Abstract
A sensitive and selective method using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (HPLC–ESI–MS) to determine the concentration of torasemide in human plasma samples was developed and validated. Tolbutamide was chosen as the internal standard (IS). The chromatography was performed on a Gl Sciences Inertsil ODS-3 column (100 mm×2.1 mm i.d., 5.0 µm) within 5 min, using methanol with 10 mM ammonium formate (60:40, v/v) as mobile phase at a flow rate of 0.2 mL/min. The targeted compound was detected in negative ionization at m/z 347.00 for torasemide and 269.00 for IS. The linearity range of this method was found to be within the concentration range of 1–2500 ng/mL (r=0.9984) for torasemide in human plasma. The accuracy of this measurement was between 94.05% and 103.86%. The extracted recovery efficiency was from 84.20% to 86.47% at three concentration levels. This method was also successfully applied in pharmacokinetics and bioequivalence studies in Chinese volunteers.
Keywords: Torasemide, HPLC–ESI–MS, Human plasma, Bioequivalence, Pharmacokinetics
1. Introduction
Torasemide (1-isopropyl-3-([4-(3-methyl-phenylamino) pyridine]-3-sulfonyl) urea, CAS: 56211–40–6, Fig. 1A) is a new high-efficiency loop diuretic, acting the same as other pyridine sulfonylurea medications in blocking the Na+–K+–2Cl− carrier to promote excretion of water on the thick ascending limb of the loop of Henle [1]. It has been successfully used to treat oedematous states associated with chronic congestive heart failure [2], renal disease [3] and hepatic cirrhosis [4], and low-dose torasemide has also been used to control arterial hypertension [5]. Research proves that torasemide is safer and better tolerated than furosemide in chronic heart failure patients and associated with a trend in reducing all-cause mortality [6]. Lower incidences of abnormal serum potassium levels and hypotension were also observed in patients receiving torasemide compared with those receiving other diuretics [7].
Fig. 1.
Structure of (A) torasemide and (B) tolbutamide (IS).
Torasemide is well absorbed and yields a bioavailability of 80%–90%. It is highly bound to protein (99%) [1]. The time of peak reaches at 1 h, and the elimination half-life varies from 3 to 4 h. Torasemide is metabolized by the hepatic cytochrome P450 system, followed by both renal and non-renal excretion of metabolites. The total amount of torasemide and metabolites in urine is 83%, including 25% of torasemide, 11% of M1, 3% of M3, and 44% of M5. Both M1 and M3 are active and probably contribute to the diuretic action of torasemide [8]. Due to its high activity, a low therapeutic dose is required.
A rapid, sensitive and reliable determination of torasemide in human plasma is essential to evaluating its pharmacokinetics in clinical trials and bioequivalence researches. Several methods have been reported including gas chromatography–tandem mass spectrography (GC–MS) [9], capillary zone electrophoresis (CZE) [10], high-performance liquid chromatography–tandem ultraviolet detection (HPLC–UV) [11], [12], [13], [14], [15], [16], [17], [18], [19] and high-performance liquid chromatography–tandem electrochemical detection (HPLC–ED) [20], [21], [22] to determine the concentration of torasemide as a single analyte [9], [12], [18], [19], [20], [21] or in combination with its important metabolites [10], [11], [13], [14], [15], [16] or with other diuretic drugs [23], [24] in plasma [11], [12], [13], [14], [15], [16], [17], [18], [19] or other biological samples [9], [13], [20], [21], [22], [23], [24]. Barroso et al. [9] applied GC–MS to determine the concentration of torasemide in human urine, which needs complicated preparation for derivatization and is not suitable for bioequivalence studies of large quantity of samples. Additionally, several studies used HPLC with low sensitivity detectors [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. These methods cannot detect the low concentration points such as the last several points in elimination phase, which will lead to inaccurate pharmacokinetic parameters. In this case, high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS) can be a better option because it combines advantages of HPLC and MS, which allows a rapid, sensitive and selective quantification of drugs in complex biological samples. However, there are only few reports regarding the determination of torasemide concentration in human plasma using HPLC–MS.
In this study, a new method using HPLC–ESI–MS was developed to determine the concentration of torasemide in human plasma rapidly, sensitively and selectively. This method was successfully applied in pharmacokinetics and bioequivalence studies in healthy Chinese volunteers.
2. Materials and methods
2.1. Solvent and chemicals
Torasemide test tablets (Batch No: 441104) were supplied by Jiangsu D&R Pharmaceutical Corporation (Taizhou, China). Torasemide reference tablets (Batch No: 14052320) were purchased from SZYY Group Pharmaceutical Limited (Taizhou, China). Torasemide reference standard (Batch No: 100605–200401) was obtained from National Institute for Food and Drug Control (Beijing, China). Tolbutamide (internal standard, IS, Fig. 1B) standard (Batch No: 40716, 99.9% purity) was obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Glibenclamide standard (Batch No: 121633–201017) was obtained from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Methanol of chromatographic grade was purchased from Merck, Germany. Ammonium formate, ethyl acetate and hydrochloric acid were of analytical grade and purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). All other chemicals were of analytical grade. Double distilled water was prepared by passing through Milli-Q water System (Merck Millipore, Massachusetts, U.S.).
