Abstract
A simple and high throughput method was developed and validated for simultaneous determination of valproic acid and its two toxicant ene-metabolites, 2-enevalproic acid and 4-enevalproic acid in epilepsy patient plasma using liquid chromatography–tandem mass spectrometry. Probenecid was used as internal standard and solid-phase extraction was selected for sample preparation. A chromatographic separation was performed on an Agilent Poroshell SB-C18 column (50 mm×4.6 mm i.d., 2.7 μm) by an optimized gradient elution at a flow rate of 0.9 mL/min. The total run time was 7 min. Electrospray ionization was used in negative ion mode by multiple reaction monitoring of the precursor-to-product ion transitions at m/z 143.0→143.0 for valproic acid, m/z 140.9→140.9 for 2-enevalproic acid and 4-enevalproic acid for their poor fragments, and m/z 283.9→239.9 for probenecid. The results showed good linearity of valproic acid, 2-enevalproic acid and 4-enevalproic acid in their respective linear ranges. The correlation coefficients were more than 0.998. The intra- and inter-day precision of the assay was less than 11.0% and the accuracy ranged from 2% to 12%. This analytical method was successfully applied to assay plasma concentrations of valproic acid and its two ene-metabolites in epilepsy patient plasma and used for therapeutic drug monitoring.
Keywords: Liquid chromatography–tandem mass spectrometry, Valproic acid, 2-enevalproic acid, 4-enevalproic acid
1. Introduction
Valproic acid (VPA, Fig. 1A) is one of the most widely used antiepileptic drugs, which has broad-spectrum antiepileptic activity.VPA possesses the best therapeutical effect on petit mal and shows individual difference among epilepsy patients [1]. It is notable that the administration of VPA may be closely associated with rare but serious hepatotoxicity with or without hepatonecrosis [2]. Metabolism is an uppermost route of VPA elimination, only with 1%–3% of an orally administered dose excreted unchanged by urine. The bioconversion of VPA relates to three important metabolic pathways: (ⅰ) approximately 50% of VPA is mediated by UDP-glucuronosyltransferases (UGTs); (ⅱ) 40% of VPA experiences mitochondrial β-oxidation; (ⅲ) almost 10% of VPA is metabolized by CYP3A4, CYP2C19 and CYP2C9 (cytochrome P-450) into oxidative metabolites. Glucuronidation and β–oxidation are the most important metabolic pathways of VPA [3]. 2-enevalproic acid (2-ene VPA) is provided by mitochondrial beta-oxidation of VPA and the formation of 4-enevalproic acid (4-ene VPA) is mediated by CYP2C9 [4]. Moreover, the structures of 2-ene VPA (Fig. 1B) and 4-ene VPA (Fig. 1C) are similar to those of hypoglycin and 4-pentenoic acid, known as hepatic toxicants [5], [6]. VPA can be used alone or in combination with other antiepileptic drugs to treat epilepsy. Therefore, it is necessary to simultaneously monitor VPA and its toxicant, ene-metabolites in epilepsy patients. However, a small molecule with simple chemical structure is a challenge for the determination with tandem mass spectrometry. Especially, 2-ene VPA and its structural isomer 4-ene VPA both have the same precursor/product transitions, which is an analytical challenge to ensure their baseline separation.
Fig. 1.
Chemical structures of (A) VAP, (B) 2-ene VAP and (C) 4-ene VAP.
