Abstract
A novel method for simultaneous determination of kolliphor HS15 and miglyol 812 in microemulsion formulation was developed using ultra-high performance liquid chromatography coupled with a nano quantitation analytical detector (UHPLC–NQAD). All components in kolliphor HS15 and miglyol 812 were well separated on an Acquity BEH C18 column. Mobile phase A was 0.1% trifluoroacetic acid (TFA) in water and mobile phase B was acetonitrile. A gradient elution sequence was programed initially with 60% organic solvent, slowly increased to 100% within 8 min. The flow rate was 0.7 mL/min. Good linearity (r>0.95) was obtained in the range of 27.6–1381.1 μg/mL for polyoxyl 15 hydroxystearate in kolliphor HS15, 0.8–202.0 μg/mL for caprylic acid triglyceride and 2.7–221.9 μg/mL for capric acid triglyceride in miglyol 812. The relative standard deviations (RSD) ranged from 0.6% to 1.7% for intra-day precision and from 0.4% to 2.7% for inter-day precision. The overall recoveries (accuracy) were 99.7%–101.4% for polyoxyl 15 hydroxystearate in kolliphor HS15, 96.7%–99.6% for caprylic acid triglyceride, and 94.1%–103.3% for capric acid triglyceride in miglyol 812. Quantification limits (QL) were determined as 27.6 μg/mL for polyoxyl 15 hydroxystearate in kolliphor HS15, 0.8 μg/mL for caprylic acid triglyceride, and 2.7 μg/mL for capric acid triglyceride in miglyol 812. No interferences were observed in the retention time ranges of kolliphor HS15 and miglyol 812. The method was validated in terms of specificity, linearity, precision, accuracy, QL, and robustness. The proposed method has been applied to microemulsion formulation analyses with good recoveries (82.2%–103.4%).
Keywords: Kolliphor HS15, Miglyol 812, Ultra-high performance liquid chromatography (UHPLC), Nano quantitation analytical detector (NQAD)
1. Introduction
Kolliphor HS15 (also known as solutol HS15) is a potent non-ionic solubilizer and emulsifying agent. It is particularly suitable for microemulsion formulation [1]. Kolliphor HS15 mainly contains polyoxyl 15 hydroxystearate obtained from the reaction of about 15 mol of ethylene oxide with 1 mol of 12-hydroxystearic acid [2]. Polyoxyl 15 hydroxystearate consists of about 70% of polyglycol mono- and di-esters (lipophilic) and about 30% of free polyethylene glycol (PEG, hydrophilic). Miglyol 812 is a mixture of medium-chain fatty acid triglycerides obtained from the esterification of the fatty acids C8 and C10 with glycerin. Commercial miglyol 812 contains four fatty acid triglycerides, i.e., C8-content triglyceride, two mixed fatty acid triglycerides (by-product A and by-product B) and low C10-content triglyceride [3].
Decades ago, many researchers found that kolliphor HS15 and miglyol 812 not only improved the drug solubility and adsorption, but also were associated with product stability and functionality [4]. For example, Ryoo et al. [5] found when 1% (m/m) of propofol was solubilized with 8% (m/m) of kolliphor HS15, the droplet size and the amount of propofol in the microemulsion formulation remained unchanged at 40 °C for eight weeks. During the study of human epidermoid carcinoma cell line, Coon et al. [6] reported that kolliphor HS15 could be used as a potential therapeutic agent because of its effectiveness for reversing multidrug resistance in vitro and low toxicity in vivo. Sellers et al. [7] investigated the effects of miglyol 812 on rats and found that miglyol 812 produced reversible gastrointestinal effects and decreased body weight gains after oral gavage for four weeks. Zhao et al. [8] described that the treatment with caprylic triglyceride effectively attenuated progression of weakness and protected spinal cord motor neuron loss in amyotrophic lateral sclerosis (ALS) disease. Palin et al. [9] found that miglyol 812 would increase the oral absorption of cefoxitin in rats when the drug was formulated in the oil phase, possibly due to the transient effect of the fatty acid membrane fluidity. Janet et al. [10] reported that the batch variations of solutol HS15 would impact the drug product performance; therefore, her team developed an advance analytical technique using matrix-assisted laser desorption/ionization (MALDI) and ion mobility to control drug product quality.
For quality control purposes, the quantitative estimation of kolliphor HS15 and miglyol 812 in microemulsion product is paramount. To our knowledge, no analytical methods have been reported for simultaneous determination of kolliphor HS15 and miglyol 812 and very few methods have been published for individual analysis of kolliphor HS15 or miglyol 812. The Unites States Pharmacopeia–National Formulary (USP37–NF32) [11] and European Pharmacopoeia (EP-7.0) [12] describe a similar liquid chromatographic (LC) method using a refractive index (RI) detector for identifying and quantifying free PEG components from kolliphor HS15. USP and EP methods both recommend the use of a mobile phase of methanol and water (8:2, v/v) at a flow rate of 1.1 mL/min. An analytical column along with two pre-columns is used. The acceptance criteria for free PEG in kolliphor HS15 is set as 27.0%–39.0%. These two compendial methods are dedicated only for analysis of about 30% of hydrophilic (unesterified) portion and are not suitable for analyzing polyglycol mono- and di-esters (lipophilic, esterified) portion.
