Abstract
An improved and reliable ultra-performance liquid chromatography/tandem mass spectrometry (UPLC–MS/MS) method has been developed and validated for the determination of lercanidipine in human plasma. Plasma samples with lercanidipine-d3 as an internal standard (IS) were prepared by solid phase extraction on Phenomenex Strata-X cartridges using 100 µL of human plasma. Chromatographic analysis was performed on UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) column under isocratic conditions. Linear calibration curves were obtained over a wide dynamic concentration range of 0.010–20.0 ng/mL. Matrix effect was assessed by post-column infusion, post-extraction spiking and standard-line slope methods. The mean extraction recovery was >94% for the analyte and IS. Inter-batch and intra-batch precision (% CV) across five quality controls was <5.8%. Bioequivalence study was performed with 36 healthy subjects after oral administration of 10 mg of lercanidipine and the assay reproducibility was evaluated by reanalysis of 133 incurred samples.
Keywords: Lercanidipine, UPLC–MS/MS, Bioequivalence, Solid phase extraction, Human plasma
1. Introduction
Hypertension, one of the major causes of cardiovascular diseases, has affected young and elderly population in the world. Antihypertensive drugs have been proven agents to prevent cardiovascular morbidity and mortality since long ago [1], [2]. Dihydropyridine calcium channel blockers are a potent class of antihypertensive drugs and work primarily as vasodilators. They are widely used for the treatment and management of hypertension and coronary artery diseases. Lercanidipine (LER), a third-generation dihydropyridine calcium channel blocker, helps in peripheral vasodilation by preventing the entrance of calcium ions through L-type calcium channels in cell membrane [3], and has shown high efficacy for patients with high cardiovascular risk and diffuse atherosclerosis. LER has high lipophilicity, which is responsible for smooth onset and prolonged therapeutic action compared with the first- and the second-generation calcium channel blockers. It has shown lower incidence of adverse events such as lack of activation of heart rate [4], [5], [6]. LER has a plasma half life of 8–10 h which, however, does not relate to its duration of antihypertensive activity. After oral administration, LER is almost completely absorbed from the gastrointestinal tract and reaches peak plasma concentration within 1–3 h. It is approximately 98% protein bound and has a distribution volume of 2–2.5 L/kg. LER gets extensively metabolized by cytochrome P450 3A4 to inactive pyridine derivatives which are eliminated in urine and feces [4], [6].
To optimize drug therapy, reduce drug accumulation, and lessen the frequency of adverse effects, it is essential to develop reliable, rapid and sensitive bioanalytical methods. There are several methods described in the literature for the determination of LER as a single analyte [7], [8], [9], [10], [11], [12], [13] or in combination with other antihypertensive drugs [14], [15], [16], [17] in biological matrices. Enantioselective determination of LER in human plasma has been described for pharmacokinetic studies using chiral columns [7], [8]. Racemic LER has been estimated using different analytical techniques like voltammetry [9], [10], high performance liquid chromatography–ultraviolet detection (HPLC–UV) [11], liquid chromatography–tandem mass spectrometry (LC–MS/MS) [12] and ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) [13] in human serum [9], [10], human urine [10], rabbit serum [11] and human plasma [12], [13]. Salem et al. [12] presented a selective and rapid method to determine LER in the concentration range of 0.1–16 ng/mL within 10 min.
UPLC is a rapid separation technique with enhanced chromatographic efficiency compared with conventional HPLC. Unlike HPLC, this technique is beneficial to attaining higher resolution, sensitivity, and speed of analysis by optimized instrumentation operated with 1.7 µm particle size [18]. To the best of our knowledge, there is only one UPLC–MS/MS method for the determination of LER with a limit of quantitation of 0.05 ng/mL employing 1.0 mL of human plasma by liquid–liquid extraction [13]. The present work aimed to improve upon this method for routine sample analysis in a clinical laboratory. The current validated method has superior sensitivity and employs small plasma volume for processing compared with the existing UPLC–MS/MS method [13]. Additionally, its reproducibility proven through incurred sample reanalysis is free from matrix interference and highly rugged.
2. Experimental
2.1. Chemicals and materials
Reference standards of lercanidipine hydrochloride (LER, purity 99.66%) and lercanidipine-d3 hydrochloride (IS, purity 99.53%) were obtained from Clearsynth Labs (P) Ltd. (Mumbai, India). HPLC grade methanol and acetonitrile were obtained from Mallinckrodt baker, S.A.de C.V. (Estado de Mexico, Mexico). Bio-ultra grade ammonium formate and LC–MS grade formic acid were procured from Sigma-Aldrich (St. louis, MO, USA). Solid phase extraction (SPE) cartridges Phenomenex Strata™-X (30 mg, 1 mL) were purchased from Phenomenex India (Hyderabad, India). Deionized water used during the entire analysis was prepared using A Milli-Q water purification system from Millipore (Bangalore, India). Blank human plasma was obtained from Supratech Micropath (Ahmedabad, India) and stored at −20 °C until use.