2.2. Instrumentation and operation condition
2.2.1. Liquid chromatography
Liquid chromatography was performed using a Shimadzu LC-10AD HPLC system consisting of an autosampler (SIL-HTc). Chromatography was carried out with Gl Sciences Inertsil ODS-3 column (100 mm×2.1 mm i.d., 5.0 µm) using methanol: 10 mM ammonium formate (60:40, v/v) as mobile phase. The temperatures of column and autosampler were maintained at 40 °C and 15 °C, respectively. The analysis was completed in 5 min at a flow rate of 0.2 mL/min. Data acquisition and processing was accomplished using Shimadzu LC–MS solution software for LCMS-2010A system.
2.2.2. Mass spectrometry
The ESI source was set at negative ionization mode. The [M-H]−, m/z 347.00 for torasemide and [M-H]−, m/z 269.00 for tolbutamide were selected as detecting ions, respectively. The quantification was performed via peak area. MS operating conditions were optimized as follows: drying gas 1.5 L/min, curved desolvation line (CDL) temperature 250 °C, block temperature 200 °C and probe voltage 1.65 kV.
2.3. Preparation of standard solution
Standard stock solutions of torasemide (1 mg/mL) and tolbutamide (1 mg/mL) were prepared in methanol and stored at 4 °C before use. Standard working solutions of torasemide for calibration curve and quality control (QC) were prepared by diluting standard stock solution with mobile phase to 4, 20, 40, 200, 2000 ng/mL and 10 μg/mL. IS working solution was prepared by diluting standard stock solution with mobile phase to 10 μg/mL.
2.4. Sample preparation
Sample preparation was performed by liquid–liquid extraction. Two hundred microliters of plasma samples were transferred to 10 mL centrifuge tubes and extracted by 2.0 mL of ethyl acetate after addition of 10 μL of IS working solution (10 μg/mL) and 20 μL of 10% hydrochloric acid solution. The mixture was vortex-mixed for 3 min, and then centrifuged at 3500 rpm for 10 min. 1.5 mL of organic layer was transferred to another clean glass tube and evaporated under a steady stream of nitrogen to dryness in a water bath at 40 °C. The residue was re-dissolved in 200 μL of mobile phase and vortexed for 30 s. Following centrifugation (16,000 rpm, 10 min), aliquots of 5 μL of the supernatant were injected into the HPLC–ESI–MS system.
2.5. Calibration curve and QC samples
Calibration curves were prepared by spiking blank plasma with appropriate amount of torasemide working solution mentioned above to yield concentrations of 1, 3, 10, 50, 200, 800, 1500, 2000 and 2500 ng/mL. The following steps were the same as those described in Section 2.4. The blank plasma samples (with or without IS) were also analyzed to confirm absence of interferences, but the results for blank samples were not used as parts of the calibration curves.
QC samples, which were analyzed at the same time with test samples, were prepared at concentrations of 3 (low level), 800 (middle level) and 2000 ng/mL (high level). The total amount of QC samples was no less than 5% of the test samples in the same batch.
2.6. Method validation
The method was validated according to the bioanalytical method validation guidance currently accepted by US Food and Drug Administration (USFDA) [25].
2.6.1. Specificity
The specificity of this method was investigated by preparing and analyzing 6 blank plasma samples from different sources of individual humans. It was assessed by comparing the chromatograms obtained from blank plasma with those from the sample spiked with torasemide at lower limit of quantitation (LLOQ). Each blank sample was also tested for the visible interference.
2.6.2. Linearity, LLOQ and limit of determination (LOD)
Linearity was completed in 5 replicates at concentration levels of 1, 3, 10, 50, 200, 800, 1500, 2000, and 2500 ng/mL. Calibration curves were generated by plotting the peak area ratios of torasemide to tolbutamide (IS) versus concentration of torasemide and fitted to the equation y=bx+a by weighted least-squares linearity regression (w=1/y2), where y corresponds to the peak area ratio of torasemide to the IS and x refers to the concentration of torasemide added to plasma.
LLOQ was determined as the concentration with a signal-to-noise ratio of 10. Standard calibrators should not deviate by more than 15% of nominal concentrations, except at LLOQ where the standard calibrator should not deviate by more than 20%. The LOD was determined as the concentration with a signal-to-noise ratio of 3.
2.6.3. Precision and accuracy
The intra-batch precision and accuracy was measured by analyzing 5 replicates plasma samples spiked with torasemide at each QC level (3, 800 and 2000 ng/mL) in a batch, and the inter-batch precision and accuracy was determined by analyzing 5 sets of plasma samples spiked with torasemide at each QC level (3, 800 and 2000 ng/mL) in three consecutive batches on different days. The concentration of each sample was calculated using standard curve prepared and analyzed on the same day. The precision was expressed as the relative standard deviation (%RSD), and the accuracy was defined as a percentage of the measured concentration over the theoretical concentration. The acceptance deviation for precision and accuracy should not exceed 15%.
2.6.4. Extraction recovery
The extraction recovery of torasemide was determined by comparing corresponding peak area ratios of torasemide to IS obtained from extracted plasma samples (As-r, n=5) with those from processed blank plasma samples added the same amount of torasemide and IS standard solution (Ar-r, n=2). This procedure was repeated at three different concentrations of 3, 800 and 2000 ng/mL.
The extraction recovery of tolbutamide was determined by comparing peak areas of IS obtained from extracted plasma samples (As-is, n=5) with those from processed blank plasma samples added the same amount of IS standard solution (Ar-r, n=2).