Coon et al. [6] described a gel permeation chromatography (GPC) method for kolliphor HS15 under isocratic conditions and at a constant flow rate of 1.0 mL/min. Effluent fractions were monitored for absorbance at 254 nm. The buffer used was phosphate-buffered saline, pH 7.4. Gel chromatogram of kolliphor HS15 revealed one major peak at the retention time of about 7 min (approximately 70% of total) and a small peak at about 12 min. The major peak was presumptively identified as polyglycol mono- and di-esters (lipophilic portion) and the small peak as the free PEG. The GPC method did not disclose the details for quantitative estimation of kolliphor HS15, because the method focused on the reversal of multidrug resistance for various fractions of kolliphor HS15. Bhaskar et al. [13] developed a liquid chromatography/tandem mass spectrometry (LC–MS/MS) method for the determination of kolliphor HS15. The LC–MS/MS method was operated with a C18 column and 5 min generic gradient. Mobile phase A was 0.1% formic acid in water and mobile phase B was 100% acetone. This study found that the most abundant ions corresponding to kolliphor HS15 were free PEG oligomers (hydrophilic portion). Percentages of the lipophilic and hydrophilic components in kolliphor HS15 were estimated using a high performance liquid chromatography (HPLC) system equipped with an evaporative light scattering detector (ELSD). The method required a time-consuming protein extraction prior to analysis.
In our present work, kolliphor HS15 and miglyol 812 were both formulated into microemulsion products as the stabilizer and emulsifying agent. The aim of this study was to develop and validate an analytical method for simultaneous determination of kolliphor HS15 and miglyol 812 in a single run.
2. Experimental
2.1. Materials and reagents
Kolliphor HS15 raw material was purchased from BASF Company (Iselin, New Jersey, USA) and miglyol 812 raw material was provided by Sasol Germany GmbH (Eatontown, New Jersey, USA). Polyoxyl 15 hydroxystearate reference standard was purchased from U.S. Pharmacopeia Co. (Rockville, Maryland, USA). Caprylic acid triglyceride, capric acid triglyceride and polyethylene glycol were all purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA). HPLC-grade acetonitrile, 0.1% trifluoroacetic acid (TFA) in water and isopropanol (IPA) were purchased from Thermos Fisher Scientific (Fair Lawn, New Jersey, USA). Water was purified by a Millipore–Q academic water purification system (Billerica, Massachusetts, USA). All other chemicals and reagents used for the experiments were of analytical grade.
2.2. Instruments
An Acquity™ ultra-high performance liquid chromatography (UHPLC™) system (Waters, Milford, Massachusetts, USA) coupled with a nano quantity analyte detector (NQAD™, Quant, Blaine, Minnesota, USA) was used for simultaneous determination of kolliphor HS15 and miglyol 812. The UHPLC system consisted of a binary solvent manager, a sample manager and a column manager. System control and data analysis were processed with Empower II software (Waters, Milford, Massachusetts, USA). The NQAD instrument consisted of a 500 mL water bottle, a liquid nebulizer, a solvent evaporator, a patented water condensation particle counter (WCPC) and an optical detector. Three ethylene bridged hybrid (BEH) UHPLC columns including BEH C18, BEH Shield RP18, BEH phenyl and one high strength silica (HSS) T3 column (Waters, Milford, Massachusetts, USA) were selected for column screening. The mobile phases consisted of 0.1% TFA in water as the aqueous component (A) and 100% acetonitrile as the organic modifier (B). A gradient elution sequence was programed initially with 60% organic solvent, slowly increased to 100% within 8 min and held for 5 min. The flow rate was set at 0.7 mL/min and the injection volume was 10 µL. The column temperature was maintained at 30 °C and the autosampler temperature was kept at ambient temperature (about 25 °C). The NQAD was operated with default settings as follows: nitrogen (N2) gas: 28.0 psi, nebulization temperature: 40 °C, and evaporation temperature: 60 °C.
2.3. Preparation of solutions
2.3.1. Standard solutions
A combination of IPA/water (65:35, v/v) was used as the diluent for kolliphor HS15 and miglyol 812. Polyoxyl 15 hydroxystearate reference standard stock solution was prepared in diluent at a concentration of approximately 25 mg/mL. The working standard solution was prepared by pipetting 2 mL of standard stock solution into a 50 mL volumetric flask and diluted to volume with diluent at a concentration of approximately 1000 µg/mL. Caprylic acid triglyceride and capric acid triglyceride reference standard stock solutions were prepared in diluent at a concentration of approximately 4 mg/mL each. The working standard solutions of caprylic acid triglyceride and capric acid triglyceride were prepared, respectively, by pipetting 2 mL of each of standard stock solution into 50 mL volumetric flasks and diluted to volume with diluent at a concentration of approximately 160 µg/mL. The above standard stock and working solutions were stored at 4 °C until use.
2.3.2. Microemulsion placebo sample
The microemulsion placebo sample was prepared by weighing each of the components, excluding kolliphor HS15 and miglyol 812, into a 100 mL glass volumetric flask and diluted to volume with diluent. This microemulsion placebo sample was used for accuracy sample spiking.