2.2. Liquid chromatographic and mass spectrometric conditions
Waters Acquity UPLC system from Waters Corporation (MA, USA), together with Acquity UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) analytical column that was maintained at 35 °C in a column oven, was used for the analysis of LER and IS. The mobile phase consisted of 2.0 mM ammonium formate in water (pH 2.5, adjusted with formic acid) and acetonitrile (10:90, v/v) and delivered at a flow rate of 0.250 mL/min. The sample manager temperature was maintained at 4 °C and the system pressure was 7200 psi. Waters Quattro Premier XE from Waters Corporation (MA, USA) triple quadrupole mass spectrometer equipped with electrospray ionization was used for ionization and detection of LER and IS in the positive ionization mode. Optimized mass parameters for LER and IS were set as follows: cone gas flow, 85 L/h; desolvation gas flow, 740 L/h; capillary voltage, 2.2 kV; source temperature, 120 °C; desolvation temperature, 320 °C; and extractor volts, 5.00 V. The pressure of argon gas used for collision activation dissociation was 0.110 Pa. Quantitation was performed by monitoring transitions of m/z 612.2-280.1 for LER and m/z 615.2-283.1 for IS using multiple reaction monitoring (MRM) mode. Compound dependent parameters like cone voltage and collision energy were set at 70 V and 30 eV for LER and IS, respectively. Quadrupoles 1 and 3 were sustained at unit mass resolution and the dwell time was set at 100 ms. MassLynx software version 4.1 was used to control all UPLC and MS parameters.
2.3. Standard stock and spiked plasma samples
The standard stock solution of LER (100 µg/mL) was prepared by dissolving its accurately weighed amount in methanol, and intermediate stock solutions (10.0 and 0.50 µg/mL) were prepared in methanol:water (50:50, v/v). Calibration stansdards (CSs) and quality control (QC) samples were prepared by spiking blank plasma with corresponding stock solution. CSs were made at 0.010, 0.020, 0.060, 0.200, 0.400, 0.800, 2.00, 4.00, 10.0, 20.0 ng/mL concentrations, while QC samples were prepared at 16.0 ng/mL (high quality control, HQC), 8.00 ng/mL/2.40 ng/mL (medium quality control, MQC-1/2), 0.030 ng/mL (low quality control, LQC) and 0.010 ng/mL (lower limit of quantification quality control, LLOQ QC). Stock solution (100 µg/mL) of IS was prepared by dissolving 1.0 mg of lercanidipine-d3 in 10.0 mL of methanol and further diluted to prepare its working solution (40.0 ng/mL) in methanol: water (50:50, v/v). Standard stock and working solutions used for spiking were stored at 5 °C, while CSs and QC samples in plasma were kept at −70 °C until use.
2.4. Sample preparation
In order to avoid photodegradation of LER, the entire extraction process was performed under yellow light (570–580 nm). Prior to extraction, all frozen subject samples, CSs and QC samples were thawed and allowed to equilibrate at room temperature. 20 µL of internal standard was added to an aliquot of 100 µL of spiked plasma sample and vortexed for 10 s. Further, 100 µL of 2.0 mM ammonium formate in water (pH 2.5, adjusted with formic acid) was added and vortexed for another 10 s and then centrifuged at 13,148g for 5 min at 10 °C. The samples were loaded on Phenomenex StrataTM-X (30 mg, 1 mL) cartridges, previously conditioned with 1 mL methanol followed by 1 mL of 2.0 mM ammonium formate in water (pH 2.5). The samples were washed with 2×1 mL of 5% methanol in water and the cartridges were dried over nitrogen (1.72×105 Pa) at a flow rate of 2.4 L/min for 1 min. Elution of LER and IS was carried out with 0.5 mL of methanol. Then, the organic phase was evaporated to dryness under a gentle stream of nitrogen gas and the residue was reconstituted with 100 µL of mobile phase and briefly vortexed for 15 s. 10 µL was injected into the chromatographic system.
2.5. Method validation
The detailed UPLC–MS/MS method validation was based on standard guidelines [19] and similar to our previous work [20]. System suitability experiment was performed by injecting six consecutive injections using aqueous standard mixture of LER (8.0 ng/mL) and IS at the start of each batch during method validation. System performance was studied by injecting one extracted blank plasma (without analyte and IS) and one extracted LLOQ sample with IS at the beginning of each analytical batch and before re-injecting any sample during method validation. The carryover effect of the autosampler was evaluated by sequentially injecting extracted blank plasma, upper limit of quantitation (ULOQ) sample, extracted blank plasma, LLOQ sample, extracted blank plasma at the start and end of each batch.
Method selectivity was assessed for potential matrix interferences in ten batches (6 normal lots of Na-heparin plasma, 2 haemolysed plasma lots, and 2 lipemic plasma lots) of blank human plasma by extraction and inspection of the resulting chromatograms for interfering peaks. The selectivity of the method towards commonly used medications by human volunteers, involving paracetamol, chlorpheniramine maleate, diclofenac, caffeine, acetylsalicylic acid and ibuprofen, was also ascertained. Their stock solutions (100 µg/mL) were prepared by dissolving requisite amount in methanol. Further, working solutions (1.0 µg/mL) were prepared in the mobile phase and 10 µL was injected to check for any possible interference at the retention time of the analyte and IS.
Five calibration lines containing ten non-zero concentrations were used to determine linearity. A quadratic, 1/x2, least-squares regression algorithm was used to plot the peak area ratio (analyte/IS) from MRM versus concentration. The mean linear regression equations were then used to calculate the predicted concentrations in all samples within analytical runs. The correlation coefficient for each calibration curve must be ≥0.99 for LER. The lowest concentration standard on the calibration line was accepted as the LLOQ, if the analyte response was at least ten times more than that of extracted blank plasma. Reinjection reproducibility for extracted samples was also checked by reinjection of one entire analytical run after storage at 5 °C.
Intra-day accuracy and precision were evaluated by replicate analysis of plasma samples on the same day. The analytical run consisted of a calibration curve and six replicates of LLOQ, LQC, MQC-1/2 and HQC samples. The inter-day accuracy and precision were assessed by analyzing five precision and accuracy batches on three consecutive validation days. The precision (% CV) at each concentration level from the nominal concentration should not be greater than 15%. Similarly, the mean accuracy should be within 85%–115%, except for the LLOQ where it can be within 80%–120% of the nominal concentration.