Numerous techniques have been reported for the quantification of VPA in biological samples, mainly including fluorescence polarization immunoassay [7], thin layer chromatography-derivatization [8], homogeneous enzyme-linked immunoassay [9], gas chromatography [10], high performance liquid chromatography [11], [12], [13], [14], [15], [16], and high performance liquid chromatography tandem mass spectrometry (LC–MS/MS) [17]. However, the major disadvantages of these techniques were the low sensitivity and the long retention time. Gao et al. [18] used LC–MS/MS to simultaneously determinate VPA and 4-ene VPA. Because no stable ion fragments were produced during VPA, 2-ene VPA and 4-ene VPA ionization, the pseudo multiple reaction monitoring (pseudo-MRM) mode was chose to develop LC–MS/MS analytical method. However, Dziadosz et al. [19] adopted the components of the mobile phase with MRM as a novel way to analysis VPA in human serum with LC–MS/MS. In this study, we once tried to use the same method to determinate VPA, 2-ene VPA and 4-ene VPA, but this measure could not be applied to 2-ene VPA and 4-ene VPA. Because the concentrations of 2-ene VPA and 4-ene VPA are low, it is impossible to inspect signal when formic acid is added into mobile phase. Meanwhile, the concentration of VPA in pseudo-MRM mode is higher than that in MRM mode with aqueous 0.1% formic acid. Finally, pseudo-MRM was selected for determination of VPA, 2-ene VPA and 4-ene VPA. Wilimowska [20] quantified the variabity of concentrations of VPA and its selected metabolites, 2-ene VPA and 4-ene VPA. However, no specific LC–MS/MS method was reported for simultaneous determination of VPA and its two ene-metabolites, 2-ene VPA and 4-ene VPA, in biological specimens up to date.
In order to better explain pharmacodynamics and toxicokinetics of VPA, 2-ene VPA and 4-ene VPA in the body, we established a sensitive and high-throughput LC–MS/MS method for simultaneous determination of VPA, 2-ene VPA and 4-ene VPA based on solid-phase extraction (SPE) followed by gradient elution program in human plasma. Finally, the method was fully validated and successfully applied to therapeutic drug monitoring.
2. Materials and methods
2.1. Chemicals and reagents
VPA, 2-ene VPA, 4-ene VPA (purity >98.0%) and probenecid (internal standard, IS, purity >99.0%) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Acetonitrile (HPLC grade) was obtained from Fisher Scientific (Fair Lawn, USA). Formic acid (HPLC grade) was purchased from Tedia (Fairfield, OH, USA). Deionized water was obtained from a Millipore Milli-Q gradient water purification system (Molsheim, Franceo). Epilepsy patient plasma samples and heparinized blank (drug-free) human plasma samples were obtained from Changchun Blood Donor Service (Changchun, China).
2.2. Instruments
The LC–MS/MS system consists of an Agilent 1100 Series HPLC system (Agilent Technologies, Palo Alto, USA) and an Applied Biosystems Sciex Qtrap 5500 mass spectrometer (Applied Biosystems, Sciex, Ontario, Canada) equipped with an electrospray ionization (ESI) source. Acquisition and integration of data were achieved by Applied Biosystems Analyst Software, version 1.5.2.
2.3. LC–MS/MS condition
The separation of VPA, 2-ene VPA and 4-ene VPA was performed at 40 °C on an Agilent Poroshell SB-C18 column (50 mm×4.6 mm i.d., 2.7 μm) protected by a SecurityGuard C18 guard column (4 mm× 3.0 mm i.d., Phenomenex Inc., USA) with a mobile phase that constituted water (A) and acetonitrile (B) at a flow rate of 0.9 mL/min. The initial mobile phase was composed of 10% B. After 4.0 min, the composition was changed to 60% B; these conditions were maintained for 0.5 min, after which the column was quickly equilibrated with the initial mobile phase.
MS parameters were optimized by infusing a standard solution of analytes or IS into the mass spectrometer through a syringe pump. The mass spectrometer with ESI source was operated in the negative ion mode. Nebulizer, heater and curtain gas pressure were set at 40, 40 and 30 psi, respectively; dwell time was optimized at 200 ms; ionspray voltage was regulated at −4500 V; heater gas temperature was maintained at 600 °C; declustering potentials (DP) −60 V and collision energies (CE) −12 eV were found to be the best for analytes and IS. Unit resolution was used for both Q1 and Q3 mass detection. MRM scan mode was used to monitor ion transitions at m/z 143.0→143.0 for VPA, m/z 140.9→140.9 for 2-ene VPA and 4-ene VPA, and m/z 283.9→239.9 for IS. Data were collected and processed by Applied Biosystems Analyst Software.