2.3.3. System suitability samples
System suitability samples were daily prepared by pipetting 2 mL of each of polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride stock solutions into a 50 mL volumetric flask, mixed well and diluted to volume with diluent.
2.3.4. Linearity and accuracy samples
Five levels (quantitation limit (QL), 25%, 50%, 100% and 125%) of the linearity samples were prepared by serial dilutions of standard stock solutions with diluent, covering the range of QL to at least 125% of each component assay level. Three levels (50%, 100% and 125%) of the accuracy samples were prepared in triplicate by pipetting 1.0, 2.0 and 2.5 mL of each of the standard stock solutions into 50 mL volumetric flasks containing 1.0 mL of microemulsion placebo solution, respectively, and diluted to volume with diluent.
2.3.5. Microemulsion assay sample
Microemulsion assay samples were prepared by weighing (500±5.0) mg of microemulsion formulation into a 50 mL volumetric flask and diluted to volume with diluent. Each assay sample contained approximately 1000 µg/mL of kolliphor HS15 and 300 µg/mL of miglyol 812.
3. Results and discussion
3.1. Method development
Our method development strategy was implemented following Quality-by-Design (QbD) principles including diluent selection, mobile phase selection, column screening, column temperature determination and instrumental parameter determination. Method development samples were prepared using each of individual reference standard of polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride.
3.1.1. Diluent selection
The development of this method started with the selection of a diluent that was suitable not only for dissolving kolliphor HS 15 and miglyol 812, but also for being compatible with the mobile phase. If the diluent is stronger than the mobile phase, it will drag the compound through the column, resulting in peak splitting or broadening. Kolliphor HS 15 is soluble in polar solvents such as water, ethanol and isopropanol to form clear solutions, while miglyol 812 is soluble in medium polar solvents such as diethyl ether and ethyl acetate. Therefore, the diluent for kolliphor HS15 and miglyol 812 should be a mixture of polar and medium polar solvents.
Diluent selection study was performed on three combinations, i.e., water/methanol, water/IPA and water/acetonitrile. It was concluded that 65% IPA in water produced clear stock solutions for polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride. The similar clear stock solutions for polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride were obtained with a combination of 75% methanol in water or a combination of 85% acetonitrile in water. Chromatograms of the sample prepared using 65% IPA in water showed three sharp peaks. Chromatograms of the samples prepared using 75% methanol in water or 85% acetonitrile in water gave polyoxyl 15 hydroxystearate a wide broad peak. Therefore, a combination of 65% IPA in water was selected as the diluent.
3.1.2. Mobile phase selection
Proper combination of water and organic solvents with different polarities is very important for achieving ideal chromatographic separations. Knowing the polarities of kolliphor HS15 and miglyol 812, this method development started with an elution program with an aqueous, moderately polar mobile phase for kolliphor HS15 that was weakly adsorbed to the stationary phase first. The polarity of mobile phase was then decreased by increasing the percentage of organic solvent for miglyol 812. In this study, three sets of mobile phase combinations including water/methanol, 0.1% TFA in water/methanol, and 0.1% TFA in water/acetonitrile were evaluated. A gradient elution sequence was programed initially with 60% organic solvent, slowly increased to 100% within 8 min, and held constantly for 5 min to make a total run time of 13 min. This study found that a combination of water/methanol under optimized elution program gave kolliphor HS15 (polyoxyl 15 hydroxystearate) a broad peak and could not separate caprylic acid triglyceride and capric acid triglyceride due to the mismatch polarity of mobile phase with these two fatty acid triglycerides. A combination of 0.1% TFA in water/methanol showed the similar chromatogram as water/methanol mobile phase, because the organic solvent programmed in most of elution time was methanol. It implied that the polarity of kolliphor HS15 was less than the polarity of the combination of water or 0.1% TFA in water and methanol, yielding a broad and tailing peak for kolliphor HS15. The third combination (0.1% TFA in water/acetonitrile) was evaluated. It gave a very nice peak shape for polyoxyl 15 hydroxystearate and well separated caprylic acid triglyceride and capric acid triglyceride peaks. Therefore, a combination of 0.1% TFA in water/acetonitrile was selected as the mobile phase for further study.
3.1.3. Column screening
Column selection for chromatographic analysis was also an important step in method development. This study utilized a chromatographic basic rule “like attracts like” and focused on the polarity matching among column stationary phase, mobile phase, kolliphor HS15 and miglyol 812. Kolliphor HS15 is a relatively polar compound and soluble in water. Miglyol 812 is a medium polar compound and soluble in isopropanol. When an aqueous, moderately polar mobile phase was used, the column stationary phase should be a non-polar material for relatively polar or medium polar components in kolliphor HS15 and miglyol 812.
In this study, three UHPLC BEH columns (namely BEH C18, BEH Shield RP18, BEH phenyl) and one HSS T3 column were evaluated for column screening. The columns used in the study were all 2.1 mm × 100 mm i.d., 1.7 μm particle size, with the exception of the HSS T3 column, which has 1.8 μm particle size. The column temperature was kept at 30 °C. The chromatographic parameters were first optimized to achieve good retention, high resolution and better peak shapes for the components in kolliphor HS15 and miglyol 812.
