whitney U test;
rheumatoid arthritis, wrist fracture, renal dialysis, osteoarthritis at the wrist or collagen vascular disease.
Abbreviations: BCTQ, Boston carpal tunnel questionnaire score; CMT, Charcot-Marie-Tooth disease; CMTSS, CMT symptom score; CTS, carpal tunnel syndrome; DASH, disability of arm, shoulder and hand questionnaire score; GJB1, gap junction protein beta 1; HNPP, hereditary neuropathy with liability to pressure palsies; MFN2, mitofusin 2; MPZ, myelin protein zero; PMP22, peripheral myelin protein 22; SD, standard deviation; SSS, symptom severity score; FSS, functional status score.
CMT Subtypes and CTS
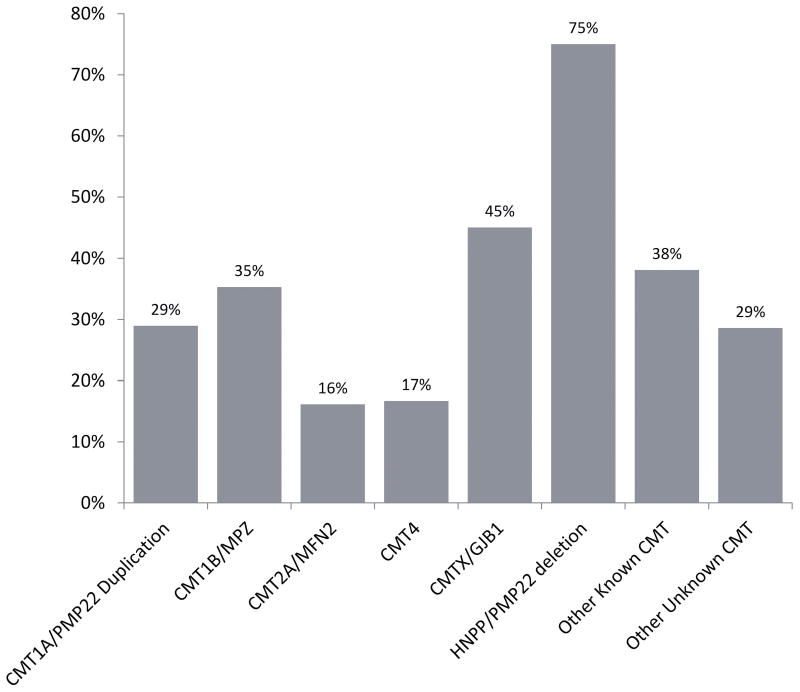
Overall, respondents with HNPP had the highest prevalence of CTS, followed by respondents with CMTX1, other known CMT, CMT1B, CMT1A, unknown CMT and CMT2A (Fig. 1). The distribution of CMT genetic subtypes in the respondents (Table 1) was comparable to the distribution in the RDCRN contact registry.
Figure 1.
Composition of CMT subtypes among the respondents with a history of CTS.