2.6.5. Stability
Stability was evaluated under different conditions that were set during sample analysis. The stability of standard stock solution of torasemide and IS was evaluated at 4 °C for 10 days. The stability of standard working solution of torasemide and IS was evaluated at room temperature for 8 h. The short-time stability of plasma samples was evaluated at room temperature for 8 h. The residue stability was evaluated by analyzing re-dissolved residue placed at room temperature for 24 h and 48 h, respectively. The post-preparative stability was evaluated by re-analyzing extracted plasma samples placed in the autosampler at 15 °C for 24 h. The freeze and thaw stability was evaluated by analyzing plasma sample undergoing freeze (−20 °C) and thaw (room temperature) cycles on three consecutive days. The long-time stability of plasma samples was evaluated at −20 °C for 15 days. Every plasma sample stability was repeated 3 times at 3 concentration levels (3, 800, 2000 ng/mL), while standard solution stability was repeated 3 times only at LLOQ level.
2.6.6. Matrix effect
To assess ionization interference from endogenous compounds co-eluted with the analyte, matrix effect was carried out. Matrix effects of torasemide or IS were evaluated by comparing the peak areas of torasemide or IS spiked with the post-extraction residue of the blank plasma with those of standard solutions at three concentration levels (3, 800, 2000 ng/mL). This procedure was repeated 5 times. If the ratio was between 115% and 85%, an exogenous matrix effect was negligible.
2.6.7. Co-eluting effect
As the retention time of torasemide and IS is close, torasemide was eluted from HPLC column into the mass spectrometer at the same time as the IS, which may affect the ionization of the torasemide or IS. In other words, the mass spectrum response of torasemide may decrease due to electrospray ionization competitive inhibition, which may vary depending on the concentration. Therefore, it is necessary to investigate the co-eluting effect. Co-eluting effect of torasemide was calculated by comparing the peak areas of torasemide with IS and without IS. Co-eluting effect of IS was calculated by comparing the peak areas of IS with torasemide and without torasemide. Three concentration levels (1, 800, 2500 ng/mL) were repeated 5 times. The acceptance deviation for co-eluting effect should not exceed 15%.
2.7. Method application
We recruited 24 healthy Chinese male volunteers aged from 18 to 40 years and with a BMI between 19 to 24. All the volunteers gave their written consent for their participation in the study after having been informed of all aspects of the study, especially the potential risks. The study protocols were approved by Jiangsu Province Hospital of Traditional Chinese Medicine (Nanjing, China), in accordance with the principles of the Declaration of Helsinki and the recommendations of the State Food and Drug Administration of China.
The study was carried out with an open randomized, balanced, two-period crossover study. Each volunteer received a single oral dose of 10.0 mg of torasemide test tablets or reference tablets in a random order in cycle, with 200 mL of water after an overnight fast of 12 h. Venous blood samples were collected at the time of 0, 10, 20, 30, 45 min and 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0, 12.0, 14.0 and 24.0 h after oral administration of the medicine. All samples were collected into heparin tubes and immediately centrifuged at 3500 rpm for 15 min and stored at −20 ◦C until analysis.
2.8. Pharmacokinetic and statistical analysis
Pharmacokinetic analysis and statistical analysis were performed by DAS2.0 software. Pharmacokinetic parameter of torasemide was estimated from the concentration–time data using non-compartmental methods. Pharmacokinetic parameters, AUC and Cmax, were used to evaluate bioequivalence through analysis of variance and two one-side t tests after the natural logarithm conversion. The Wilcoxon signed rank test was used for the non-parametric analysis to determine differences in Tmax. The 90% confidence intervals of the T/R-ratios of logarithmically transformed data were within the accepted range of 80%–125% for AUC0-τ and AUC0-∞, and 75%–133% for Cmax.
2.9. Incurred sample reanalysis
Several random samples from the highest plasma concentration closed to Tmax or the lowest plasma concentration at the end of elimination phase were reanalyzed again in each assay period. The percentage difference of the results was determined with the equation (Repeat-Original)/Mean×100%, where repeat and original refers to the concentration of torasemide measured two times. The acceptance criterion between two measurements should not exceed ±20% [26].
3. Results and discussion
3.1. HPLC–MS method development
To develop a rapid, simple and sensitive method for determination of torasemide, different options were evaluated to optimize chromatography separation and MS detection parameters.
The chromatography condition was optimized to improve signal, decrease base-line noise, avoid interference of matrix and reduce run time. Optimization of chromatography conditions included the option of columns type like Shimadzu Shim-pack VP-ODS column (250 mm×2.0 mm i.d., 4.6 µm), Gl Sciences Inertsilph-3 column (100 mm×2.1 mm i.d., 3.0 µm) or Gl Sciences InertsilODS-3 column (100 mm×2.1 mm i.d., 5.0 µm) and different kinds and concentrations of mobile phase like water, formic acid, ammonium formate and ammonium acetate together with methanol in different proportions. All the columns provided adequate response. However, Gl Sciences Inertsilph-3 column cannot separate torasemide and endogenous compounds effectively, and the endogenous compounds were still detected above 10 min, which would have an effect on next sample assay. Shimadzu Shim-pack VP-ODS almost provided a baseline separation of analytes and endogenous compounds, but the retention time for the target analyte was above 5 min. Thus, neither columns were considered for further experiment. The best chromatography was achieved on Gl Sciences Inertsil ODS-3 column within 5 min with good peak shape, due to its small dead volume and excellent bonding and endcapping techniques, and nearly having no interference on analytes. In addition, methanol: 10 mM ammonium formate (60:40, v/v) was selected as optimized mobile phase as it showed best response during method development. Further, tolbutamide (CAS: 64–77–7) and glibenclamide (CAS: 10238–21–8) were tested as IS, and tolbutamide showed a more appropriate retention time to finish the total analysis within 5 min. The retention time of torasemide and IS was 3.68 min and 3.60 min, respectively.