Ion suppression/enhancement effects on the method sensitivity were evaluated by post column analyte infusion experiment. Briefly, a standard solution containing LER (at ULOQ level) was infused post-column into the mobile phase at 5 µL/min employing infusion pump. Aliquots of 10 µL of extracted control blank plasma sample were then injected into the column by the autosampler and chromatograms were acquired for the analyte and IS.
Extraction recovery of LER and IS from human plasma was evaluated in six replicates by comparing the mean peak area responses of pre-extraction fortified samples with those of post-extraction fortified samples. Absolute matrix effect was assessed by comparing the mean area response of post-extraction fortified samples with mean area of solutions prepared in mobile phase solutions (neat standards) at HQC, MQC-1/2 and LQC levels [21]. IS-normalized matrix factors (MFs, analyte/IS) were calculated to assess the variability of the results due to matrix effects. Relative matrix effect was assessed from the precision (% CV) values of the slopes of the calibration curves prepared from eight different plasma lots/sources, which included one haemolysed plasma and one lipemic plasma. To prove the absence of matrix effect, % CV should be less than 3%–4% for method applicability to support clinical studies [22].
Stability tests were conducted in stock solutions of LER and IS for short-term and long-term stability at 25 °C and 5 °C, respectively. The acceptance criterion was ±10.0% deviation from the nominal value. All stability results for spiked plasma samples were evaluated by measuring the area ratio response (LER/IS) of stability samples against freshly prepared comparison standards at LQC and HQC levels. Bench top, processed sample at room temperature and in cooling chamber at 5 °C, autosampler stability, freeze-thaw (−20 °C and −70 °C) and long-term (−20 °C and −70 °C) stability of the analyte in plasma were studied at LQC and HQC levels using six replicates. The samples were considered stable if the deviation from the mean calculated concentration of freshly prepared quality control samples was within ±15.0%.
Method ruggedness was verified using two precision and accuracy batches. The first batch was analyzed on two different columns of the same make but different batch number, while the second batch was analyzed by two different analysts who did not participate in method validation. The ability to dilute samples which could be above the upper limit of the calibration range was validated by analyzing six replicate samples containing 40 ng/mL of LER in the screened plasma after five-/ten-fold dilution respectively. The precision and accuracy of dilution reliability was determined by comparing the samples against freshly prepared calibration curve standards.
2.6. Bioequivalence study and incurred sample reanalysis
The study was an open-label, randomized, balanced, crossover, two-treatment, two-period and two-sequence design to determine the bioavailability, intra-subject variability and bioequivalence between a single dose of 10 mg of LERCAN® (Aspen Pharmacare, South Africa) and 10 mg of ZANIDIP® (Medley S.A. Industria Farmaceutica, Brazil) LER tablet formulations in 36 adult (aged 18–45 years) healthy volunteers under fed conditions. Each subject was checked to be healthy through medical history, physical examination and routine laboratory tests. All the subjects were informed about the objectives and possible risks of the study and were given a written consent. The study protocol was approved by an independent ethics committee constituted as per Indian Council of Medical Research (ICMR) and was performed in accordance with International Conference on Harmonization, E6 Good Clinical Practice guidelines [23]. The subjects were orally administered a single dose of the formulations after recommended wash out period of 7 days with 240 mL of water. Further, under fed conditions, the subjects were given high-fat and high-calorie breakfast (consisting of 250 mL milk with 5 g sugar, 35 g walnuts, two slices of bread with cheese and two cheese cutlets, containing 969 cal in total) 30 min before giving the drug under investigation. After administration, blood samples were collected at 0.00 (pre-dose), 0.50, 1.00, 1.33, 1.67, 2.00, 2.33, 2.67, 3.00, 3.33, 3.67, 4.00, 4.50, 5.00, 6.00, 7.00, 8.00, 10.00, 12.00, 16.00, 20.00, 24.00 and 48.00 h for test and reference formulations. Plasma was separated by centrifugation and kept frozen at −70 °C until analysis. During entire analysis, subjects had a standard diet while water intake was unmonitored. Non-compartmental model using WinNonlin software version 5.2.1 (Pharsight Corporation, Sunnyvale, CA, USA) was used to evaluate pharmacokinetic parameters of LER. To determine whether the formulations were pharmacokinetically equivalent, Cmax, AUC0–48 and AUC0–inf, and their ratios (test/reference) using log transformed data were assessed. The drug formulations were considered pharmacokinetically equivalent if the difference between the compared parameters was statistically nonsignificant (P≥0.05) and the 90% confidence intervals (CI) for these parameters were within 80%–125%. An incurred sample reanalysis (ISR) was also done by selecting 133 samples which were near the Cmax and the elimination phase in the pharmacokinetic profile of the drug. The results obtained were compared with the data obtained previously for the same sample following the same procedure. The percent change value between the two measurements should be within ±20% as reported previously [24].
3. Results and discussion
3.1. UPLC–MS/MS method optimization
Method development was initiated to improve upon a previously published UPLC–MS/MS method for higher sensitivity, lower plasma volume for processing, minimized consumption of toxic organic solvent and reduction in overall analysis time [13]. Thus, the extraction procedure, mass spectrometry parameters, and chromatographic conditions were suitably optimized.