Respondents with and without CTS
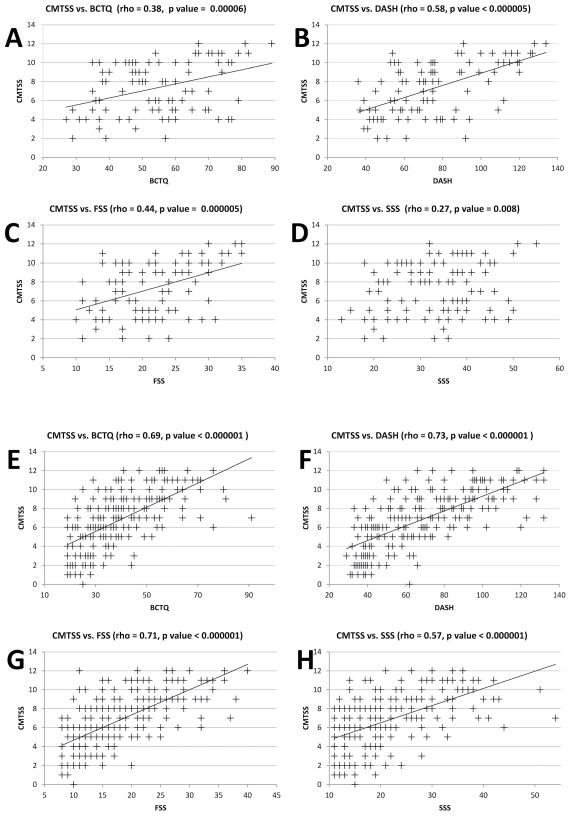
Individuals with CMT and CTS reported a higher frequency of medical comorbidities (rheumatoid arthritis, wrist fracture, renal disease requiring dialysis, osteoarthritis at the wrist or collagen vascular disease) than those without reported CTS (Table 1). There was no significant difference in reported diabetes or thyroid disease between respondents with and without CTS. CMTSS was equivalent between the group with a diagnosis of CTS and the group with no history of CTS. The BCTQ score and its two components SSS and FSS were significantly different between respondents from the two groups. DASH was also significantly different between respondents from the two groups, albeit to a lesser extent in comparison to BCTQ, SSS and FSS. The difference in the SSS component of the BCTQ was the most significant between the groups (Table 1). Correlation analyses of the CMTSS score with the BCTQ, DASH, SSS and FSS in the two groups showed that the SSS had only a weak correlation to the CMTSS in respondents with CMT and CTS with a Spearman’s rho of 0.27 (Fig. 2). There was no correlation between Age and BCTQ, DASH, SSS and FSS. A weak correlation was found with Age and CMTSS in the CMT patients with no history of CTS (p=0.002, rho=0.21).
Figure 2.
Scatter plots with Spearman’s correlation analysis of Charcot-Marie-Tooth (CMT) symptom severity (CMTSS) with the Boston Carpal Tunnel Questionnaire (BCTQ), Disability of Arm, Shoulder and Hand (DASH), Carpal Tunnel Symptom Severity Score (SSS) and Carpal Tunnel Functional Status Score (FSS) in CMT patients with Carpal Tunnel Syndrome (CTS) (A-D) and CMT patients without CTS (E-H). BCTQ, FSS and DASH showed moderate to strong correlation to CMTSS scores in all CMT patients. The SSS component of BCTQ showed a weak correlation to CMTSS in CMT patients with CTS (D).
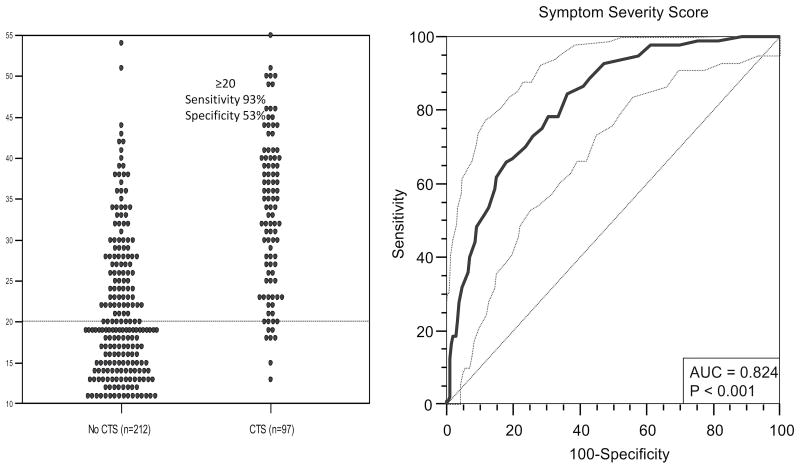
The frequency of SSS was compared between respondents with a CTS diagnosis and respondents with no history of CTS through a dot plot and a receiver operating characteristic plot (ROC). When SSS of ≥ 20 was used as a threshold to indicate CTS disease, a high sensitivity and moderate specificity were achieved (Fig. 3). Given that high sensitivity is sought for screening purposes, the cutoff of SSS ≥ 20 was chosen to provide for a screening tool in patients with inherited neuropathy.