2.4. Preparation of calibration standards and quality control (QC) samples
The stock solutions of VPA, 2-ene VPA and 4-ene VPA (1 mg/mL) were successively prepared in ultra-pure water, stored in a refrigerator at 4 °C prior to use, and then mixed correspondingly to get mixed stock solution. Mixed working solutions of VPA, 2-ene VPA and 4-ene VPA were prepared by diluting prepared mixed stock solution to 20, 40, 60, 80, 100 and 125 µg/mL for VPA, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.25 µg/mL for 2-ene VPA, and 0.02, 0.04, 0.06, 0.08, 0.10 and 0.125 µg/mL for 4-ene VPA with drug-free human plasma. QC samples were prepared independently in the same way with concentrations of 30, 80 and 125 µg/mL for VPA, 1.5, 4.0 and 6.25 µg/mL for 2-ene VPA and 0.03, 0.08 and 0.125 µg/mL for 4-ene VPA. A stock solution of probenecid of 1 mg/mL was also prepared in ultra-pure water and diluted with drug-free human plasma subsequently to obtain an IS working solution of 0.05 µg/mL.
2.5. Sample preparation
Frozen human plasma samples from subjects were allowed to thaw in a water-bath at room temperature and vortex-mixed before processing. A 50 µL aliquot of plasma sample (calibration standards, QC samples or test samples) was mixed with 50 µL of IS working solution (0.05 µg/mL), and then diluted with 500 µL of 0.2% formic acid. The mixture was vortexed for 1 min and then loaded on Waters Oasis HLB 1 mL/10 mg cartridge which had been conditioned with 1.0 mL of methanol and equilibrated with 1.0 mL of 0.2% formic acid. Finally, the cartridges were washed with 1.0 mL water and eluted from the cartridge by 2.0 mL of a mixture of acetonitrile and water (4:1, v/v); then 20 µL of the eluent was injected into the LC–MS/MS system.
2.6. Method validation
The developed method was fully validated for selectivity, cross-talk, carryover, linearity, accuracy, precision, matrix effects, recovery and stability according to the Food and Drug Administration (FDA) guidance [21]. Selectivity of the method was performed using six different sources of blank plasma samples and ensuring no interfering peaks could be observed at retention time of the analytes and IS. Cross-talk was assessed among MS/MS channels by separately injecting a high concentration solution of calibration curve of VPA, 2-ene VPA, 4-ene VPA and IS and then monitoring the responses in other MS/MS channels. Carryover was evaluated by injecting an upper limit of quantitation (ULOQ) followed by a blank sample. The calibration standards were obtained by weighted (1/x2) least-square linear regression based on the peak area ratios of analytes to IS versus the nominal analyte concentration. To ensure linearity, the coefficient (r2) should be more than 0.99; meanwhile, the deviations of the calculated concentrations should be less than 15% of the nominal concentration and a ±20% deviation at the lower limit of quantification (LLOQ) was accepted. The accuracy and intra- and inter-day precision were evaluated on six replicates of QC samples at low, medium and high concentrations on three consecutive days. The accuracy was demanded to be less than 15%, and both intra- and inter-day precisions were required to be below 15% except for the LLOQ, at which ±20% was permitted.
To evaluate matrix effects and recovery of analytes and IS, three mixtures should be prepared. Matrix effects were evaluated by comparing peak areas of the analytes and IS in post-extraction spike, which was obtained by the SPE with blank plasma samples, and then mixing the solvent standards with those solvent standard solutions. Extraction recovery was obtained by comparing peak areas of QC samples with those of post-extraction spikes at three different concentrations (low, medium and high).
Stability of the analytes was assessed by analyzing QC samples placed on storage for 60 days at −20 °C, for 2 h at room temperature (25 °C) as well as after three freeze/thaw cycles. Stability of pre-extraction spike on storage in autosampler vials at room temperature for 2 h was also evaluated.
2.7. Clinical application
The validated LC–MS/MS method was applied to determine the concentration of VPA, 2-ene VPA and 4-ene VPA in epilepsy patient plasma refereed to therapeutic drug monitoring. Experimental procedures were compliant with the ethical regulations of Jilin University.
