Abstract
A liquid chromatography–high resolution mass spectrometry (LC–HRMS) method was developed and validated for the determination of piperine (PPR) on dried blood spots (DBS). DBS samples were prepared by spiking the whole blood with analyte to produce 30 µL of blood spots on specimen collection cards. Chromatographic separation was achieved on an Atlantis dC18 column using acetonitrile and water (0.1% formic acid) (85:15, v/v) as mobile phase in an isocratic mode of elution at a flow rate of 0.75 mL/min. MS detection was carried out in electrospray positive ion mode for the target ions and monitored at m/z 286.1465 for PPR and 272.1303 for the internal standard (IS). The developed method exhibited a linear dynamic range over 0.01–2000 ng/mL for PPR on DBS. The overall extraction recovery of PPR from DBS was 92.5%. Influence of hematocrit and spot volume on DBS was also evaluated and found to be well within the acceptable limits. The method was successfully applied to pharmacokinetic studies of PPR in rats.
Keywords: Dried blood spot, LC–HRMS, Piperine, Pharmacokinetics, Trichostachine
1. Introduction
Piperine (PPR), a major alkaloid of Piper longum and Piper nigrum, has been reported to have several pharmacological actions. It exhibits antioxidant, anti-inflammatory, antidiarrheal, anti-convulsant, antimutagenic, hypolipidemic, bile secretion-promoting and tumor-inhibiting activities [1], [2]. It is also a known antidepressant of the central nervous system [3], [4]. In addition, it has been reported to enhance the bioavailability of several drugs [5], [6]. Because of these multiple biological effects, bioanalysis and pharmacokinetic studies of PPR have become a focus of research. Several analytical methods were reported in the literature for the determination of PPR alone or in combination with other bioactive compounds in biological fluids, including high performance thin layer chromatography (HPTLC) [7], high performance liquid chromatography (HPLC) [8], [9], liquid chromatography–nuclear magnetic resonance–mass spectrometry (LC–NMR–MS) [10], liquid chromatography–tandem mass spectroscopy (LC–MS/MS) [11], [12] and ultra-fast liquid chromatography (UFLC) [13], [14]. In all the above methods, sample treatment has been carried out by protein precipitation. However, these assays require a relatively large blood volume (typically >0.5 mL) to generate sufficient plasma volume for analysis. In contrast, dried blood spots (DBS), the collection of blood samples on absorbent paper requires minimum blood volumes, which can easily be obtained by finger or heel prick, and obviates plasma centrifugation to simplify sample preparation. Compared with conventional whole blood samples, the collection of whole blood samples on filter paper shows distinct advantages, including longer lifespan of samples with reduced need for refrigeration, less invasiveness, greater cost-effectiveness, easy shipment and storage, and reduction of infection risks by deactivation of potential pathogens on the filter paper.
Thus, DBS has become a subject of interest for the quantification of various drugs for pharmacokinetic studies as the bioanalytical dry matrix offers several advantages over conventional venous blood sampling. This also facilitates significant advantages when ethical considerations hamper large volume sampling in premature infants and the elderly. Also, a number of publications on the use of DBS for the analysis of a variety of drugs including antimalarials [15], antiepileptics [16], antiretrovirals [17], antidiabetics [18] and antihypertensives [19] have been reported. The successful usage of DBS has also been reported in toxicokinetic [20], therapeutic drug monitoring [21] and pharmacokinetics studies [22] as well.
To the best of our knowledge, no studies have been reported for quantification of PPR, the principal bioactive compound in Piper nigrum and Piper longum, using DBS sampling. Medicinal plant extracts which are used in ayurvedic formulation usually contain very minute amount of bioactive compounds. It is also common that these extracts may contain a number of molecules which sometimes have the same molecular weight. In the present study, we employed high–resolution mass spectrometry (HRMS) because it offers high sensitivity, mass resolution and mass accuracy (<5 ppm). HRMS in general has been improved over the last couple of years and is now competitive with triple quadrupole MS for quantitation. Here, we described the details of our attempts to develop and validate a simple, sensitive and selective LC–HRMS method for the quantification of PPR on DBS and discussed its potential utility.