3.1.1. Mass spectrometry
Measurement of LER and IS levels in human plasma was performed using electrospray ionization (ESI) in the positive ionization mode for UPLC–MS/MS analyses to attain high sensitivity and a good linearity in regression curves. The full scan Q1 mass spectra in the positive mode gave predominantly protonated precursor ions at m/z 612.2 and 615.2 for LER and IS, respectively. Fragmentation of protonated precursor ions gave highly intensive and consistent product ions at m/z 280.1 and 283.1 for LER and IS, respectively. The source dependent and compound dependent parameters were suitably optimized to obtain a consistent and sufficient response for the analyte. Thus, MRM transitions of m/z 612.2/280.1 for LER and m/z 615.2/283.1 for IS were finalized for quantitation. A dwell time of 100 ms was adequate and there was no cross talk between MRMs of LER and IS.
3.1.2. Liquid chromatography
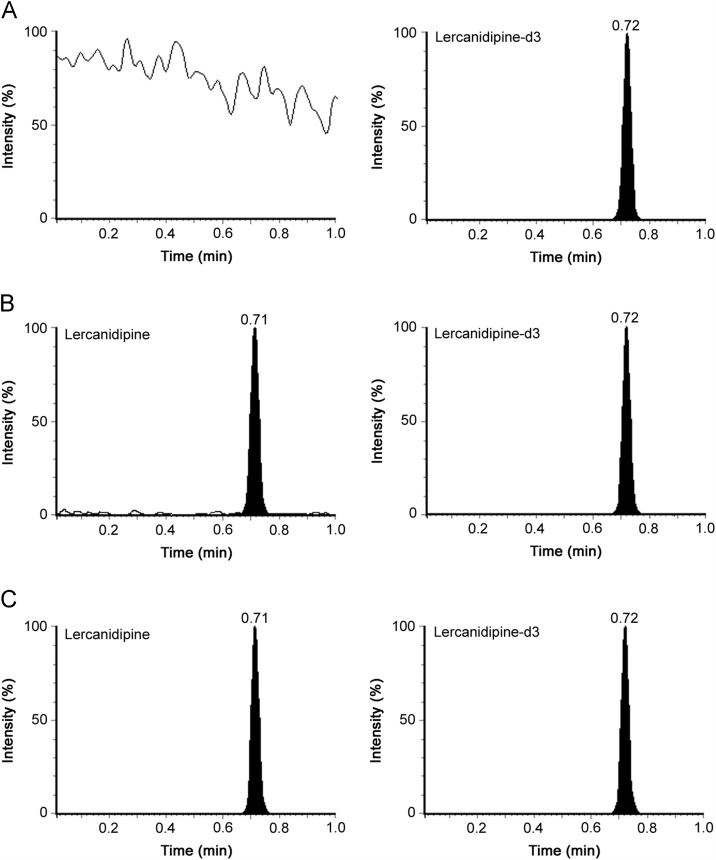
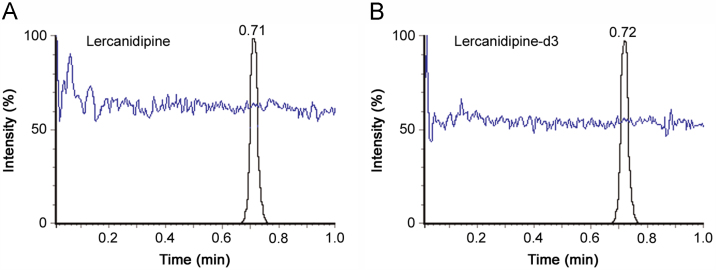
Chromatographic analysis was initiated to obtain adequate retention, sharp peak shape and short run time for LER and IS, with minimum matrix interference and solvent consumption. Selection of proper mobile phase and its pH was also investigated in the present work. Different combinations of acetonitrile/methanol and acidic modifiers (formic acid, glacial acetic acid, ammonium formate) were tested for adequate response and peak shape on Waters Acquity UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) column. It was observed that higher acetonitrile content enabled shorter run time and better peak shape, while the acidic additive helped to obtain adequate response for the analyte and IS. Nevertheless, the best chromatographic conditions were obtained using 2.0 mM ammonium formate in water, pH 2.5 adjusted with formic acid-acetonitrile (10:90, v/v) as the mobile phase under isocratic conditions. It ensured sufficient retention and elution of the analyte and IS within 1.0 min. The retention time for LER and IS was 0.71 and 0.72 min, respectively. Representative UPLC–MS/MS chromatograms of blank plasma fortified with IS, LER at LLOQ and an actual subject sample at Cmax illustrate the selectivity of the proposed method (Fig. 1). Use of deuterated IS, lercanidipine-d3, helped to compensate any possible variability during extraction and LC–MS/MS analysis. Additionally, there were no interfering signals at the retention time of LER or IS due to commonly used medications by human volunteers. Post-column infusion chromatogram showed no ion suppression or enhancement at the retention time of LER and IS (Fig. 2).
Fig. 1.
Representative MRM ion-chromatograms of (A) blank plasma with working solution of lercanidipine-d3 (m/z 615.2→283.1) (IS), (B) lercanidipine (m/z 612.2→280.1) at LLOQ and IS, and (C) lercanidipine in real subject sample at Cmax and IS after administration of 10 mg dose of lercanidipine hydrochloride, LERCAN®.
Fig. 2.
Injection of extracted blank human plasma during post-column infusion of (A) lercanidipine at ULOQ level and (B) lercanidipine-d3.