3. Results and discussion
3.1. Optimization of mass spectrometry, chromatography and sample preparation
VPA and its ene-metabolites, 2-ene VPA and 4-ene VPA, contain carboxy side chain. The negative ion mode was adopted in the LC–MS/MS method since it produced higher intensity signals than the positive ion mode. In this study, the abundance of deprotonation ion [M–H]- was the maximum in the Q1 MS spectra of the analytes and IS, but the poor fragmentation leading to low sensitivity is a challenge of bioanalysis. Pseudo-MRM is an effective way to overcome this with the same Q1 and Q3 ions and low collision energy. As a result, the response of baseline and noise of analytes was very high (>1.0e4 cps) for VPA, 2-ene VPA and 4-ene VPA, which brought great difficulties for sensitivity and chromatographic separation. Finally, ion transitions were observed at m/z 143.0→143.0 for VPA, m/z 140.9→140.9 for 2-ene VPA and 4-ene VPA, and m/z 283.9→239.9 for IS.
Because 2-ene VPA and 4-ene VPA possess the same transitions, rapid and complete chromatographic separation is the most important issue that must be addressed. Several available C18 columns including Zorbax SB-C18, Zorbax SB-Aq, Zorbax extend-C18 and Poroshell SB-C18 were tested. At last, Agilent Poroshell SB-C18 column (50 mm×4.6 mm i.d., 2.7 μm) was selected due to the approved baseline separation of 2-ene VPA and 4-ene VPA. The composition of the mobile phase was optimized through several trials in order to achieve high sensitivity and symmetric peak shapes in analysis of biological samples. The best result was obtained with a mobile phase consisting of acetonitrile and water. Using this mobile phase with Agilent Poroshell SB-C18 column, VPA, 2-ene VPA and 4-ene VPA could be simultaneously determined with a short run time of only 7 min with gradient elution.
For the sample preparation, we have tried protein precipitation (PPT), liquid–liquid extraction (LLE) and SPE. Asymmetric peaks with poor sensitivity and significant matrix effects were produced by PPT using acetonitrile, methanol and perchloric acid. LLE with several different organic solvents (dichloromethane, diethyl ether, hexane, ethyl acetate and isopropanol with different ratio mixtures) was tried. Due to the great polarity difference between VPA and its alkene metabolites, the recovery was not stable and sustainable for the three analytes. Finally, SPE was found to give reproducible extraction efficiency of analytes and IS from acidified samples with almost no interference, low matrix effects and high sensitivity. But deprotonation of VPA, 2-ene VPA and 4-ene VPA having carboxy functional group eliminates the interaction with SPE column. In order to ensure the three analytes keeping molecular state and increase their retention behavior in C18 solid phase extraction column, SPE column was equilibrated with 1.0 mL of 0.2% formic acid and plasma samples were diluted with 500 µL of 0.2% formic acid.
3.2. Selection of IS
Probenecid was selected as IS due to the similarity of retention time and extraction efficiency and its adequate ionization response in the negative ion mode.
3.3. Method validation
3.3.1. Specificity
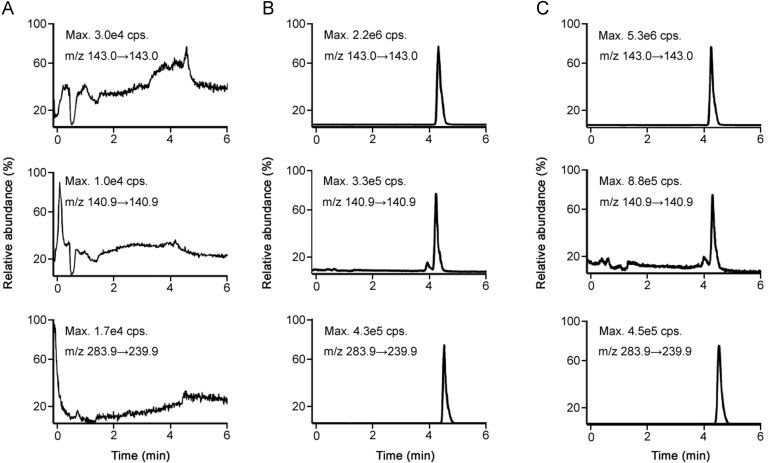
As shown in the chromatograms of drug-free plasma (Fig. 2A), no significant interfering peaks from the endogenous substances can be observed at the retention times of the analytes and IS. Fig. 2B shows the chromatograms of a calibration standard of the analytes and IS with a concentration of LLOQ. Fig. 2C shows the chromatograms of plasma samples from an epilepsy patient 12 h after continuous oral administration of 0.5 g sodium valproate with IS added. No interference from endogenous substances in plasma at the retention times of the analytes and IS was observed, suggesting the selectivity of the analytes for the developed method was satisfactory.