2. Experimental
2.1. Chemicals and materials
PPR and trichostachine (TCS) were isolated from Piper nigrum fruits in our laboratory. Their structures were identified by NMR and MS analysis and compared with reported data [23]. HPLC grade methanol, acetonitrile and formic acid were obtained from Merck Specialties Pvt. Ltd. (Mumbai, India). Water used in the entire analysis was prepared on a Milli–Q water purification system procured from Millipore (Bangalore, India). FTA DMPK-C cards were supplied by Whatmann (Whatman, GE Healthcare, NJ). Sample tubes were obtained from Tarsons (Kolkata, India). A centrifuge (model 2–16P) supplied by Sigma (Zurich, Switzerland) and a Harris punch and cutting mat supplied by Whatmann (Stanford, ME, USA) were employed. Sachets of silica gel and sealing plastic bags for the storage of blood spot cards were purchased from the local market. The repeater multi pipette used for spotting blood was obtained from Tarsons (Kolkata, India). EDTA coated capillaries from Sarstedt (Leicester, UK) were used.
2.2. Liquid chromatography–high resolution mass spectrometry (LC–HRMS)
The chromatographic system consisting of an Agilent 1200 series LC instrument (Agilent Technologies, USA) coupled with quadrupole time–of–flight (Q–TOF) mass spectrometer (Q–TOF LC/MS 6510 series classic G6510A, Agilent Technologies, USA) equipped with an ESI source was used. Chromatography was performed on Waters Atlantis dC18 (150 mm×4.6 mm, 5 μm) column at 25 °C using acetonitrile and water (containing 0.1% formic acid) (85:15, v/v) as mobile phase in an isocratic elution mode at a flow rate of 0.75 mL/min, and the injection volume was 10 µL. The data acquisition was under the control of Mass Hunter workstation software. The typical operating source conditions for MS scan in positive ESI mode were optimized: the ionization voltage 80 V, the capillary voltage 3000–3500 V, the skimmer 60 V, nitrogen used as the drying (300 °C; 9 L/min) and nebulizer (45 psi) gas. External calibration of the TOF–MS was performed daily before the analysis.
2.3. Preparation of stock solutions, calibration standards (CSs) and quality control (QC) samples
PPR and internal standard (IS) (approximately 10 mg) were dissolved in 10 mL of methanol in a volumetric flask to give a 1 mg/mL stock solution. Working solutions containing all analytes were freshly prepared in methanol : water (50:50, v/v) to produce dilutions of 20,000, 15,000, 10,000, 5000, 2500, 1000, 500, 250, 100, 20 and 0.1 ng/mL. CSs were prepared by spiking different samples of fresh blood (900 µL) with 100 µL of each of the above-mentioned working solutions to yield final concentrations of 2000, 1500, 1000, 500, 250, 100, 50, 25, 10, 2 and 0.01 ng/mL of PPR in the blood. A zero PPR blood sample (blank) was prepared by spiking fresh blood (900 µL) with 100 µL of methanol : water (50:50, v/v). QC samples were prepared by diluting the appropriate working standards with whole blood to give concentrations of 10 (low QC), 400 (medium QC) and 1200 (high QC) ng/mL of PPR. QC and CS samples thus prepared were employed for use in the validation of the method and also for stability studies. These DBS QCs were stored at room temperature in sealed plastic bag containing desiccant for further use.
2.4. DBS sample preparation
Using a calibrated pipette, 30 µL of the respective whole blood samples collected at regular intervals was spotted onto FTA blood spot cards to prepare the DBS samples. The samples were set to dry for 3 h at room temperature and stored at 4 °C until required for analysis.
2.5. DBS sample extraction
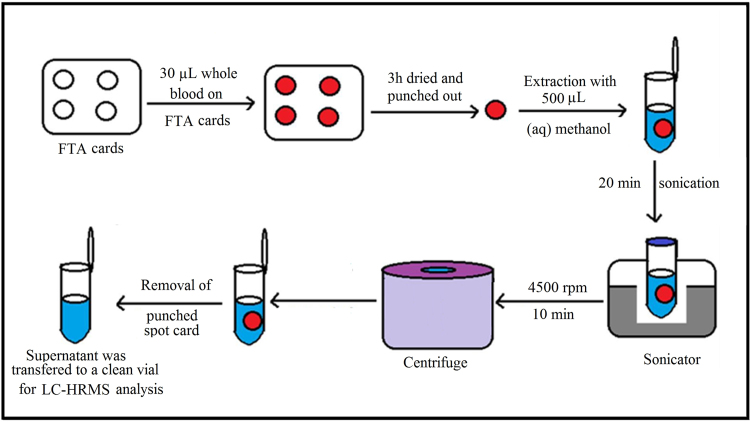
A 10 mm disk was punched from the center of the DBS sample and transferred to a microcentrifuge tube. A 500 µL volume of extraction solvent (50% aqueous methanol) consisting of IS (250 ng/mL) was added, and the tube was vortex mixed for 10 min. The contents were centrifuged for 10 min, and the supernatant was transferred to an autosampler vial for analysis by LC–HRMS. The extraction procedure is shown schematically in Fig. 1.