Because of its good robustness and stability, the BEH C18 was selected as the prime column for the screening. The BEH C18 incorporated trifunctional ligand bonding chemistries and utilized new, proprietary endcapping processes for good peak shapes. The BEH C18 was designed for the analysis of polar or medium polar compounds [14]. In the experiment, the BEH C18 eluted three sharp peaks with minimal peak tailing for polyoxyl 15 hydroxystearate at a retention time of about 2.1 min, caprylic acid triglyceride at about 7.6 min, and capric acid triglyceride at about 9.8 min. The BEH C18 column also afforded a good resolution between caprylic acid triglyceride and capric acid triglyceride. It demonstrated that the BEH C18 column chemistry closely matched the polarity of kolliphor HS 15 and miglyol 812. The chromatogram obtained from the BEH C18 column screening preliminarly concluded that this column was appropriate and met the method requirement. However, additional column screening was continued for the purposes of developing more useful methods for future trouble shooting.
The second column evaluated was the HSS T3. The column chemistries of HSS T3 and BEH C18 are similar. The HSS T3 column contained 100% silica particles and utilized T3 bonding process to retain and separate polar compounds. The HSS T3 column was designed for separation of polar compounds because of its superior polar-compound retention and aqueous mobile phase compatibility. In this study, the HSS T3 column could separate kolliphor HS15, caprylic acid triglyceride and capric acid triglyceride with excellent peak symmetry. However, the retention time of polyoxyl 15 hydroxystearate was delayed about 0.5 min and the peak area was decreased by 25%, possibly due to the difference of particle sizes. It implied that the HSS T3 column might attract traces of components in its stationary phase.
The third column studied was the BEH Shield RP18 column. The stationary phase of BEH Shield RP18 column combined the hydrophobicity alkyl ligand and the hydrophilicity polar group. It is dedicated for complement of BEH C18 and BEH C8 phases. In the experiment, the BEH Shield RP18 column could not separate the peaks of caprylic acid triglyceride and capric acid triglyceride, proving that the polarity of BEH Shield RP18 was not matched to that of medium chain fatty acid triglyceride components.
The BEH phenyl column was the fourth column screening, which contained phenyl ligand and utilized a trifunctional C6 alkyl ether between the phenyl ring and the silyl functionality, which was dedicated for the analysis of polymer additives. In this study, the BEH phenyl column could well separate polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride with better peak shapes, but the resolution between caprylic acid triglyceride and capric acid triglyceride reduced when compared with the BEH C18. It implied that this column was not suitable for miglyol 812, because this resolution (<1.1) would not have spaces for the two by-products components between caprylic acid triglyceride and capric acid triglyceride.
Based on the above optimized methods for column screening, the results proved that the BEH C18 column afforded the best retention and separation of all components in kolliphor HS15 and miglyol 812. Hence, the BEH C18 column was selected for further study.
3.1.4. Column temperature determination
Three column temperatures were evaluated during method development, namely, ambient temperature (approximately 20 °C), 30 °C and 45 °C. Determination was carried out based on a visual check of chromatogram and a comparison of peak areas. The ambient column temperature (approximately 20 °C) often increased the UHPLC system back pressure (>15,000 psi) because the lower column temperature increased the mobile phase viscosity. Furthermore, higher mobile phase viscosity also reduced the diffusion in the chromatographic system, giving broad peaks. In general, higher temperature has proven effective for improving the overall chromatographic performance, but the column temperature of 45 °C eluted components faster and decreased the resolution of four component peaks in miglyol 812, resulting in a poor separation of miglyol 812. Interestingly, a comparison of three major peak areas in kolliphor HS15 and miglyol 812 showed that the differences were less than 3.1% when using 30 °C and 45 °C for column separation. Hence, the column temperature of 30 °C was determined for further study.
3.1.5. Nano quantitation analyte detector (NQAD)
Kolliphor HS15 and miglyol 812 contain very weak ultraviolet (UV) absorbance and cannot be analyzed using UV detection. The NQAD detector is a new aerosol-based detector and incorporates four chambers: nebulization, evaporation, aerosol particle counter and an optical detection. The NQAD detector begins by continuously nebulizing the liquids eluted from the LC column into various sizes of droplet mists containing solvents and sample particles. In the evaporation chamber, the solvents are evaporated by nitrogen flow and the sample particles are suspended in air. In the third chamber, a WCPC condenses the water vapor onto the particle surfaces to enlarge them to a size large enough for counting. In the end, the number of sample particles is detected individually by an optical detector and converted to an analog output signal as a chromatogram. Unlike other aerosol-based detectors that measure a cloud of particles, the NQAD detector counts each individual droplet [15]. It is minimally affected by baseline drift and maximally increases the method sensitivity. In this study, the temperatures of nebulization and evaporation chambers were evaluated. We found that a slight change in temperatures of the nebulization and evaporation would not significantly impact the method detection. The NQAD detector was then operated with typical settings as follows: nitrogen (N2) gas: 28.0 psi, nebulization temperature: 40 °C, and evaporation temperature: 60 °C.