Mass spectrometric condition was optimized so as to achieve stable responses of analytes. In this study, ESI was selected as the ionization source. Both positive and negative ionization modes were evaluated to obtain optimum response of torasemide and IS. It was found that better signal intensity was achieved with positive ion mode. However, the endogenous compounds also have a good response, which would have an effect on analytes. Instead of developing a method with excessive high sensitivity, we tried to develop a bioanalytical method with good stability and high signal-to-noise ratio. Thus, negative ion mode was selected to ensure a better selectivity with no interference. Probe voltage was improved to increase sensitivity. Finally, torasemide and IS were detected at m/z 347.00 and m/z 269.00, respectively. The ion full-scan spectra of torasemide and IS are shown in Fig. 2.
Fig. 2.
Negative ion electrospray mass scan spectra of (A) torasemide and (B) tolbutamide.
3.2. Extraction optimization
The extraction was tried by both precipitating proteins and liquid–liquid extraction. Plasma was first spiked with precipitating reagents like methanol, isopropanol and butanol. However, there was significant interference observed from the plasma matrix in the retention time of torasemide. Then, liquid–liquid extraction with different solvents like ethyl acetate, ether, cyclohexane, hexane and dichloromethane with different acid–base properties was tested. The extraction efficiency of different reagents is listed in Table 1, which indicated ethyl acetate and dichloromethane should be chosen for further study. An orthogonal experiment of extraction efficiency of ethyl acetate and dichloromethane was monitored in different pH conditions. Table 1 shows that acid reagent performed higher extraction. Fig. 3 shows extraction efficiency with different volumes of acid solvent. The maximum response was obtained for both torasemide and IS by adding 20 μL of acid reagents. In final protocol, ethyl acetate with 20 μL of 10% HCl was chosen.
Table 1.
Extraction efficiency of several extraction reagents.
| Reagents | Acid-base properties | Extraction efficiency (%) |
|
|---|---|---|---|
| Torasemide | IS | ||
| Hexane | Neutral | Not found | Not found |
| Cyclohexane | Neutral | Not found | Not found |
| Ether | Neutral | Not found | 8 |
| Dichloromethane | Neutral | 4 | 64 |
| Dichloromethane | Base (50 μL of saturation NaHCO3) | 6 | 42 |
| Dichloromethane | Acid (50 μL of 10% hydrochloric acid) | 18 | 91 |
| Ethyl acetate | Neutral | 28 | 56 |
| Ethyl acetate | Base (50 μL of saturation NaHCO3) | 35 | 35 |
| Ethyl acetate | Acid (50 μL of 10% hydrochloric acid) | 61 | 84 |
Fig. 3.
Extraction efficiency with different volumes of 10% HCl.
3.3. Method validation
3.3.1. Specificity
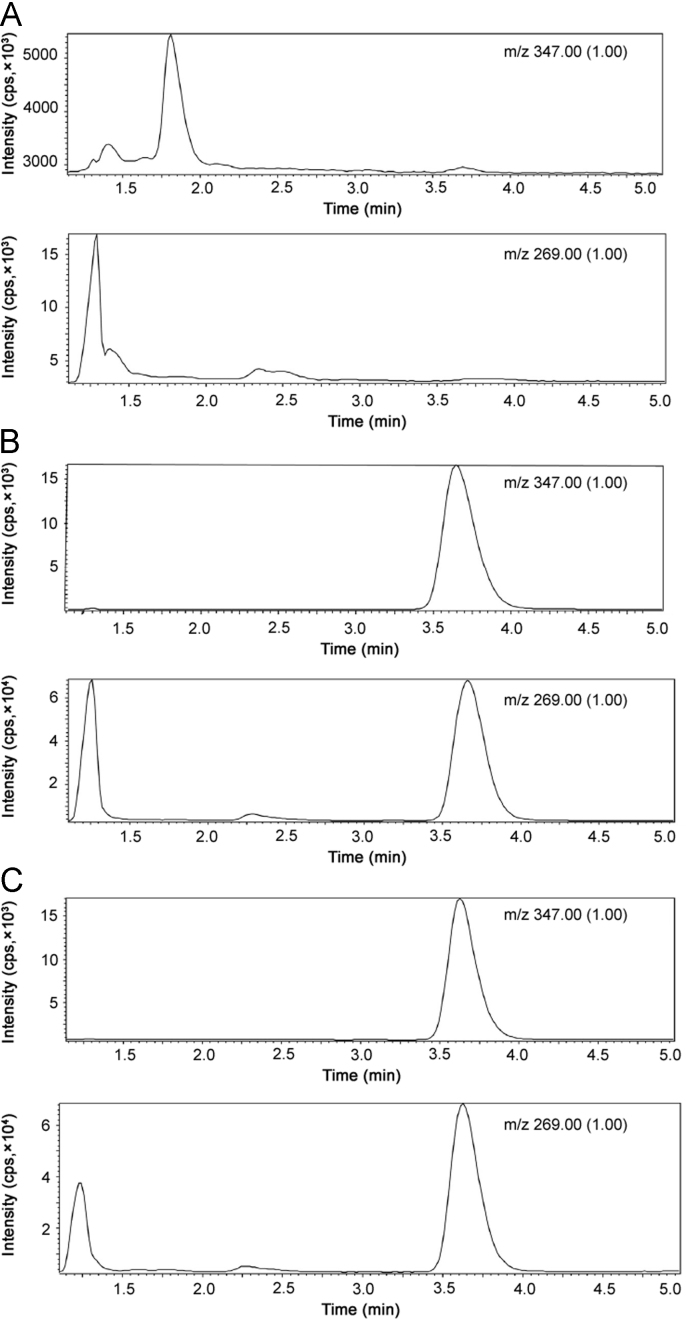
Six different sources of blank plasma samples were detected. The representative chromatograms of blank plasma samples, the plasma spiked with torasemide and IS and the plasma samples from volunteers are shown in Fig. 4. The endogenous substances of blank plasma samples were not interferences in the analyte.