Fig. 1.
Extraction of PPR from dried blood spots.
2.6. Validation procedures
Validation of the developed method was performed to evaluate the following parameters: selectivity, linearity, precision, accuracy, lower limit of quantification (LLOQ) as well as stability of QC samples. DBS also has its own unique set of properties that should be assessed, which include spot volume, hematocrit and dilution effects [24].
3. Results and discussion
3.1. Selection of IS
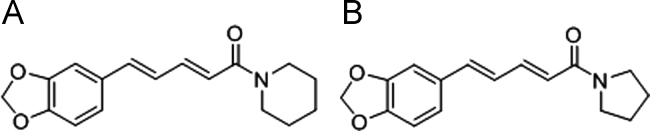
Selection of appropriate IS is an important aspect to deal with sample matrix effects. An ideal IS should be a structurally similar analog or stable isotope–labeled compound. TCS was chosen as the IS for the quantification of PPR due to its similarity in structure (Fig. 2), ionization response and extraction recovery in ESI–MS and a similar elution pattern.
Fig. 2.
Chemical structures of (A) PPR and (B) IS.
3.2. Optimization of chromatographic conditions
Chromatographic conditions, especially the composition of mobile phase, play a critical role in achieving good chromatographic behavior and appropriate ionization. Different mobile phases (methanol–water, acetonitrile–water with or without formic acid or ammonium acetate) were investigated using Atlantis dC18 (150 mm×4.6 mm, 5 µm) column to optimize the analytical performance. It was observed that acetonitrile was found to be better in terms of resolution and peak shapes as compared with methanol. Using acetonitrile–water (containing 0.1% formic acid) good peak shape, considerable response and baseline separation were achieved. The mobile phase was operated at a flow rate of 0.75 mL/min allowing a short run time of 3.0 min, without compromising the chromatographic selectivity.
3.3. Optimization of mass conditions
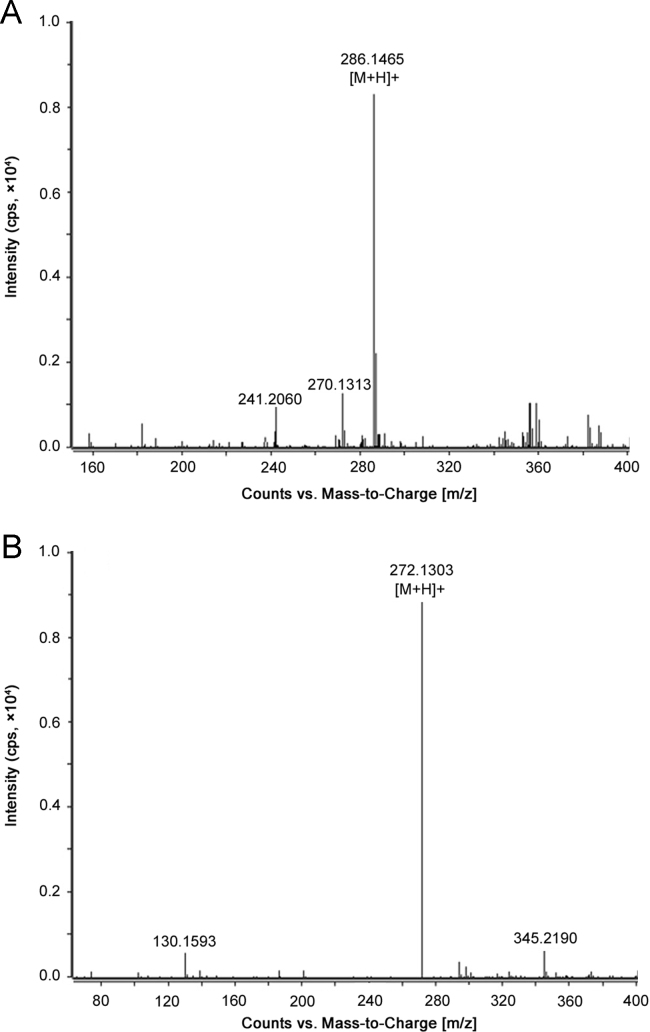
MS parameters were tuned in positive ionization mode for PPR and the IS. The MS parameters were optimized to maximize the response. Acetonitrile offered a higher response than methanol and was therefore chosen as the organic modifier in the eluent. Addition of 0.1% formic acid to the mobile phase increased MS response. Since extracted ion chromatograms (EICs), based on the accurate mass measurement, were to be used for quantification of PPR, it