3.2. Method validation
The proposed method was validated for specificity, linearity, accuracy, precision, sensitivity and robustness as per ICH method validation guidelines [16].
3.2.1. Specificity
Microemulsion placebo solution was injected each time prior to study and checked for the potential interference of peaks at the retention times of polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride.
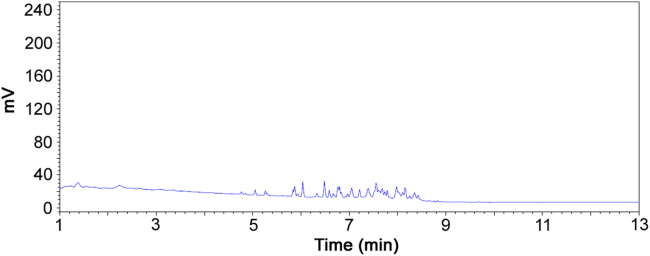
A typical microemulsion placebo solution chromatogram is shown in Fig. 1 and no interfering peaks were found at the retention times of kolliphor HS 15 and miglyol 812.
Fig. 1.
Microemulsion placebo solution chromatogram.
3.2.2. System suitability
System suitability test is an integral part of the method validation and used to confirm that the resolution, peak tailing and reproducibility were adequate for the analysis performed.
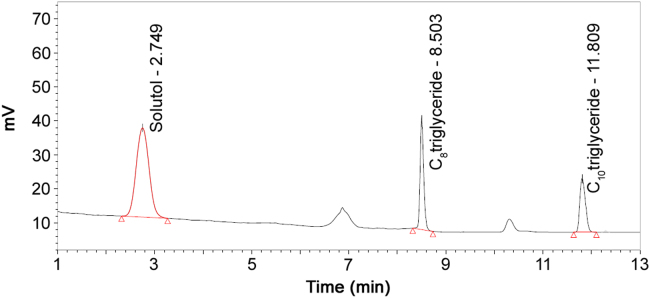
A typical system suitability chromatogram is shown in Fig. 2. It shows that all three major components were well separated with symmetric peak shapes. Two unidentified peaks were confirmed to be free PEG from polyoxyl 15 hydroxystearate by co-injection of free PEG reference standard. The detailed results of system suitability samples are shown in Table 1.
Fig. 2.
System suitability chromatogram.
Table 1.
Results of system suitability samples.
| Components | Retention time (min) | Resolution | Tailing factor | RSD (%) (n=6) |
|---|---|---|---|---|
| Polyoxyl 15 hydroxystearate | 2.75 | – | 1.13 | 0.7 |
| Caprylic acid triglyceride | 8.50 | 10.4 | 1.35 | 0.4 |
| Capric acid triglyceride | 11.81 | 16.6 | 1.09 | 0.6 |
Table 1 shows that the average relative standard deviation (RSD, n=6) of peak areas was 0.7% for polyoxyl 15 hydroxystearate, 0.4% for caprylic acid triglyceride, and 0.6% for capric acid triglyceride. The resolution of polyoxyl 15 hydroxystearate/caprylic acid triglyceride peaks was 10.4 and the resolution of caprylic acid triglyceride/capric acid triglyceride peaks was 16.6. The tailing factors of the three component peaks were less than 2.0.
3.2.3. Linearity
Linearity samples were serially diluted from each individual standard stock solution to obtain five concentration levels, covering QL to 125% of the assay level. The peak areas at each level of polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride were calculated to assess the method linearity. Calibration curves were constructed for each individual standard by plotting the concentration of each individual standard versus peak area responses. The regression equations were calculated from the calibration graphs, along with the standard deviations of the slope and intercept on the ordinate. The detailed results are shown in Table 2.
Table 2.
Linearity sample concentrations and results.
| Components | Concentration (µg/mL) |
Regression equations (Y=ax+b) | Correlation coefficient (r) | ||||
|---|---|---|---|---|---|---|---|
| QL | 25% | 50% | 100% | 125% | |||
| Polyoxyl 15 hydroxystearate | 27.6 | 276.2 | 552.4 | 1104.8 | 1381.1 | Y=901.8 x+8736.9 | 0.9980 |
| Caprylic acid triglyceride | 0.8 | 40.4 | 121.2 | 161.8 | 202.0 | Y=2590.7x+ 25,819 | 0.9920 |
| Capric acid triglyceride | 2.7 | 44.4 | 88.7 | 177.5 | 221.9 | Y=1179.0x +3352 | 0.9825 |
3.2.4. Accuracy/recovery
Analytical recovery experiments were performed by adding known amount of polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride standard stock solutions into a 50 mL flask each containing 1.0 mL of microemulsion placebo solution. The average recoveries of three levels (50%, 100% and 125%) were 100.4%, 98.0% and 100.0%, respectively, for polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride. The results demonstrated that the method has sufficient capability for the accurate quantification of kolliphor HS15 and miglyol 812 samples. Detailed accuracy/recovery results are given in Table 3.
Table 3.