Figure 3.
A dot plot showing the distribution of CTS symptom severity score (SSS) in subjects with a diagnosis of CTS and those with no history of CTS and a threshold of SSS ≥20 used as indicative of CTS disease. A receiver operating characteristic (ROC) plot with area under the curve (AUC) of 0.824 is obtained, when SSS is used as a screening test for CTS. At SSS ≥ 20 as a threshold, there is a sensitivity of 93% (0.86–0.97, 95% CI), specificity 53% (0.46–0.60, 95% CI), negative predictive value (NPV) 94% (0.88–0.98, 95% CI) and positive predictive value (PPV) 47% (0.40–0.55, 95%CI) with Fisher exact 2-tailed p <0.0000001.
CTS therapy outcomes
The SSS component of the BCTQ (identified in the survey as pre-or post-therapy) was used to evaluate CTS therapy outcomes in respondents with a history of CTS. There was no significant reduction in the mean calculated SSS in respondents with no therapy and injection therapy. There was however a significant reduction in the mean SSS in patients who used splint therapy or underwent surgical intervention for CTS. The reduction in the mean SSS was also apparent in respondents with CMT1A after splint use or surgical intervention for CTS. There was no statistically significant reduction in the mean SSS in respondents with CMTX1 with splint use or surgical intervention. Splint use in respondents with HNPP was not associated with a reduction in the mean SSS and only one patient with HNPP and CTS reported having a surgical intervention for CTS (Table 2).
Table 2.
CTS therapy outcomes measured through the symptom severity score (SSS) in participants with CMT and CTS.
| Characteristics | n | SSS (Pre-) | SSS (Post-) | p-value |
|---|---|---|---|---|
| No Treatment, mean ± SD | 12 | 25 ± 5 | 24 ± 6 | 0.1† |
| Splint, mean ± SD | 35 | 34 ± 10 | 31 ± 9 | < 0.006† |
| Injection, mean ± SD | 6 | 36 ± 10 | 33 ± 13 | 0.5† |
| Surgery, mean ± SD | 44 | 34 ± 9 | 24 ± 8 | <0.000001† |
| CMT1A (splint), mean ± SD | 12 | 37 ± 9 | 32 ± 10 | <0.02† |
| CMT1A (surgery), mean ± SD | 25 | 34 ± 9 | 24 ± 8 | 0.0001† |
| HNPP (splint), mean ± SD | 8 | 36 ± 17 | 36 ± 12 | 0.7† |
| HNPP (surgery), mean ± SD | 1 | 31 | 22 | - |
| CMTX1 (splint), mean ± SD | 5 | 30 ± 11 | 26 ± 8 | 0.3† |
| CMTX1 (surgery), mean ± SD | 4 | 40 ± 7 | 26 ± 6 | 0.07 |
Wilcoxon signed-rank test
The satisfaction rate in respondents with CTS who underwent surgical intervention was high with 92% reporting being either very satisfied or satisfied with the procedure. Among those with a history of surgical therapy 68% reported that they would try CTS release again.
Discussion
The two respondent groups in the study; namely, CMT patients with a CTS diagnosis and those with no history of CTS matched in terms of age, gender ratio and CMT severity as measured by the CMTSS. Overall, the group with CTS comprised 31% of the respondents. This rate of reported CTS disease in patients with inherited neuropathies is much higher than in the general population (estimated to be as high as 5.8%)7 but is consistent with a previous report of higher prevalence of median neuropathy at the wrist in CMT.22 In considering the prevalence of CTS in various subtypes of CMT, patients with HNPP were unique among inherited neuropathy subtypes in having a higher prevalence of CTS (three times more likely) compared to having HNPP and no CTS. This is consistent with previous reports of a high prevalence of CTS in patients with HNPP.23 Contrary to a prior report,24 we did not find a higher prevalence of CTS in CMTX1 patients, as compared to other forms of CMT. Although diabetes and thyroid disease have been recognized as risk factors for developing CTS in the general population,25 we did not identify an increase in the prevalence of CTS when diabetes or thyroid disease were reported in patients with inherited neuropathies. Interestingly, CTS was significantly less frequent in respondents who did not have comorbidities such as rheumatoid arthritis, wrist fracture, renal dialysis, osteoarthritis at the wrist or collagen vascular disease. Thus, having one or more of these comorbidities appears to increase the risk of having CTS in patients with inherited neuropathies.