Fig. 4.
Chromatograms of torasemide and the IS resulting from analysis of (A) blank plasma, (B) blank plasma spiked with torasemide and IS, and (C) volunteer plasma. The retention time of torasemide and the IS was 3.68 and 3.60 min, respectively.
3.3.2. Linearity, LLOQ and LOD
The method performed excellent linear response over the concentration range of 1–2500 ng/mL by weighted least-squares (w=1/y2) linear regression analysis. The mean standard curve was typically described by the equation: C=R×325.0–0.4386, with the correlation coefficient≥0.9984, where R corresponds to the peak area ratio of torasemide to the IS, and C refers to the concentration of torasemide in plasma. The use of the weighted regression resulted in less than 15% deviation between the nominal and experimental concentrations calculated by the equation. Results of five representative standard curves for HPLC–MS determination of torasemide are given in Table 2. The LLOQ for torasemide was about 1 ng/mL. Fig. 5 shows the chromatogram of LLOQ for torasemide with the signal-to-noise ratio exceeding 10. The LOD was about 0.5 ng/mL.
Table 2.
Results of five representative calibration curves for HPLC–MS determination of torasemide.
| Concentration added (ng/mL) | Back-calculated concentration (ng/mL) | Mean (ng/mL) | RSD (%) | Mean accuracy (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 0.97 | 0.98 | 0.97 | 1.01 | 0.98 | 0.98 | 1.67 | 98.20 |
| 3 | 3.19 | 3.17 | 3.18 | 2.89 | 3.24 | 3.13 | 4.44 | 104.47 |
| 10 | 10.70 | 10.03 | 10.53 | 10.15 | 9.76 | 10.23 | 3.71 | 102.34 |
| 50 | 55.09 | 52.84 | 53.28 | 50.97 | 50.11 | 52.46 | 3.75 | 104.92 |
| 200 | 209.84 | 205.74 | 214.17 | 210.06 | 205.42 | 209.05 | 1.72 | 104.52 |
| 800 | 766.57 | 783.48 | 773.88 | 810.69 | 801.13 | 787.15 | 2.34 | 98.39 |
| 1500 | 1383.82 | 1381.15 | 1350.44 | 1411.66 | 1407.41 | 1386.90 | 1.77 | 92.46 |
| 2000 | 1745.05 | 1877.51 | 1770.01 | 1867.53 | 1841.87 | 1820.39 | 3.27 | 91.02 |
| 2500 | 2352.83 | 2541.92 | 2456.21 | 2598.71 | 2645.29 | 2518.99 | 4.63 | 100.76 |
Fig. 5.
Chromatogram of torasemide resulting from analysis of 1ng/mL (LLOQ) of torasemide in human plasma. The retention time of torasemide and IS was 3.68 min and 3.60 min, respectively.
3.3.3. Precision and accuracy
The intra- and inter-batch precision and accuracy of the method are shown in Table 3. The intra- and inter-batch precision and accuracy ranged from 4.70% to 10.07% and from 94.05% to 103.86% at three QC levels, respectively.
Table 3.
The inter- and intra-batch precision and accuracy of the method for the determination of torasemide.
| Concentration added (ng/mL) | Intra-batch (n=5) |
Inter-batch (n=15) |
||||
|---|---|---|---|---|---|---|
| Concentration measured (mean±SD) (ng/mL) | Precision (% RSD) | Accuracy (%) | Concentration measured (mean±SD) (ng/mL) | Precision (% RSD) | Accuracy (%) | |
| 3 | 2.85±0.14 | 4.92 | 94.87 | 3.00±0.30 | 10.07 | 99.90 |
| 800 | 830.91±59.69 | 7.18 | 103.86 | 791.04±57.01 | 7.32 | 98.88 |
| 2000 | 1880.91±88.39 | 4.70 | 94.05 | 1932.59±157.29 | 8.14 | 96.63 |
3.3.4. Extraction recovery
The extraction efficiency of torasemide and IS in human plasma was consistent, precise and reproducible. The mean extraction recoveries of torasemide at each QC level (3, 800 and 2000 ng/mL) were 86.47%±8.67%, 85.54%±2.86% and 84.20%±2.63%, respectively, and the mean extraction recovery of IS was 99.36%±5.98% at the concentration used in the analysis (10 μg/mL).