3.1.3. Plasma extraction
Due to high lipophilicity of LER, liquid–liquid extraction (LLE) has been used extensively for quantitative extraction of LER from human plasma [7], [12], [13], [14], [15]. However, in our effort to minimize usage of toxic organic solvents which is one of the limitations of LLE, SPE with Phenomenex StrataTM-X cartridges was employed. Two previous methodologies have employed SPE on Oasis HLB [17] and mixed mode C8/cation exchange cartridges [14] with satisfactory recovery for LER from human plasma. To improve the extraction recovery, 2.0 mM ammonium formate in water (pH 2.5, adjusted with formic acid) was added to plasma sample before loading for conditioning of cartridges. The mean extraction recovery obtained was highly consistent as well as quantitative across four QC levels.
3.2. Assay performance and validation results
The precision (% CV) for system suitability test was observed in the range of 0.08%–0.15% for the retention time and 0.75%–1.34% for the area response of LER and IS. The observed signal-to-noise ratio for system performance was ≥24 for both LER and IS. The column and autosampler carryover evaluation showed negligible carry-over in blank plasma (≤0.13% of LLOQ sample) after the injection of ULOQ sample. The method selectivity was assessed in ten different batches of blank plasma to check for the interference of endogenous matrix components in the quantitation of LER. There was no major interference at the retention time of the analyte in any of the batches.
LER showed good linearity (r2≥0.9996) through the studied concentration range of 0.010–20 ng/mL. The accuracy and precision values for the calibration curve standards ranged from 98.3% to 101.3% and 0.65% to 2.60%, respectively. The limit of detection (LOD) and LLOQ of the method for LER was 0.003 and 0.010 ng/mL at a signal-to-noise ratio of 24 and 10, respectively. The reinjection reproducibility expressed as % CV in the measurement of retention time for LER was ≤0.74 for 100 injections on the same column.
The intra and inter-batch precision and accuracy results for LER across five QC levels are presented in Table 1. The intra-batch precision (% CV) and accuracy ranged from 2.04%–5.41% and 98.1%–102.0%, respectively. Similar to inter-batch study, the precision varied from 1.48% to 5.72% and the accuracy was within 98.3%–103.0%.
Table 1.
Precision and accuracy data for lercanidipine.
| QC level | Intra-batch (n=6; single batch) |
Inter-batch (n=30; 6 from each batch) |
||||
|---|---|---|---|---|---|---|
| Mean conc. found (ng/mL) | CV (%) | Accuracy (%) | Mean conc. found (ng/mL) | CV (%) | Accuracy (%) | |
| HQC | 15.7 | 2.49 | 98.1 | 16.2 | 1.48 | 101.2 |
| MQC-1 | 8.13 | 2.04 | 101.6 | 7.90 | 1.89 | 98.7 |
| MQC-2 | 2.37 | 2.17 | 98.7 | 2.36 | 3.52 | 98.3 |
| LQC | 0.0302 | 4.58 | 100.6 | 0.0309 | 5.72 | 103.0 |
| LLOQ QC | 0.0102 | 5.41 | 102.0 | 0.0101 | 5.33 | 101.0 |
The extraction recoveries for LER and IS are presented in Table 2. The mean extraction recovery for LER and IS varied from 94.3% to 98.6% and 94.1% to 97.5% across QC levels. Evaluation of MFs is essential as the presence of unmonitored co-eluting compounds from the matrix can affect the accuracy, precision, ruggedness and overall reliability of a validated method. MFs were checked in lipemic and haemolysed plasma samples in addition to normal heparinised plasma. MFs can be determined from the peak area response for the analyte and IS respectively, while the ratio of the two factors yields IS-normalized MF for the analyte. Further, the IS-normalized MFs using stable-isotope labeled IS should be close to unity because of the similarities in the chemical properties and elution behavior of the analyte and IS. The IS-normalized MFs ranged from 0.98 to 1.04 (Table 3). Additionally, the coefficient of variation (% CV) of the slopes of calibration lines for relative matrix effect in eight different plasma lots was 2.94% as shown in Supplementary Table 1.
Table 2.
Extraction recovery for lercanidipine and lercanidipine-d3 (n=6).
| QC level | Lercanidipine |
Lercanidipine-d3 |
||||
|---|---|---|---|---|---|---|
| Area response |
Extraction recovery (%) (B/A) | Area response |
Extraction recovery (%) (B/A) | |||
| A | B | A | B | |||
| HQC | 452,157 | 445,829 | 98.6 | 227,342 | 221,658 | 97.5 |
| MQC-1 | 225,687 | 215,305 | 95.4 | 231,376 | 217,725 | 94.1 |
| MQC-2 | 67,548 | 65,589 | 97.1 | 229,476 | 219,379 | 95.6 |
| LQC | 845 | 797 | 94.3 | 221,683 | 214,367 | 96.7 |
A: mean area response of six replicates prepared by spiking in extracted blank plasma;
B: mean area response of six replicates prepared by spiking before extraction.
Table 3.
Matrix factors for lercanidipine and lercanidipine-d3 (n=6).
| QC level | Lercanidipine |
Lercanidipine-d3 |
||||
|---|---|---|---|---|---|---|
| Area response |
Matrix factor (A/B) | Area response |
Matrix factor (A/B) | |||
| A | B | A | B | |||
| HQC | 452,157 | 438,987 | 1.03 | 227,342 | 231,982 | 0.98 |
| MQC-1 | 225,687 | 221,262 | 1.02 | 231,376 | 222,476 | 1.04 |
| MQC-2 | 67,548 | 67,468 | 1.00 | 229,476 | 231,794 | 0.99 |
| LQC | 845 | 820 | 1.03 | 221,683 | 219,488 | 1.01 |
A: Mean area response of six replicates prepared by spiking in extracted blank plasma;
B: Mean area response of six replicates prepared by spiking in mobile phase (neat samples).