was necessary to determine the accurate masses of the analyte and the IS. Standard mass spectra in the full scan range of m/z 100–1000 were obtained by injecting standard solutions of PPR (500 ng/mL) and the IS (250 ng/mL). The molecular ions [M+H]+ for PPR and the IS showed a high intensity signal at m/z 286.1465 and m/z 272.1303 (Fig.3), respectively. The mass deviation observed was 4.94 ppm for PPR and 3.81 ppm for the IS.
Fig. 3.
ESI–HRMS spectra of (A) PPR and (B) IS.
3.4. Optimization of sample preparation
In the present work, different extraction solvents were investigated for extraction of PPR and the IS from DBS. Spiked DBSs were treated with different solvents and mixtures (methanol, acetonitrile, buffer/organic solvent mixtures): pure organic solvents were proved to be unsuitable for removing PPR from the paper (extraction yields less than 50%), whereas addition of aqueous solvent (water) increased extraction of the analyte from DBS samples. Finally, a mixture of methanol/water (50:50, v/v) gave promising results in terms of sample cleaning and extraction yields (more than 90%).
3.5. Method validation
3.5.1. Linearity, selectivity and LLOQ
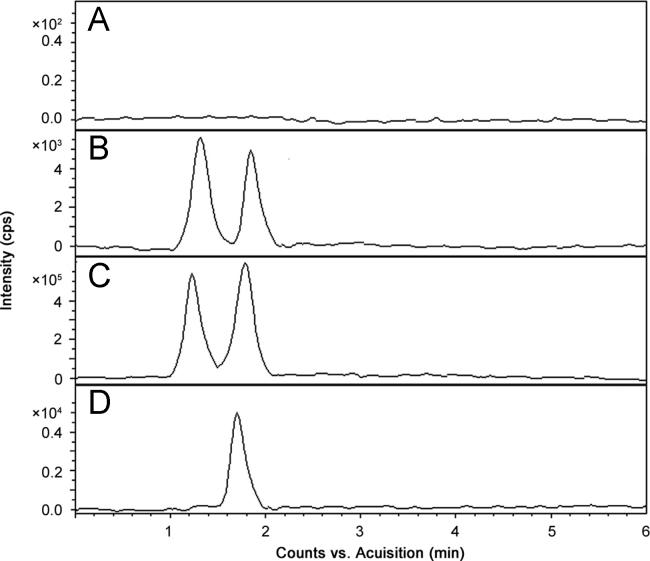
The peak area ratio of PPR to the IS in DBS sample was linear with respect to the analyte concentration over the range of 0.01–2000 ng/mL. The mean linear regression equation of calibration curve for the analyte was y=758.2x +0.091, where y is the peak area ratio of the analyte to the IS and x is the concentration of the analyte. The correlation coefficient (r2) for PPR was 0.9987 over the concentration range used. The LLOQ, the lowest concentration of the standard curve that can be measured with acceptable accuracy and precision for the analyte from the normal DBS sample, was 0.01 ng/mL. To demonstrate the selectivity of the LC–HRMS method, blank blood spots and PPR spiked blood spots were analyzed and subsequently processed. No interferences were observed at the retention time of PPR and the IS in any of the samples (Fig. 4).
Fig. 4.
Representative LC–MS chromatograms of (A) a blank DBS and DBS samples spiked with IS and PPR at (B) LLOQ, (C) higher limit of quantification (HLOQ) and (D) 4.0 h after administration of a 15 mg/kg oral dose of PPR.