3.3.5. Stability
The results of stability tests of standard stock solution, standard working solution, as well as short-time, post-preparation, residue, freeze and thaw, and long-time plasma samples at different QC levels (n=3) are shown in Tables 4 and 5. The results indicated that the condition of sample analysis does not affect the stability of torasemide.
Table 4.
Stability of torasemide and IS in standard solution (n=3).
| Condition of sample analysis | Torasemide (4 ng/mL) |
IS (10 μg/mL) |
||
|---|---|---|---|---|
| Peak area measured (mean) | RSD (%) | Peak area measured (mean) | RSD (%) | |
| Measured immediately (0 h) | 7,332 | 4.24 | 5,948,450 | 2.49 |
| Standard working solution (room temperature, 8 h) | 7,707 | 5.27 | 6,181,236 | 2.93 |
| Standard stock solution (4 °C, 10 days) | 8,008 | 4.23 | 6,102,100 | 2.51 |
Table 5.
Stability of torasemide in human plasma at different QC levels (n=3).
| Condition of sample analysis | Concentration measured (mean±SD) (ng/mL) |
||
|---|---|---|---|
| 3 ng/mL | 800 ng/mL | 2000 ng/mL | |
| Measured immediately (0 h) | 3.13±0.36 | 749.26±70.43 | 2093.47±107.95 |
| Short-term stability (8 h) | 2.98±0.35 | 704.88±23.14 | 1940.60±40.34 |
| Residue stability (24 h) | 3.21±0.26 | 769.29±44.98 | 2268.68±24.06 |
| Residue stability (48 h) | 2.74±0.20 | 739.03±62.49 | 2105.73±218.95 |
| Long-term stability (15 days) | 3.33±0.06 | 803.27±57.07 | 2212.88±100.74 |
| Post-preparative stability (24 h) | 3.04±0.10 | 804.82±34.30 | 2152.68±71.85 |
| Freeze and thaw stability (first time) | 3.33±0.12 | 815.70±87.47 | 2196.63±57.21 |
| Freeze and thaw stability (second time) | 2.79±0.11 | 787.85±28.88 | 2230.56±59.67 |
| Freeze and thaw stability (third time) | 3.36±0.05 | 839.40±45.24 | 2106.65±150.40 |
3.3.6. Matrix effect
To assess ionization interference from endogenous compounds co-eluted with the analyte, matrix effect was carried out. The mean matrix effect for torasemide at concentrations of 3, 800 and 2000 ng/mL was 102.72%±8.99%, 113.22%±1.49% and 109.11%±2.76%, respectively. The mean matrix effect for IS (10 μg/mL) at three concentration levels of torasemide was 101.38%±5.36%, 106.65%±4.57% and 92.23%±2.93%, respectively. Therefore, it clearly proved that matrix effect of plasma was negligible in this method. And this method was robust enough and gave accurate and consistent results when applied in real subject samples.
3.3.7. Co-eluting effect
The co-eluting effect for torasemide at three concentration levels (1, 800, 2500 ng/mL) was from 96.57% to 98.25%, and for IS from 95.33% to 103.92% (Table 6). The results indicated that the elution peak of IS during the run had no effect on the quantification of torasemide.
Table 6.
Results of co-eluting effects of torasemide and IS at different concentration levels (n=5).
| Concentration (ng/mL) | Torasemide |
IS |
||
|---|---|---|---|---|
| Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | |
| 1 | 97.38 | 9.49 | 102.34 | 1.93 |
| 800 | 96.57 | 0.66 | 95.33 | 0.91 |
| 2500 | 98.25 | 2.61 | 103.92 | 2.54 |
3.4. Method application
The optimized HPLC–ESI–MS method was applied in the analysis of torasemide in human plasma after oral administration of torasemide tablets with the dose of 10 mg by 24 healthy male volunteers. All the volunteers completed the study. No one presented clinically significant adverse effects during the present study, and all of them were discharged in good health after repeating physical examination and laboratory analysis after period 2.
The accuracy and precision of three QC level samples in the two-period crossover study was from 96.89%±4.08% to 107.14%±3.96% (Supplementary Table 1). The mean concentration of torasemide in plasma sample versus time plot of 24 h is shown in Fig. 6. Pharmacokinetic parameters of the test tablets and reference tablets are listed in Table 7. The pharmacokinetic parameters including Tmax and Cmax after oral administration of torasemide with the dose of 10 mg by healthy volunteers were similar to those in the study by Lesne [27], which were 0.5–1.5 h and 800–3200 ng/mL respectively. However, t1/2 and AUC of torasemide have a deviation from our research. It results from the lower sensitivity of determination method which cannot detect last several points of elimination accurately.
Fig. 6.
Mean drug plasma concentration-time curve of torasemide in 24 healthy volunteers after oral administration.
Table 7.
Pharmacokinetic parameters of torasemide in 24 male volunteers after oral administration.
| Parameters | Test tablets | Reference tablets |
|---|---|---|
| Cmax (ng/mL) | 1408.29±337.27 | 1487.86±360.24 |
| Tmax (h) | 0.9±0.4 | 1.0±0.5 |
| t1/2 (h) | 4.43±0.57 | 4.43±0.60 |
| MRT (h) | 3.90±0.60 | 4.01±0.72 |
| AUC 0-τ (ng h/mL) | 3886.86±865.99 | 3906.06±761.72 |
| AUC 0-∞ (ng h/mL) | 3936.57±903.93 | 3956.96±789.98 |
The results from the analysis of variance found that formulation and period had no significant effect on AUC0-τ, AUC0-∞ or Cmax at the significance level of 0.05. The result from Wilcoxon signed rank test found that Tmax had no significantly different. Therefore, it can be concluded that the test and reference tablets are bioequivalent for both the rate and the extent of absorption.