Stability of LER in plasma under various conditions at two QC levels with the values for percent change is shown in Table 4. Samples for short-term and long-term stock solution stability remained unchanged up to 24 h and 48 days, respectively, for LER and IS. Bench top stability of LER in plasma was established up to 16 h and was stable for minimum of five freeze-thaw cycles at −20 °C and −70 °C. Autosampler stability of the spiked quality control samples maintained at 4 °C was determined up to 78 h without significant drug loss. For long-term stability, the spiked plasma samples stored at −20 °C and −70 °C were found stable for a minimum period of 164 days.
Table 4.
Stability of lercanidipine in plasma under various conditions (n=6).
| Storage conditions | QC level | Nominal conc. (ng/mL) | Mean stability sample±SD (ng/mL) | Change (%) |
|---|---|---|---|---|
| Bench top stability at room temperature, 16 h | HQC | 16.0 | 15.56±0.24 | −3.13 |
| LQC | 0.030 | 0.0304±0.0003 | 1.33 | |
| Freeze-thaw stability after 5th cycle at −20 °C | HQC | 16.0 | 15.84±0.35 | −1.25 |
| LQC | 0.030 | 0.0303±0.0007 | 1.00 | |
| Freeze-thaw stability after 5th cycle at −70 °C | HQC | 16.0 | 16.31±0.31 | 1.88 |
| LQC | 0.030 | 0.0296±0.0006 | −1.33 | |
| Processed sample stability at room temperature, 14 h | HQC | 16.0 | 16.20±0.33 | 1.25 |
| LQC | 0.030 | 0.0301±0.0006 | 0.33 | |
| Autosampler stability at 4 °C, 78 h | HQC | 16.0 | 16.42±0.57 | 2.50 |
| LQC | 0.030 | 0.0297±0.0005 | −1.00 | |
| Processed sample stability (wet extract), at 2–8 °C , 73 h | HQC | 16.0 | 15.90±0.29 | −0.63 |
| LQC | 0.030 | 0.0299±0.0007 | −0.33 | |
| Long-term stability at −20 °C, 164 days | HQC | 16.0 | 15.67±0.53 | −2.50 |
| LQC | 0.030 | 0.0302±0.0004 | 0.67 | |
| Long-term stability at −70 °C, 164 days | HQC | 16.0 | 15.79±0.41 | −1.88 |
| LQC | 0.030 | 0.0304±0.0007 | 1.33 |
.
For method ruggedness, the precision (% CV) and accuracy values for different columns ranged from 1.4% to 3.2% and 96.8% to 102.2%, respectively, across four QC levels. Similarly, the precision and accuracy results for different analysts were within 1.8%–2.9% and 97.6%–104.5%, respectively. The precision (% CV) for dilution reliability of 1/5th and 1/10th dilution was between 1.66% and 2.63%, while the accuracy results were within 97.1%–103.7%.
3.3. Application to a bioequivalence study and incurred sample reanalysis
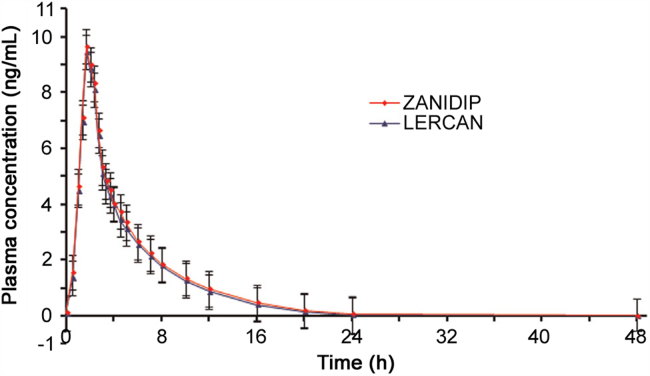
The UPLC–MS/MS method was successfully applied to determine LER concentration in human plasma samples after oral administration of a single 10 mg dose of LERCAN® and ZANIDIP® formulations. The present study was conducted under fed conditions as it has been shown that food increases the bioavailability of LER hydrochloride by three folds [25]. Fig. 3 presents the mean plasma concentration vs. time profile of LER in 36 healthy subjects.
Fig. 3.
Mean plasma concentration-time profile of lercanidipine after oral administration of 10 mg of LERCAN® and 10 mg of ZANIDIP® tablet formulations by 36 healthy Indian volunteers.
The mean pharmacokinetic parameters obtained for both the formulations are compiled in Table 5. The Cmax, t1/2, AUC0–t, and AUC0–inf values were comparable with a similar study with 10 mg of LER hydrochloride [25]; however, the Tmax values were approximately half of those obtained in the present work. This could be related to genetic difference, type of food, gender, age, weight, and other renal and hepatic functions. Nevertheless, there was no obvious statistical difference between the two formulations in any parameter. The ratios of mean log-transformed Cmax, AUC0–48, and AUC0–inf and their 90% CIs were all within the defined bioequivalence range of 80%–125% (Table 5). These observations validate the bioequivalence of the two products in terms of rate and extent of absorption. Moreover, there was no adverse event during the study. About 2700 samples, which included CSs, QCs and subject samples, were analyzed in a period of 9 days and the precision and accuracy were well within the acceptable limits. Further, the reproducibility test performed with 133 incurred samples showed the percent change within ±10% of the initial analysis results, which confirms the reproducibility of the proposed method.
Table 5.