The accuracy results of samples.
| Components | Level (%) | Conc. added (µg/mL) | Average conc. found (µg/mL) (n=3) | Average recovery (%) (n=3) | Overall average recovery (%) (n=9) | RSD (%) (n=9) |
|---|---|---|---|---|---|---|
| Polyoxyl 15 hydroxystearate | 50 | 552.4 | 552.5 | 100.0 | 100.4 | 1.5 |
| 100 | 1104.8 | 1120.5 | 101.4 | |||
| 125 | 1381.0 | 1376.4 | 99.7 | |||
| Caprylic acid triglyceride | 50 | 80.8 | 78.1 | 96.7 | 98.0 | 2.0 |
| 100 | 161.6 | 157.7 | 97.6 | |||
| 125 | 202.0 | 201.2 | 99.6 | |||
| Capric acid triglyceride | 50 | 88.7 | 83.5 | 94.1 | 100.0 | 5.8 |
| 100 | 177.5 | 183.4 | 103.3 | |||
| 125 | 221.9 | 227.5 | 102.5 |
3.2.5. Precision
Intra- and inter-day precisions were evaluated. The intra-day precision was evaluated using the results of six preparations each containing three components, polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride. The inter-day precision was evaluated by analyzing the same sample in duplicate for two days. Detailed intra-day and inter-day precision results are given in Table 4.
Table 4.
The intra-day and inter-day precision results.
| Components | Precision (RSD%) |
Difference (%) | |
|---|---|---|---|
| Intra-day | Inter-day | ||
| Polyoxyl 15 hydroxystearate | 1.7 | 0.4 | −0.3 |
| Caprylic acid triglyceride | 1.6 | 2.7 | 1.7 |
| Capric acid triglyceride | 0.6 | 0.4 | −4.3 |
The intra-day RSD values of all three components were lower than 2.0% and the inter-day RSD values of all three components were lower than 3.0%. The differences of the mean from two days were −0.3%, 1.7% and −4.3%, respectively, for polyoxyl 15 hydroxystearate, caprylic acid triglyceride and capric acid triglyceride.
3.2.6. Detection limit (DL) and QL
Method sensitivity was assessed at DL and QL. The DL and QL of kolliphor HS15 and miglyol 812 were determined by injecting progressively low concentration of the reference standard solution under the chromatographic conditions (Table 5). The lowest concentration assayed was regarded as QL where the signal-to-noise ratio was at least 10:1. The DL was defined as a signal-to-noise ratio of 3:1.
Table 5.
Results of detection limit (DL) and quantification limit (QL).
| Components | DL (µg/mL) | QL (µg/mL) |
|---|---|---|
| Polyoxyl 15 hydroxystearate | 5.5 | 27.6 |
| Caprylic acid triglyceride | 0.4 | 0.8 |
| Capric acid triglyceride | 0.9 | 2.7 |
3.2.7. Robustness
Robustness was evaluated by deliberating variations in UHPLC–NQAD method parameters, one at a time. In this study, four experimental conditions were slightly varied, including flow rate, column temperature, nebulizer temperature and evaporation temperature. Robustness results are summarized in Table 6.
Table 6.
Robustness test results.
| Parameters | Polyoxyl 15 hydroxystearate |
Caprylic acid triglyceride |
Capric acid triglyceride |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak area | Diff. (%) | Peak area | Diff. (%) | Peak area | Diff. (%) | |||||||||||
| Flow rate (mL/min) | ||||||||||||||||
| 0.7 | 1,459,980 | 611,306 | 607,004 | |||||||||||||
| 0.6 | 1,499,094 | 2.68 | 535,450 | −12.41 | 617,440 | 1.72 | ||||||||||
| Column temperature (°C) | ||||||||||||||||
| 30 | 1,183,531 | 557,103 | 498,911 | |||||||||||||
| 45 | 1,174,089 | −0.80 | 554,693 | −0.43 | 483,495 | −3.09 | ||||||||||
| Evaporation temperature in NQAD (°C) | ||||||||||||||||
| 45 | 1,313,528 | 451,385 | 456,791 | |||||||||||||
| 60 | 1,307,247 | −0.48 | 449,095 | −0.51 | 464,495 | 1.69 | ||||||||||
| Nebulization temperature in NQAD (°C) | ||||||||||||||||
| 35 | 1,307,247 | 449,895 | 463,749 | |||||||||||||
| 40 | 1,295,625 | −0.89 | 463,539 | 3.03 | 474,011 | 2.21 | ||||||||||
Robustness results demonstrated that a little variation of method parameters would not significantly change the test results, except for the result of caprylic acid triglyceride under flow rate change. When the flow rate was varied from 0.7 to 0.6 mL/min, the peak area of caprylic acid triglyceride was reduced about 12.41%. As a result, its retention time was delayed about 0.5 min and the peak was completely detached from the bottom of free PEG 2 peak.
3.3. Method applications
The analytical method was successfully developed and has been applied in analyses of kolliphor HS15, miglyol 812 and microemulsion formulation samples.
3.3.1. Analysis of kolliphor HS15 raw material
Due to the difficulty of lipophilic analysis, current available methods for kolliphor HS15 are applicable only to the analysis of non-esterified hydrophilic components, such as USP–NF and Ph. Eur. Methods [11], [12].