3.5.2. Accuracy and precision
Intra-day assay precision and accuracy were calculated using six determinations of the three DBS QCs during a single analytical run. Inter-day assay precision and accuracy were evaluated by analyzing the six QCs once a day for three consecutive days. The precision and accuracy were presented as RSD (%) and RE (%), respectively. All values obtained were well within acceptance criteria for assay validations and were within the pre-defined 15% limits required. The data of intra-day and inter-day accuracy and precision of the method are summarized in Table 1.
Table 1.
Intra-day and inter-day precision and accuracy for the detection of PPR in rat plasma (n=6).
| Analyte | Conc. added (ng/mL) | Intra-day |
Inter-day |
||||
|---|---|---|---|---|---|---|---|
| Conc. found (Mean±SD) (ng/mL) | Precision (RSD, %) | Accuracy (RE, %) | Conc. found (Mean±SD) (ng/mL) | Precision (RSD, %) | Accuracy (RE, %) | ||
| 10 | 10.21±0.16 | 1.56 | −2.1 | 10.35±0.21 | 2.02 | −3.5 | |
| Rat-DBS | 400 | 398.11±1.84 | 0.46 | 0.47 | 403.27±2.28 | 0.56 | −0.81 |
| 1200 | 1203.41±10.36 | 0.86 | −0.28 | 1197.48±11.27 | 0.94 | 0.21 | |
3.5.3. Recovery and matrix effect
The extraction recoveries of PPR and the IS were determined by comparing the peak areas of extracted QC samples (n=6) with the peak areas of pure QC samples of the same concentrations. The recoveries of PPR and the IS were determined at three concentration levels of QC (low, medium and high concentrations) as given in Table 2. The matrix effect was evaluated by comparing the chromatographic peak areas of neat solutions of the analyte and the IS spiked into extracted blank DBS samples (n=6) at low, medium and high concentration levels with those of the standard solutions at the same concentrations. The results were consistent over the tested concentration levels and found to be within acceptable limits (95%–105%). Thus, the matrix effects were found to be insignificant and did not affect the accuracy of the proposed LC–HRMS method.
Table 2.
The recovery and matrix effect of PPR and the IS (n=6).
| Analyte | Conc. added (ng /mL) | Recovery (%) |
Matrix effect (%) |
||
|---|---|---|---|---|---|
| Mean±SD | RSD | Mean±SD | RSD | ||
| PPR | 10 | 93.15±1.25 | 1.34 | 96.37±3.02 | 3.13 |
| 400 | 92.64±2.34 | 2.52 | 95.07±1.87 | 1.96 | |
| 1200 | 92.81±2.14 | 2.30 | 93.92±2.33 | 2.48 | |
| IS | 50 | 94.54±1.73 | 1.82 | 93.18±.1.26 | 1.35 |
3.5.4. Stability
The results obtained from stability studies indicated that PPR DBSs at three concentration levels in six replicates were stable at ambient temperature (25 °C) for 24 h. Again the long-term stability studies of the sample at 4 °C for 7 days and also at −20 °C for 30 days did not alter the analyte. The stability test results are given in Table 3. It can be seen from Table 3 that the results were well within the acceptance limits.
Table 3.
Stability of PPR in rat plasma (n=6).
| Stability tested | Conc. added (ng/mL) | Conc. found (Mean±SD) (ng/mL) | Precision (RSD, %) | Accuracy (RE, %) |
|---|---|---|---|---|
| At 25 °C for 24 h | 10 | 9.78±0.44 | 4.49 | 2.20 |
| 400 | 402.56±1.98 | 0.49 | −0.64 | |
| 1200 | 1195.69±3.21 | 0.26 | 0.43 | |
| At 4 °C for 7 days | 10 | 10.35±0.38 | 3.67 | −3.50 |
| 400 | 397.21±2.56 | 0.64 | 0.69 | |
| 1200 | 1204.27±2.84 | 0.23 | −0.35 | |
| At −20 ºC for 30 days | 10 | 9.81±0.41 | 4.17 | 1.90 |
| 400 | 405.23±1.58 | 0.38 | −1.30 | |
| 1200 | 1198.07±3.74 | 0.31 | 0.16 |
3.6. Blood spot size
The effect of blood spot volume was assessed at two QC levels of 100 and 500 ng/mL in replicate (n=5) at various spot volumes (25, 30 and 35 µL) spotted on DBS cards. A 10 mm diameter disk was punched from the center of each sample to avoid possible problems arising from punch location, and the samples were subsequently extracted. Concentrations assessed from each spot were compared with the standard spot volumes, and a linear regression equation was generated from 30 µL of DBS samples. The accuracy and precision data were within the 15% limit for 25 and 35 µL spot volume sizes at the two tested concentrations (Table 4), indicating that the amount of blood spotted did not affect the distribution of PPR across the FTA card for quantification.