Mean pharmacokinetic parameters (±SD) and comparison of treatment ratios and 90% CIs of natural log (Ln)-transformed parameters following oral administration of 10 mg of lercanidipine tablet formulation by 36 healthy Indian subjects under fed condition.
| Parameter | Mean |
LERCAN/ZANIDIP (%) | 90% CI (Lower–upper) | Power | Intra-subject variation (% CV) | |
|---|---|---|---|---|---|---|
| LERCAN | ZANIDIP | |||||
| Cmax (ng/mL) | 9.16±1.46 | 9.39±1.84 | 97.55 | 94.04–101.13 | 0.9996 | 6.05 |
| AUC0–48 (h ng/mL) | 17.8±4.05 | 19.0±4.51 | 94.05 | 91.23–96.82 | 0.9993 | 5.01 |
| AUC0–inf (h ng/mL) | 21.1±5.82 | 22.8±6.06 | 92.61 | 88.41–96.60 | 0.9998 | 7.43 |
| Tmax (h) | 1.67±0.28 | 1.73±0.26 | – | – | – | – |
| t1/2 (h) | 3.36±0.96 | 3.54±0.64 | – | – | – | – |
| Kel (1/h) | 0.12±0.02 | 0.13±0.01 | – | – | – | – |
3.4. Comparison with reported methods
The current method is more sensitive and employs less plasma volume for processing compared with all other procedures for determination of LER in human plasma. Further, the consumption of organic diluent for extraction and chromatography is significantly less than that of available methods. The on-column loading of LER at ULOQ was only 0.20 ng per sample injection. Methods reported for the analysis of LER by LC–MS/MS are based on its enantioselective determination in human plasma [7], simultaneous determination with other antihypertensive drugs for toxicokinetic studies [17], rapid screening for forensic and clinical studies [14] and for pharmacokinetic study with healthy subjects [12] or patients [13]. The present method with a linear range of 0.01–20 ng/mL adequately covers the therapeutic plasma concentration range of 1.2–13.6 ng/mL for LER. The LLOQ of 0.01 ng/mL was established to have a better assessment of LER pharmacokinetics, especially during the elimination phase. To the best of our knowledge, this is the first report on bioequivalence study in healthy Indian subjects. Additionally, the ISR results have proved the reproducibility of method which has not been reported in any previous study on LER. A detailed summary of all liquid chromatography–tandem mass spectrometry methods developed for LER in human plasma is presented in Table 6 to highlight the merits of the present method.
Table 6.
Comparison of salient features of the present method with reported LC–MS/MS methods for lercanidipine in human plasma.
| Sr. no. | LLOQ (ng/mL) | Linear range (ng/mL) | Sample processing volume (µL) | Extraction technique | Maximum on-column analyte loading per injection (ng) | Organic solvent consumption per sample analysis (mL) | Post-column infusion study; matrix effect; application | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1a | 0.025 | 0.025–50.0 | 1000 | LLE with hexane-isopropanol | 20.0 | ~17.5 | Yes; –; Stereoselective analysis of 10 mg of lercanidipine tablet, Zanidip in one healthy volunteer | [7] |
| 2 | 0.10 | 0.10–16.0 | 1000 | LLE with hexane-ethyl acetate | 9.6 | ~27.2 | –; Yes; Bioequivalence study with 20 mg of lercanidipine tablet in 36 healthy volunteers | [12] |
| 3b | 0.05 | 0.050–30.0 | 500 | LLE with methyl tert-butyl ether | 3.0 | ~4.2 | –; Yes; Bioavailability study with 10 mg of lercanidipine tablet, Zanidip in 3 patients | [13] |
| 4c | 1.00 | – | 1000 | Automated SPE on mixed-mode C8/cation exchange cartridges | 2.72 | ~9.0 | –; –; Screening of 11 dihydropyridine calcium channel blockers in plasma for forensic and clinical studies | [14] |
| 5d | 1.00 | 1.0–2000 | 200 | SPE on Oasis HLB C18 cartridiges | 8.0 | ~22.0 | –; Yes; Toxicokinetic study in 24 healthy beagle dogs | [17] |
| 6 b | 0.010 | 0.010–20.0 | 100 | SPE on Phenomenex Strata-X cartridges | 0.20 | ~1.9 | Yes; Yes; Bioequivalence study with 10 mg of lercanidipine tablets, Zanidip and Lercan in 36 healthy volunteers; Yes | PW |
LLOQ: lower limit of quantitation; LLE: liquid–liquid extraction; SPE: solid phase extraction; PW: present work.
Enantiomeric determination.
UPLC–MS/MS method.
Together with 10 dihydropyridine calcium channel blockers.
Along with benazepril and benazeprilat.
4. Conclusion
A selective, sensitive and rugged UPLC–MS/MS method for the quantitation of LER in human plasma has been developed and fully validated as per USFDA guidelines. The method presents perceived advantage over the existing UPLC–MS/MS method in terms of lower sample requirement for processing and five-fold higher sensitivity. Although the chromatographic analysis time is identical to the existing UPLC–MS/MS method, the consumption of organic solvent (for chromatography and extraction) in the present work is considerably lower. Moreover, matrix effect is extensively evaluated through post-column infusion, post-extraction spiking and calibration line slope methods. Additionally, incurred sample reanalysis study was also performed to prove the method reproducibility which has not been reported in existing procedures for LER. Finally, the present method shows adequate sensitivity and selectivity for the quantification of LER in human plasma in clinical study with healthy volunteers.
Acknowledgments
One of the authors, Darshan V. Chaudhary, would like to thank UGC-BSR (F 4-1/2009 (BSR)/7-74/2007), New Delhi, for research fellowship, F 4-1/2009 (BSR)/7-74/2007, and Department of Chemistry, Gujarat University, for supporting this work.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
Supplementary data associated with this article can be found in the online version at 10.1016/j.jpha.2015.09.001.