Respondents with CMT and a diagnosis of CTS had higher BCTQ, SSS, FSS and DASH scores than the group without CTS. The SSS component of BCTQ is however unique in that it is more resistant to the confounding effects of symptomatology of CMT as demonstrated through the weak correlation with CMTSS in patients with CMT and CTS. As such, the 11 questions encompassing the SSS can be used with some confidence in assessing CTS disease severity in patients with CMT. Although it has been suggested that patients with SSS < 23 should be considered to have their symptoms under control,26 our data indicates that using SSS less than 20 might be more appropriate as a screening tool in patients with inherited neuropathy given that high sensitivity is sought for screening purposes. A SSS of less than 20 decreases the likelihood of superimposed CTS in the setting of a hereditary neuropathy with a negative predictive value (NPV) of 94% (0.88–0.98, 95% CI). While the DASH questionnaire has been used in the assessment of outcomes in CTS patients,15,27 this study demonstrates that there is a strong correlation between DASH and CMT symptoms that degrades its utility in assessing CTS symptoms in patients with underlying hereditary neuropathy.
With the use of the SSS as a patient reported outcome measure (PROM) to assess various CTS therapies in CMT patients, we found that splint and surgical interventions were associated with a significant reduction in the reported CTS symptoms. More specifically, splint and surgical therapies were shown to be effective in reducing CTS symptoms in patients with CMT1A (the most common subtype), while splint and surgical therapies were not shown to be effective in reducing symptom severity in HNPP and CMTX (Table 2). Contrary to other CMT subtypes, the majority of patients with HNPP reported no improvement in their CTS symptoms with splint therapy. The apparent lack of response to CTS therapies in HNPP and CMTX1 patients should be interpreted with caution, given the small sample size in these two subtypes. Finally, the satisfaction rate in respondents with CTS who underwent surgical intervention was very high with 93% reporting being either very satisfied or satisfied with the outcome.
A limitation of this study is that the INC RDCRN contact registry and by extension our sample, may not accurately represent the general CMT population. While we did not find any statistically significant difference between the respondents with CTS and those without CTS in terms of age, gender distribution or CMT severity, we cannot exclude the possibility that responding registry participants have a different clinical profile than the general CMT population. Although a diagnosis of CTS was not required for participation in this study, a sampling bias favoring a higher CTS prevalence cannot be excluded. A further limitation of the study was that the specifics of the CTS diagnosis (e.g. physical examination and neurophysiological testing) were not available to us for independent confirmation. Another potential limitation of this study relates to the possibility of subject recall bias as they were reporting on symptoms that might have changed over the years especially prior and post CTS therapy.
In conclusion, the rate of reported CTS is high across CMT subtypes as compared with those described for the general population, while HNPP is associated with an increased prevalence of CTS in comparison to other CMT subtypes. The CTS severity score (SSS), a component of BCTQ, appears to be a useful screening tool in assessing CTS in patients with inherited neuropathy. Splint therapy and surgical intervention was associated with significant improvement in carpal tunnel symptoms as measured through the SSS in patients with inherited neuropathy.
Acknowledgments
The authors would like to thank all the participants in this Inherited Neuropathies Consortium (INC) Contact Registry study; without whom this study would not be possible. The INC (2U54NS065712-07) is a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. The INC is funded through a collaboration between NCATS and the NINDS. Study Funded by NIH-RDCRN U54 (U54NS065712).