Table 4.
Effect of varying blood spot size on accuracy and precision of assay of PPR.
| PPR concentration in whole blood (ng/mL) | DBS volume (µL) | Conc. found (mean ±SD) (ng/mL) (n=6) | Accuracy (RE, %) | Precision (RSD, %) |
|---|---|---|---|---|
| 100 | 25 | 97.28±2.35 | 2.72 | 2.41 |
| 30 | 97.91±2.81 | 2.09 | 2.86 | |
| 35 | 98.57±2.73 | 1.43 | 2.76 | |
| 500 | 25 | 503.54±3.17 | −0.70 | 0.62 |
| 30 | 496.59±4.72 | 0.68 | 0.95 | |
| 35 | 498.27±5.24 | 0.34 | 1.05 |
3.7. Effect of hematocrit
It is necessary to test the effect of hematocrit (Hct) on the determination of active analytes in the blood. Hct has a significant effect on blood viscosity. Variability in viscosity leads to differences in flux and diffusion properties of blood through the DBS card used for sample collection. This can directly affect the accuracy of determinations of analytes in DBS samples. Hct is normally about 0.31–0.50 for rats [25]. At a high Hct value, the distribution of blood sample through the paper/card might be poor, resulting in a smaller blood spot when compared with the blood sample with a low Hct. The effects of 20%, 35% and 50% Hct were tested with 10 mm spot size at 1000 ng/mL of PPR. The measured PPR concentrations were compared with the results obtained from DBS samples with Hct of 35%, and the results are given in Table 5. The results revealed that %RSD was within the acceptance criteria irrespective of Hct values.
Table 5.
Influence of hematocrit value on precision and accuracy of the assay of PPR (1000 ng/mL).
| Parameters | Hematocrit (%) |
||
|---|---|---|---|
| 20 | 35 | 50 | |
| Mean concentration (ng/mL) | 975.21 | 984.37 | 992.07 |
| SD | 8.35 | 6.54 | 4.51 |
| Precision (RSD, %) | 0.85 | 0.66 | 0.45 |
| Accuracy (RE, %) | 2.47 | 1.56 | 0.79 |
| Percent difference from 35% Hct | −0.91 | 0 | 0.77 |
3.8. Animal experiments
The applicability of the developed bioanalytical method (LC–HRMS) for PPR in DBS was demonstrated by the results obtained from pharmacokinetic studies conducted in six male Wistar rats weighing 200±20 g approximately which were fasted overnight before and 4 h after PPR dosing (The study was approved by the Animal Ethical Committee of Indian Institute of Chemical Technology, Hyderabad). Each rat received an oral dose of 15 mg/kg of PPR in 50% propylene glycol/50% Milli Q water (v/v) formulation at a concentration of 1 mg/mL of PPR. Blood samples (30 µL) were collected from prick from the tail. Serial blood samples were spotted onto the FTA bloodspot cards at 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12 and 24 h post-dose. The samples were processed, as per the procedure detailed in Section 2.5, and analyzed.