3.5. Results from incurred sample reanalysis
The reproducibility of the determination was studied by reanalysis of incurred samples (Appendix A, Appendix A). The percentage difference in two assay periods was from −18.56% to 15.81%, which was within the acceptance criterion of ±20% [24]. The result showed that the reported concentrations of torasemide in human plasma measured by this method were reliability.
3.6. Comparison with other methods
A detailed comparison of methods for the determination of torasemide in human biological samples is showed in Table 8. The developed method of the study is more sensitive than all other methods developed for determination of torasemide as a single analyte in human plasma [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. In addition, the proposed method uses less plasma (200 μL) than many other reported methods, and has better human compliance for volunteers in bioequivalence and pharmacokinetic studies.
Table 8.
Comparison of the methods developed for torasemide determination in human biological samples.
| Instrumentation | Extraction technique* | Sample volume (μL) | Linear range (ng/mL) | Retention time (min) | Type of biological samples | Application | Refs. |
|---|---|---|---|---|---|---|---|
| GC–MS | D+LLE | 2000 | 50–5000 | 18.4 | urine | Determination of torasemide in urine obtained from a health volunteer after a single dose | [9] |
| HPLC–ED | SPE | 1000 | 9–7000 | 17.1 | urine | Analysis the torasemide and its metabolite M5 in urine sample after a single dose | [21] |
| HPLC–UV | SPE | 1000 | 20–1000 | 11 | plasma | Determination of torasemide and its important metabolites | [11] |
| HPLC–UV | LLE | 500 | 50–5000 | 1.8 | plasma | Measurement of the torasemide concentration in plasma from healthy subject after a single dose | [16] |
| HPLC–UV | PP | 1000 | 100–4000 | 5.00 | plasma | Bioequivalence study of 20 mg torasemide in 12 healthy volunteers | [12] |
| UPLC–UV | SPE | 1000 | 10–1000 | 18 | Plasma & urine | Determination of torasemide and its two metabolites in plasma and urine | [13] |
| HPLC–UV | SPE | 1000 | 60–3000 | 10 | plasma | Determination of torasemide and its two metabolites in plasma | [15] |
| HPLC–UV | PP | 275 | 300–60000 | 6.8 | plasma | Determination of torasemide and spironolactone in plasma | [17] |
| HPLC–UV | LLE | 500 | 20–5000 | 8.4 | plasma | Bioequivalence study of 10 mg torasemide in 28 healthy volunteers | [18] |
| HPLC–UV | LLE | 500 | 2000–20000 | 3.9 | serum | Pharmaceutical research of torasemide in 5 hypertensive patients | [19] |
| HPLC–ESI–MS | LLE | 200 | 1–2500 | 3.68 | plasma | Bioequivalence study of 10 mg torasemide in 24 healthy volunteers & reanalysis of 54 incurred samples | This study |
D: derivatization; LLE: liquid–liquid extraction; SPE: solid phase extraction; PP: protein precipitation
4. Conclusion
Only few methods were reported on the determination of the concentration of torasemide in human plasma by HPLC–MS. In this research, a sensitive and selective HPLC–ESI–MS method for the determination of torasemide in human plasma samples was developed. This method used fewer plasma samples to bring out wider linear range and better human compliance. This method was validated according to bioanalytical method validation and successfully applied in pharmacokinetic and bioequivalence studies on torasemide in human volunteers.
Acknowledgments
We thank all co-workers in Jiangsu Province Hospital of Traditional Chinese Medicine and Chinese Pharmaceutical University. The study was funded by Jiangsu D&R Pharmaceutical Corporation (Taizhou, PR China).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2015.11.002.
Contributor Information
Yuan Tian, Email: tiancpu@sina.com.
Zunjian Zhang, Email: zunjianzhangcpu@hotmail.com.