Abbreviations
- BCTQ
Boston carpal tunnel questionnaire score
- CMT
Charcot-Marie-Tooth disease
- CMTSS
CMT symptom score
- CTS
carpal tunnel syndrome
- DASH
disability of arm, shoulder and hand questionnaire score
- GJB1
gap junction protein beta 1
- HNPP
hereditary neuropathy with liability to pressure palsies
- MFN2
mitofusin 2
- MPZ
myelin protein zero
- PMP22
peripheral myelin protein 22
- SD
standard deviation
- SSS
symptom severity score
- FSS
functional status score
Footnotes
Ethical Publication Statement: We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures
F. Panosyan received fellowship support from the Inherited Neuropathies Consortium. D. Marking, C. Kirk, M. Reilly, S. Scherer and M. Shy report no disclosures relevant to the manuscript. D. Herrmann received grant support from the NINDS (U54NS065712), FARA, MDA, and have received funds for consulting or Adjudication activities from Medpace, Guidepoint Global, and LAM-Therapeutics.
References
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6(2):98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 2.Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurology. 2009;8(7):654–667. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- 3.Videler AJ, Beelen A, Aufdemkampe G, de Groot IJ, Van Leemputte M. Hand strength and fatigue in patients with hereditary motor and sensory neuropathy (types I and II) Archives of Physical Medicine and Rehabilitation. 2002;83(9):1274–1278. doi: 10.1053/apmr.2002.34282. [DOI] [PubMed] [Google Scholar]
- 4.Miller MJ, Williams LL, Slack SL, Nappi JF. The Hand in Charcot-Marie-Tooth Disease. Journal of Hand Surgery-British and European Volume. 1991;16B(2):191–196. doi: 10.1016/0266-7681(91)90175-n. [DOI] [PubMed] [Google Scholar]
- 5.Harding AE, Thomas PK. The Clinical-Features of Hereditary Motor and Sensory Neuropathy Type-I and Type-Ii. Brain. 1980;103(JUN):259–280. doi: 10.1093/brain/103.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Videler AJ, Beelen A, van Schaik IN, de Visser M, Nollet F. Limited Upper Limb Functioning Has Impact on Restrictions in Participation and Autonomy of Patients with Hereditary Motor and Sensory Neuropathy 1a. Journal of Rehabilitation Medicine. 2009;41(9):746–750. doi: 10.2340/16501977-0419. [DOI] [PubMed] [Google Scholar]
- 7.Atroshi I, Gummesson C, Johnssson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. Jama-Journal of the American Medical Association. 1999;282(2):153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 8.Earle N, Zochodne DW. Is carpal tunnel decompression warranted for HNPP? J Peripher Nerv Syst. 2013;18(4):331–335. doi: 10.1111/jns5.12047. [DOI] [PubMed] [Google Scholar]
- 9.Piscosquito G, Reilly MM, Schenone A, Fabrizi GM, Cavallaro T, Santoro L, et al. Responsiveness of clinical outcome measures in Charcot-Marie-Tooth disease. European Journal of Neurology. 2015;22(12):1556–1563. doi: 10.1111/ene.12783. [DOI] [PubMed] [Google Scholar]
- 10.Shy ME, Siskind C, Swan ER, Krajewski KM, Doherty T, Fuerst DR, et al. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68(11):849–855. doi: 10.1212/01.wnl.0000256709.08271.4d. [DOI] [PubMed] [Google Scholar]
- 11.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A Self-Administered Questionnaire for the Assessment of Severity of Symptoms and Functional Status in Carpal-Tunnel Syndrome. Journal of Bone and Joint Surgery-American Volume. 1993;75A(11):1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hudak PL, Amadio PC, Bombardier C, Boland A, Fischer T, Flatow EL, et al. Development of an upper extremity outcome measure: The DASH (Disabilities of the Arm, Shoulder, and Head) American Journal of Industrial Medicine. 1996;29(6):602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Eklund E, Svensson E, Hager-Ross C. Hand function and Disability of the Arm, Shoulder and Hand in Charcot-Marie-Tooth disease. Disability and Rehabilitation. 2009;31(23):1955–1962. doi: 10.1080/09638280902874170. [DOI] [PubMed] [Google Scholar]