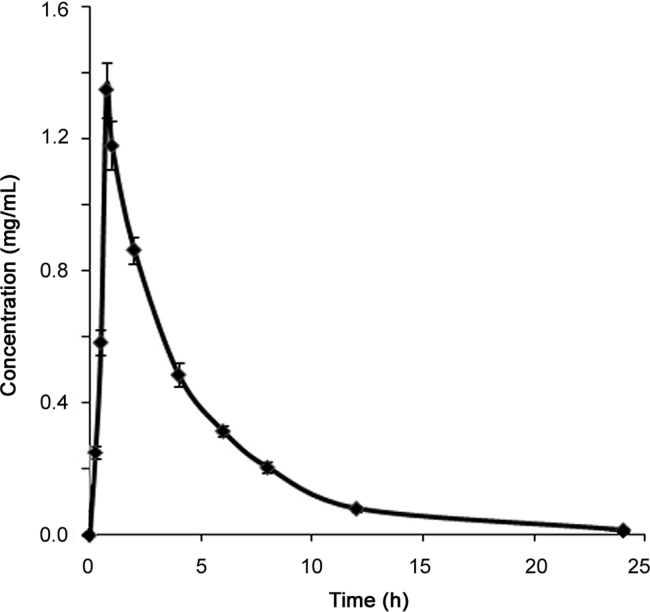
3.9. Pharmacokinetics
The mean concentration–time data were subjected to noncompartmental pharmacokinetic analysis using linear trapezoidal rule. Fig. 5 shows the mean plasma concentration–time profile of PPR. The pharmacokinetic parameters such as Cmax, Tmax, t1/2, AUC0−t and AUC0–∞ for PPR are summarized in Table 6. The pharmacokinetic data obtained from DBS were further compared with that from earlier reports for PPR using a conventional biomatrix i.e., plasma [12]. The data obtained in this investigation were in very close agreement, thus confirming the validity of the DBS method.
Fig. 5.
Mean plasma concentration–time profile of PPR after administration of an oral dose of 15 mg/kg of PPR to male Wistar rats (Data are expressed as mean±SD (n=6)).
Table 6.
Pharmacokinetic parameters of PPR after oral administration at a dose of 50 mg/kg to rats (n=6).
| Pharmacokinetic parameters | DBS (Mean±SD) | Plasma (Mean±SD) |
|---|---|---|
| Cmax (ng/mL) | 1454±84 | 1659±147 |
| Tmax (h) | 0.7± 0.3 | 0.5± 0.4 |
| t1/2 (h) | 2.8±0.5 | 2.6±0.2 |
| AUC0−t (ng h/mL) | 4931±196 | 5688±449 |
| AUC0−∞ (ng h/mL) | 4864±238 | 5699±448 |
4. Conclusion
We demonstrated that a full scan LC–HRMS assay met the validation acceptance criteria in terms of accuracy, precision, selectivity, sensitivity and matrix effect. The LC–HRMS method developed was rapid and provided high-sensitivity determination of PPR in DBS samples. The developed DBS method has several advantages such as non-invasive, requiring only a micro-volume blood sample (typically ≤50 µL) and simple to perform compared with conventional venous blood sampling. The fully validated LC–HRMS method is simple, highly sensitive, specific, robust, and has been successfully applied to pharmacokinetic study in rat DBS.
Acknowledgments
The authors are grateful to Dr M. Laxmikantam, Director, CSIR–IICT, for providing facilities to perform this work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Katragadda Suresh Babu, Email: suresh@iict.res.in.
Potturi Sita Devi, Email: sitadeviiict@gmail.com.
References
- 1.Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Food. Sci. Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- 2.Bae G.S., Kim M.S., Jung W.S. Inhibition of lipopolysaccharide-induced inflammatory responses by piperine. Eur. J. Pharmacol. 2010;642:154–162. doi: 10.1016/j.ejphar.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.A., Hong S.S., Han X.H. Piperine from the fruits of piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005;53:832–835. doi: 10.1248/cpb.53.832. [DOI] [PubMed] [Google Scholar]
- 4.Li S., Wang C., Wang M. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Atal C.K., Zutshi U., Rao P.G. Scientific evidence on the role of Ayurvedic herbals on bioavailability of drugs. J. Ethnopharmacol. 1981;4:229–232. doi: 10.1016/0378-8741(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 6.Bano G., Raina R.K., Zutshi U. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 1991;41:615–617. doi: 10.1007/BF00314996. [DOI] [PubMed] [Google Scholar]
- 7.Bhat B.G., Chandrasekhara N. Determination of piperine in biological tissues by thin-layer chromatography and ultraviolet absorption densitometry. J. Chromatogr. 1985;338:259–263. doi: 10.1016/0378-4347(85)80096-7. [DOI] [PubMed] [Google Scholar]
- 8.Bajad S., Singlab A.K., Bedia K.L. Liquid chromatographic method for determination of piperine in rat plasma: application to pharmacokinetics. J. Chromatogr. B. 2002;776:245–249. doi: 10.1016/s1570-0232(02)00352-5. [DOI] [PubMed] [Google Scholar]
- 9.Bajad S., Johri R.K., Sing J. Simple high-performance liquid chromatography method for the simultaneous determination of ketoconazole and piperine in rat plasma and hepatocyte culture. J. Chromatogr. A. 2002;949:43–47. doi: 10.1016/s0021-9673(01)01260-2. [DOI] [PubMed] [Google Scholar]