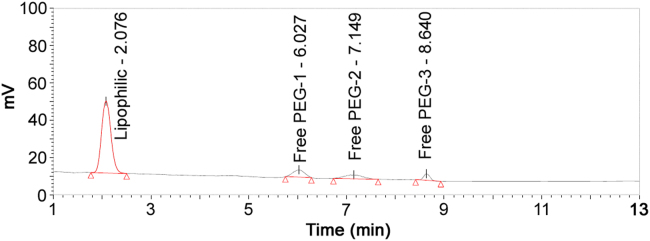
This UHPLC–NQAD method has a capability of direct determination of both lipophilic and hydrophilic parts without sample derivatization. A short gradient sequence was programed and gave a good separation between lipophilic and hydrophilic parts (Fig. 3).
Fig. 3.
Chromatogram of kolliphor HS15 raw material.
Three free PEG components were identified and confirmed by co-injection of free PEG reference standard solution. Content (%) of each component was estimated by area normalization method, where the percentage of each component was divided by summing up peak areas of all the components to 100%. Retention time of lipophilic component peak was about 2.1 min and three free PEG components peaks were about 6.0, 7.1 and 8.6 min. Content (%) of lipophilic component was found to be 67.8%, and three free PEG components were 14.7%, 10.7% and 6.9%. The content (%) of total free PEG components was 32.3% and in match with USP–NF acceptance criteria of 27.0% to 39.0% for free PEG.
3.3.2. Analysis of miglyol 812 raw material
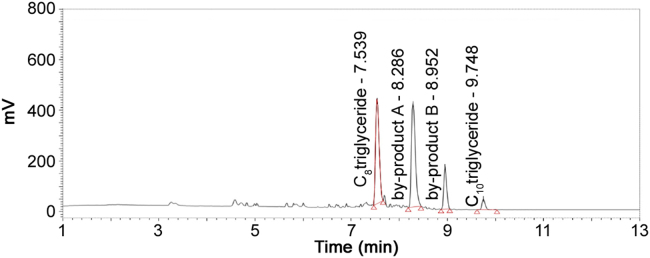
Miglyol 812 raw material is a mixture of caprylic triglyceride/capric acid triglyceride obtained by the esterification of caprylic acid and capric acid with glycerin. The ratio of caprylic acid and capric acid participated in esterification with glycerin is about 60:40(v/v). The resulted triglyceride mixture contained four components, caprylic triglyceride (C8–C8–C8), by-product A (C8–C8–C10), by-product B (C8–C10–C10) and capric triglyceride (C10–C10–C10). Caprylic triglyceride/capric acid triglyceride contain very weak UV absorbance and cannot be directly detected using UV. In this UHPLC–NQAD method, a short gradient elution gave a good separation for all four component peaks (Fig. 4).
Fig. 4.
Chromatogram of miglyol 812 raw material.
Components of caprylic triglyceride and capric triglyceride in miglyol 812 raw material were identified and confirmed by individually co-injecting caprylic triglyceride and capric triglyceride reference standard solution. Contents (%) of four triglyceride peaks were estimated by area normalization method, where the percentage of each triglyceride was divided by summing up peak area of all the triglycerides to 100%. The retention time of four triglyceride peaks was about 7.6, 8.3, 9.0 and 9.8 min and their contents (%) were 39.9%, 43.5%, 12.8%, and 3.8%, respectively.
3.3.3. Analysis of microemulsion formulation
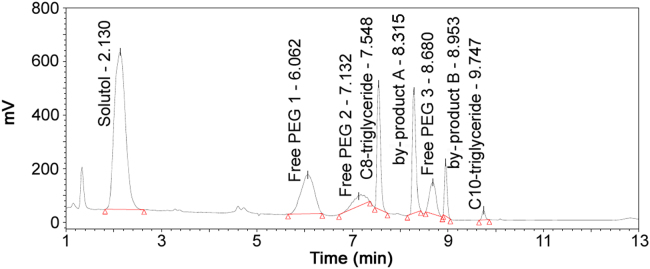
A sample of microemulsion product formulated with Kolliphor HS15 and miglyol 812 was analyzed using this UHPLC–NQAD method and the chromatogram was obtained as shown in Fig. 5. It shows that all components including three free PEGs in kolliphor HS15 and two by-products in miglyol 812 were well separated. The content (%) of each component is given in Table 7, Table 8. Recovery (%) of each component in microemulsion formulation was calculated using the same component found in kolliphor HS15 and miglyol 812 raw materials.
Fig. 5.
Chromatogram of microemulsion formulation.
Table 7.
Recoveries of all components in kolliphor HS15.
| Components | Kolliphor HS15 raw material (%) | Microemulsion formulation (%) | Recovery (%) |
|---|---|---|---|
| Lipophilic | 67.7 | 69.6 | 102.8 |
| Free PEG 1 | 14.7 | 15.2 | 103.4 |
| Free PEG 2 | 10.7 | 8.8 | 82.2 |
| Free PEG 3 | 6.9 | 6.4 | 92.8 |
| Total free PEG | 32.3 | 30.4 | 94.1 |
Table 8.