- 14.Murphy SM, Herrmann DN, McDermott MP, Scherer SS, Shy ME, Reilly MM, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16(3):191–198. doi: 10.1111/j.1529-8027.2011.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appleby MA, Neville-Smith M, Parrott MW. Functional outcomes post carpal tunnel release: a modified replication of a previous study. J Hand Ther. 2009;22(3):240–248. doi: 10.1016/j.jht.2009.03.001. quiz 249. [DOI] [PubMed] [Google Scholar]
- 16.Wintman BI, Winters SC, Gelberman RH, Katz JN. Carpal tunnel release. Correlations with preoperative symptomatology. Clin Orthop Relat Res. 1996;(326):135–145. [PubMed] [Google Scholar]
- 17.Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074–1081. doi: 10.1016/S0140-6736(09)61517-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaye JJ, Reynolds JM. Carpal tunnel syndrome: using self-report measures of disease to predict treatment response. Am J Orthop (Belle Mead NJ) 2007;36(4):E59–62. [PubMed] [Google Scholar]
- 19.Jarvik JG, Comstock BA, Heagerty PJ, Haynor DR, Fulton-Kehoe D, Kliot M, et al. Magnetic resonance imaging compared with electrodiagnostic studies in patients with suspected carpal tunnel syndrome: predicting symptoms, function, and surgical benefit at 1 year. J Neurosurg. 2008;108(3):541–550. doi: 10.3171/JNS/2008/108/3/0541. [DOI] [PubMed] [Google Scholar]
- 20.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128–146. [PubMed] [Google Scholar]
- 21.Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the disabilities of the arm, shoulder, and hand, the carpal tunnel questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg Am. 2003;28(2):250–254. doi: 10.1053/jhsu.2003.50043. [DOI] [PubMed] [Google Scholar]
- 22.Guerre J, Gutierrez A. Prevalence of median entrapment neuropathy at the wrist in a patient with Charcot-Marie-Tooth disease. Muscle & Nerve. 2015;52:S67–S67. [Google Scholar]
- 23.Potocki L, Chen KS, Koeuth T, Killian J, Iannaccone ST, Shapira SK, et al. DNA rearrangements on both homologues of chromosome 17 in a mildly delayed individual with a family history of autosomal dominant carpal tunnel syndrome. Am J Hum Genet. 1999;64(2):471–478. doi: 10.1086/302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arthur-Farraj PJ, Murphy SM, Laura M, Lunn MP, Manji H, Blake J, et al. Hand weakness in Charcot-Marie-Tooth disease 1X. Neuromuscular Disorders. 2012;22(7):622–626. doi: 10.1016/j.nmd.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpitskaya Y, Novak CB, Mackinnon SE. Prevalence of smoking, obesity, diabetes mellitus, and thyroid disease in patients with carpal tunnel syndrome. Annals of Plastic Surgery. 2002;48(3):269–273. doi: 10.1097/00000637-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Storey PA, Fakis A, Hilliam R, Bradley MJ, Lindau T, Burke FD. Levine-Katz (Boston) Questionnaire analysis: means, medians or grouped totals? Journal of Hand Surgery-European Volume. 2009;34E(6):810–812. doi: 10.1177/1753193408104560. [DOI] [PubMed] [Google Scholar]
- 27.Greenslade JR, Mehta RL, Belward P, Warwick DJ. Dash and boston questionnaire assessment of carpal tunnel syndrome outcome: What is the responsiveness of an outcome questionnaire? Journal of Hand Surgery-British and European Volume. 2004;29B(2):159–164. doi: 10.1016/j.jhsb.2003.10.010. [DOI] [PubMed] [Google Scholar]