- 10.Bajad S., Coumar M., Khajuria R. Characterization of a new rat urinary metabolite of piperine by LC/NMR/MS. Eur. J. Pharm. Sci. 2003;19:413–421. doi: 10.1016/s0928-0987(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu H.L., Luo R., Chen X.Q. Tissue distribution profiles of three antiparkinsonian alkaloids from Piper longum L. in rats determined by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2013;928:78–82. doi: 10.1016/j.jchromb.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Basu S., Patel V.B., Jana S. Liquid chromatography tandem mass spectrometry method (LC–MS/MS) for simultaneous determination of piperine, cinnamic acid and gallic acid in rat plasma using a polarity switch technique. Anal. Methods. 2013;5:967–976. [Google Scholar]
- 13.Kakarala M., Dubey S.K., Tarnowski M. Ultra-low flow liquid chromatography assay with ultraviolet (UV) detection for piperine quantitation in human plasma. J. Agric. Food. Chem. 2010;58:6594–6599. doi: 10.1021/jf100657r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Bi Y., Luo R. Simultaneous UFLC–ESI–MS/MS determination of piperine and piperlonguminine in rat plasma after oral administration of alkaloids from Piper longum L.: application to pharmacokinetic studies in rats. J. Chromatogr. B. 2011;879:2885–2890. doi: 10.1016/j.jchromb.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Blessborn D., Römsing S., Annerberg A. Development and validation of an automated solid-phase extraction and liquid chromatographic method for determination of lumefantrine in capillary blood on sampling paper. J. Pharm. Biomed. Anal. 2007;45:282–287. doi: 10.1016/j.jpba.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 16.la Marca G., Malvagia S., Filippi L. Rapid assay of topiramate in dried blood spots by a new liquid chromatography-tandem mass spectrometric method. J. Pharm. Biomed. Anal. 2008;48:1392–1396. doi: 10.1016/j.jpba.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Koal T., Burhenne H., Römling R. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:2995–3001. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
- 18.Swales J.G., Gallagher R.T., Denn M. Simultaneous quantitation of metformin and sitagliptin from mouse and human dried blood spots using laser diode thermal desorption tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011;55:544–551. doi: 10.1016/j.jpba.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Rao R.N., Bompelli S., Maurya P.K. High-performance liquid chromatographic determination of anti- hypertensive drugs on dried blood spots using a fluorescence detector--method development and validation. Biomed. Chromatogr. 2011;25:1252–1259. doi: 10.1002/bmc.1599. [DOI] [PubMed] [Google Scholar]
- 20.Barfield M., Spooner N., Lad R. Application of dried blood spots combined with HPLC-MS/MS for the quantification of acetaminophen in toxicokinetic studies. J. Chromatogr. B. 2008;870:32–37. doi: 10.1016/j.jchromb.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 21.van der Heijden J., de Beer Y., Hoogtanders K. Therapeutic drug monitoring of everolimus using the dried blood spot method in combination with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2009;50:664–670. doi: 10.1016/j.jpba.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Déglon J., Thomas A., Daali Y. Automated system for on-line desorption of dried blood spots applied to LC/MS/MS pharmacokinetic study of flurbiprofen and its metabolite. J. Pharm. Biomed. Anal. 2011;54:359–367. doi: 10.1016/j.jpba.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Parmar V.S., Jain S.C., Bisht K.S. Phytochemistry of the genus Piper. Phytochem. 1997;46:597–673. [Google Scholar]
- 24.FDA . US Department of Health and Human Services; FDA, CDER, CVM: 2001. Guidance for Industry. Bioanalytical Method Validation. . <www.fda.gov/cder/guidance/index.htm>. [Google Scholar]
- 25.Denniff P., Spooner N. The effect of hematocrit on assay bias when using DBS samples for the quantitative bioanalysis of drugs. Bioanalysis. 2010;2:1385–1395. doi: 10.4155/bio.10.103. [DOI] [PubMed] [Google Scholar]