Appendix A. Supplementary material
Supplementary material
References
- 1.Knauf H., Mutschler E. Clinical pharmacokinetics and pharmacodynamics of torasemide. Clin. Pharmacokinet. 1998;34:1–24. doi: 10.2165/00003088-199834010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Han L.N., Guo S.L., Lin X.M. Torasemide reduces dilated cardiomyopathy, complication of arrhythmia, and progression to heart failure. Genet. Mol. Res. 2014;13:7262–7274. doi: 10.4238/2014.September.5.11. [DOI] [PubMed] [Google Scholar]
- 3.Klütsch K., Grosswendt J., Haecker W. Single dose comparison of torasemide and furosemide in patients with advanced renal failure. Arzneimittel-Forsch. 1988;38:200–204. [PubMed] [Google Scholar]
- 4.Friedel H.A., Buckley M.M.T. Torasemide, Drugs. 1991;41:81–103. doi: 10.2165/00003495-199141010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Preobrazhenskiĭ D.V., Nekrasova N.I., Khoseva E.N. Torasemide is the effective loop diuretic for long-term therapy of arterial hypertension. Kardiologiia. 2010;51:67–73. [PubMed] [Google Scholar]
- 6.DiNicolantonio J.J. Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol. 2012;8:707–728. doi: 10.2217/fca.12.54. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Fu Y., Cao Q.M. Torasemide efficacy and safety studies in the treatment of acute heart failure. Chin. J. Med. Guide. 2013;7:1200–1203. [Google Scholar]
- 8.Neugebauer G., Besenfelder E., Mollendorff E.V. Pharmacokinetic and metabolism of torsemide in man. Arzneimittel-Forsch. 1988;38:164–168. [PubMed] [Google Scholar]
- 9.Barroso M.B., Meiring H.D., Jong A.D. Gas chromatographic–mass spectrometric analysis of the loop diuretic torasemide in human urine. J. Chromatogr. B. 1997;690:105–113. doi: 10.1016/s0378-4347(96)00392-1. [DOI] [PubMed] [Google Scholar]
- 10.Akesolo U., Gonzalez L., Jimenez R.M. Multivariate optimisation of a cyclodextrin-assisted-capillary zone electrophoretic method for the separation of torasemide and its metabolites. J. Chromatogr. A. 2003;990:271–279. doi: 10.1016/s0021-9673(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt S., Meineke I., Brockmöller J. Improved solid-phase extraction and HPLC measurement of torasemide and its important metabolites. J. Chromatogr. B. 2006;831:31–35. doi: 10.1016/j.jchromb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Khan I.J., Loya P., Saraf M.N. A simplified HPLC method for quantification of torsemide from human plasma and its application to a bioequivalence study. Indian. J. Pharm. Sci. 2008;70:519–522. doi: 10.4103/0250-474X.44609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.March C., Farthing D., Wells B. Solid-phase extraction and liquid chromatography of torsemide and metabolites from plasma and urine. J. Pharm. Sci. 1990;79:453–460. doi: 10.1002/jps.2600790520. [DOI] [PubMed] [Google Scholar]
- 14.Karnes H.T., Farthing D., Besenfelder E. Solid phase extraction with automated elution and HPLC of torsemide and metabolites from plasma. J. Liq. Chromatogr. 1989;12:180–189. [Google Scholar]
- 15.Besenfelder E. The determination of torasemide and metabolites in plasma by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1987;5:259–266. doi: 10.1016/0731-7085(87)80029-8. [DOI] [PubMed] [Google Scholar]
- 16.Liu K.H., Lee Y.K., Ryu J.Y. Simple and sensitive assay of torasemide in human plasma by high-performance liquid chromatography using a monolithic silica column. Chromatography. 2004;60:639–643. [Google Scholar]
- 17.Subramanian V., Nagappan K., Mannemala S.S. Optimization and validation of a sensitive HPLC–PDA method for simultaneous determination of torasemide and spironolactone in human plasma using central composite design. Acta. Chim. Slov. 2015;61:633–641. doi: 10.17344/acsi.2014.1262. [DOI] [PubMed] [Google Scholar]
- 18.Kang H.A., Yoon H., Lee Y.B. Bioequivalence of Torad tablet 5 mg to Torem tablet 5 mg (torasemide 5 mg) J. Pharm. Invest. 2013;43:153–159. [Google Scholar]
- 19.Thulasamma P., Venkateswarlu P. Development and validation of RP-HPLC method for the quantitative estimation of torsemide in pharmaceutical dosage forms and human serum. Int. J. Chem. Tech. Res. 2014;6:1103–1109. [Google Scholar]
- 20.Barroso B., Alonso R., Jiménez R. Quantitative analysis of the loop diuretic torasemide in tablets and human urine by HPLC-EC. J. Liq. Chromatogr. Rel. Technol. 1996;19:179–186. [Google Scholar]
- 21.Barroso M.B., Alonso R.M., Jiménez R.M. Simultaneous determination of torasemide and its major metabolite M5 in human urine by high-performance liquid chromatography-electrochemical detection. J. Chromatogr. Sci. 2001;39:491–496. doi: 10.1093/chromsci/39.11.491. [DOI] [PubMed] [Google Scholar]
- 22.Fernández M., Alonso R.M., Jiménez R.M. Differential-pulse adsorptive stripping voltammetry of the diuretic torasemide at a hanging mercury drop electrode. Analyst. 1994;119:319–322. [Google Scholar]
- 23.Ventura R., Nadal T., Alcalde P. Fast screening method for diuretics, probenecid and other compounds of doping interest. J. Chromatogr. A. 1993;655:233–242. [Google Scholar]
- 24.Thieme D., Grosse J., Lang R. Screening, confirmation and quantitation of diuretics in urine for doping control analysis by high-performance liquid chromatography–atmospheric pressure ionisation tandem mass spectrometry. J. Chromatogr. B. 2001;757:49–57. doi: 10.1016/s0378-4347(01)00058-5. [DOI] [PubMed] [Google Scholar]
- 25.FDA. Guidance for industry: bioanalytical method validation. U.S. department of health and human services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), and Center for Veterinary Medicine (CVM), 2013.
- 26.Yadav M., Shrivastav P.S. Incurred sampler analysis (ISR): a decisive tool in bio-analytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
- 27.Lesne M. Comparison of the pharmacokinetics and pharmacodynamics of torasemide and furosemide in healthy volunteers. Arzneimittel-Forsch. 1988;38:160–163. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material