Appendix A. Supplementary material
Supplementary material
References
- 1.Pruijm M.T., Maillard M.P., Burnier M. Patient adherence and the choice of antihypertensive drugs: focus on lercanidipine. Vasc. Health Risk Manag. 2008;4:1159–1166. doi: 10.2147/vhrm.s3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghi C., Santi F. Fixed combination of lercanidipine and enalapril in the management of hypertension: focus on patient preference and adherence. Patient Preference Adherence. 2012;6:449–455. doi: 10.2147/PPA.S23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hair P.I., Scott L.J., Perry C.M. Fixed-dose combination lercanidipine/enalapril. Drugs. 2007;67:95–106. doi: 10.2165/00003495-200767010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Borghi C. Lercanidipine in hypertension. Vasc. Health Risk Manag. 2005;1:173–182. [PMC free article] [PubMed] [Google Scholar]
- 5.Fogari R., Mugellini A., Zoppi A. Differential effects of lercanidipine and nifedipine GITS on plasma norepinephrine in chronic treatment of hypertension. Am. J. Hypertens. 2003;16:596–599. doi: 10.1016/s0895-7061(03)00901-4. [DOI] [PubMed] [Google Scholar]
- 6.Bang L.M., Chapman T.M., Goa K.L. Lercanidipine: A Review of its efficacy in the management of hypertension. Drugs. 2003;63:2449–2472. doi: 10.2165/00003495-200363220-00013. [DOI] [PubMed] [Google Scholar]
- 7.Jabor V.A.P., Coelho E.B., Ifa D.R. Enantioselective determination of lercanidipine in human plasma for pharmacokinetic studies by normal-phase liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2003;796:429–437. doi: 10.1016/j.jchromb.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Jabor V.A.P., Coelho E.B., Lanchote V.L. Enantioselective pharmacokinetics of lercanidipine in healthy volunteers. J. Chromatogr. B. 2004;813:343–346. doi: 10.1016/j.jchromb.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Altun Y., Uslu B., Ozkan S.A. Electroanalytical characterization of lercanidipine and its voltammetric determination in pharmaceuticals and human serum on boron-doped diamond electrode. Anal. Lett. 2010;43:1958–1975. [Google Scholar]
- 10.Ozturk F., Tasdemir I.H., Erdogan D.A. A new voltammetric method for the determination of lercanidipine in biological samples. Acta Chim. Slov. 2011;58:830–839. [PubMed] [Google Scholar]
- 11.Charde S., Kumar L., Saha R. Development and validation of high‐performance liquid chromatographic method for estimation of lercanidipine in rabbit serum. Anal. Lett. 2007;40:2128–2140. [Google Scholar]
- 12.Salem I.I., Idrees J., Tamimi J.I. Al. Selective and rapid liquid chromatography mass spectrometry method for the determination of lercanidipine in human plasma. J. Chromatogr. B. 2004;803:201–207. doi: 10.1016/j.jchromb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Kalovidouris M., Michalea S., Robola N. Ultra-performance liquid chromatography/tandem mass spectrometry method for the determination of lercanidipine in human plasma. Rapid Commun. Mass Spectrom. 2006;20:2939–2946. doi: 10.1002/rcm.2693. [DOI] [PubMed] [Google Scholar]
- 14.Mueller C.A., Gonzalez A.B., Weinmann W. Screening for dihydropyridine calcium channel blockers in plasma by automated solid-phase extraction and liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2004;39:639–646. doi: 10.1002/jms.630. [DOI] [PubMed] [Google Scholar]
- 15.Baranda A.B., Etxebarria N., Jimenez R.M. Development of a liquid–liquid extraction procedure for five 1,4-dihydropyridines calcium channel antagonists from human plasma using experimental design. Talanta. 2005;67:933–941. doi: 10.1016/j.talanta.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez O., Alonso R.M., Ferreirós N. Development of an LC–MS/MS method for the quantitation of 55 compounds prescribed in combined cardiovascular therapy. J. Chromatogr. B. 2011;879:243–252. doi: 10.1016/j.jchromb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen K., Zhang J., Liu S. Simultaneous determination of lercanidipine, benazepril and benazeprilat in plasma by LC–MS/MS and its application to a toxicokinetics study. J. Chromatogr. B. 2012;899:1–7. doi: 10.1016/j.jchromb.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Swartz M.E. UPLC: an Introduction and review. J. Liq. Chromatogr. Relat. Technol. 2005;28:1253–1263. [Google Scholar]
- 19.Guidance for Industry, Bionanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), 2001.
- 20.Sharma P., Patel D.P., Sanyal M. Simultaneous analysis of oxybutynin and its active metabolite N-desethyl oxybutynin in human plasma by stable isotope dilution LC–MS/MS to support a bioequivalence study. J. Pharm. Biomed. Anal. 2013;84:244–255. doi: 10.1016/j.jpba.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 22.Matuszewski B.K. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J. Chromatogr. B. 2006;830:293–300. doi: 10.1016/j.jchromb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Guidance for Industry: ICH E6 Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), 1996.
- 24.Yadav M., Shrivastav P.S. Incurred sample reanalysis (ISR): a decisive tool in bioanalytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
- 25.Barchielli M., Dolfini E., Farina E., P. Clinical pharmacokinetics of lercanidipine. J. Cardiovasc. Pharmacol. 1997;29(Suppl. 2):S1–S15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material