Recoveries of all components in miglyol 812.
| Components | Miglyol 812 raw material (%) | Microemulsion formulation (%) | Recovery (%) |
|---|---|---|---|
| C8-triglyceride | 39.9 | 41.1 | 103.0 |
| By-product A | 43.5 | 43.2 | 99.3 |
| By-product B | 14.4 | 13.8 | 95.8 |
| C10-triglyceride | 2.1 | 2.0 | 95.2 |
Recoveries (%) of four components in kolliphor HS15 were 82.2%–103.4% and recoveries of four components in miglyol 812 was 95.2%–103.0%. The results indicated that no component in kolliphor HS15 and miglyol 812 was significantly lost during the formulation of microemulsion product. Furthermore, it proved this method was reliable, accurate and effective for simultaneous quantification of kolliphor HS15 and miglyol 812 in microemulsion formulation.
4. Conclusion
A rapid, sensitive and robust UHPLC–NQAD method has been developed and validated for simultaneous determination of kolliphor HS15 and miglyol 812 in microemulsion formulation. All components were well separated using Acquity BEH C18 column with a short gradient elution chromatography. Good selectivity and sensitivity were achieved by this UHPLC–NQAD method. The validated method has been successfully applied to assay of microemulsion formulation sample and the results proved that method is acceptable and reliable.
Acknowledgments
The authors would like to thank Tina Masiuk, Thomas Zakszewski, and Kristel Dewitt for their technical support and productive discussions.
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
References
- 1.Technical information, Kolliphor HS15, Macrogol 15 Hydroxystearate Ph. Eur., Polyoxyl 15 Hydroxystearate USP, Pharma Ingredients & Services, BASF, Ludwigshafen, Germany, 2012, pp. 1–8.
- 2.B.O. Mashkevich, Drug Delivery Research Advances, Nova Science Publishers Inc., New York, 2007, pp. 99–100.
- 3.Product information, Miglyol 810, 812, 818, 829, 840 Neutral Oils for Pharmaceutical and Cosmetics, Sasol Germany GmbH, Witten, Germany, 2004, pp. 1–7.
- 4.Golightly L.K., Smolinske S.S., Bennett M.L. Pharmaceutical excipients adverse effects associated with inactive ingredients in drug products. Med. Toxicol. Advers. Drug. Exp. 1988;2:128–165. [PubMed] [Google Scholar]
- 5.Ryoo H.K., Park C.W., Chi S.C. Development of propofol-loaded microemulsion systems for parenteral delivery. Arch. Pharm. Res. 2005;28:1400–1404. doi: 10.1007/BF02977908. [DOI] [PubMed] [Google Scholar]
- 6.Coon J.S., Knudson W., Clodfelter K. Solutol HS15, nontoxic polyethylene esters of 12-hydroxystearic acid, reverses multi-drug resistance. Cancer Res. 1991;51:897–902. [PubMed] [Google Scholar]
- 7.Sellers R.S., Antman M., Phillips J. Effects of miglyol 812 on rats after 4 weeks of gavage as compared with methyl cellulose/tween 80. Drug. Chem. Toxicol. 2005;28:423–432. doi: 10.1080/01480540500262839. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W., Varghese M., Vempati P. Caprylic triglyceride as a novel therapeutic approach to effectively improve the performance and attenuate the symptoms due to the motor neuron loss in ALS disease. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0049191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palin K.J., Phillips A.J., Ning A. The oral adsorption of cefoxitin from oil and emulsion vehicles in rats. Int. J. Pharm. 1986;33:99–104. [Google Scholar]
- 10.H. Janet, M. Liz, U. Diana, et al., The analysis of solutol HS15 using MALDI and ion mobility, in: Proceedings of the18th International Mass Spectrometry Conference, Bremen, Germany, 2009, PMM–484.
- 11.United States Pharmacopeia/National Formulary, 36th Ed., United States Pharmacopeia Commission, Rockville, USA, 2015, pp. 6126–6129.
- 12.European Pharmacopoeia (Ph Eur.), European Pharmacopoeia Commission, Strasbourg, France, 2005, pp. 1941–1942
- 13.Bhaskar V.V., Middha A., Tiwari S. Liquid chromatography tandem mass spectrometry for quantitative estimation of polyethyleneglycol 400 and its applications. J. Chromatogr. B. 2013;926:68–76. doi: 10.1016/j.jchromb.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 14.New L.S., Chan E. Evaluation of BEH C18, BEH HILIC, and HSS T3 (C18), column chemistries for the UHPLC–MS–MS analysis of glutathione, glutathione disulfide and ophthalmic acid in mouse liver and human plasma. J. Chromatogr. Sci. 2008;46:209–214. doi: 10.1093/chromsci/46.3.209. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson J.P., Li J., Farrell W. Comparison of the response of four aerosol detectors used with ultra-high pressure liquid chromatography. J. Chromatogr. A. 2011;1218:1646–1655. doi: 10.1016/j.chroma.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 16.ICH guideline, Q2B, Validation of analytical procedures: methodology, in: Proceedings of the International Conference on Harmonization, Rockville, USA, 1996, pp. 1–10.