Fig. 2.
Representative chromatograms of VPA, 4-ene VPA and IS in human plasma. (A) blank plasma sample, (B) blank plasma sample spiked with VPA, 2-ene VPA, 4-ene VPA at LLOQ and IS at 0.50 μg/mL, and (C) an epilepsy patient's plasma sample collected 12 h after the oral administration of 0.5 g sodium valproate.
3.3.2. Cross-talk and carryover
ULOQ solutions of VPA, 2-ene VPA, 4-ene VPA and IS were injected successively, and no peaks were observed in other MS/MS channels at the retention times of the analytes and IS. No peaks of the analytes or IS from the blank sample subsequently injected after a ULOQ sample were observed, indicating no carryover from residues in rotary sampling/switching valves.
3.3.3. Linearity
The linearity of VPA was within a concentration range of 20–125 μg/mL with a typical regression curve of y=5.72×10–5 x+0.328 (r=0.9981). The calibration curve of 2-ene VPA was linear over the concentration range of 1.0−6.25 μg/mL with a typical regression curve of y=5.72×10–5x+0.328 (r=0.9981). The calibration range of 4-ene VPA was 0.02–0.125 μg/mL with a typical regression curve of y=6.02 ×10–4x+0.00762 (r=0.9984). It was sufficient for therapeutic drug monitoring based on oral administration while at LLOQ the accuracy and precision was less than 12%.
3.3.4. Precision and accuracy
The precision and accuracy data of the method are summarized in Table 1 which shows that the intra- and inter-day accuracy was ≤11.0% and ≤12.0%, respectively.
Table 1.
Intra- and inter-day accuracy and precision for determination of VPA, 2-ene VPA and 4-ene VPA (three days; six replicates for each day).
| Analytes | Concentration added (μg/mL) | Precision (RSD, %) |
Accuracy (RE, %) |
||
|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | ||
| VPA | 30 | 0.6 | 0.8 | 5.0 | 5.5 |
| 80 | 8.3 | 10.0 | 3.8 | 4.0 | |
| 100 | 5.2 | 11.0 | 2.0 | 3.2 | |
| 2-ene VPA | 1.5 | 1.4 | 2.0 | 4.0 | 5.0 |
| 4.0 | 2.7 | 3.6 | 5.0 | 5.4 | |
| 5.0 | 7.2 | 9.0 | 2.0 | 2.3 | |
| 4-ene VPA | 0.03 | 3.7 | 7.0 | 0.5 | 1.0 |
| 0.08 | 6.4 | 9.0 | 11.0 | 12.0 | |
| 0.10 | 6.0 | 10.0 | 5.2 | 6.3 |
3.3.5. Matrix effect
The matrix effect of the analytes and IS was 97.8%–100.1%, indicating that ion suppression or enhancement from the plasma matrix was negligible under the current conditions. The matrix effect is represented in Table 2.
Table 2.
Matrix effect and recovery for the determination of VPA, 2-ene VPA, 4-ene VPA and IS in human plasma.
| Analytes | Concentration (μg/mL) | Matrix effect (%) | Recovery (%) |
|---|---|---|---|
| VPA | 30 | 97.9 | 105.0 |
| 80 | 98.1 | 96.9 | |
| 100 | 99.0 | 101.5 | |
| 2-ene VPA | 1.5 | 100.1 | 105.2 |
| 4.0 | 99.4 | 97.0 | |
| 5.0 | 98.6 | 96.4 | |
| 4-ene VPA | 0.03 | 99.9 | 103.0 |
| 0.08 | 99.4 | 106.7 | |
| 0.1 | 97.8 | 101.4 | |
| IS | 0.5 | 98.4 | 98.1 |
3.3.6. Recovery
The recoveries of the analytes and IS were determined by comparing the peak areas of QC samples with those of the post-extraction spikes at the corresponding concentrations. The recoveries of the analytes and IS are shown in Table 2.
3.3.7. Stability
The stability of the analytes in human plasma was evaluated with QC samples stored for 60 days at −20 °C and 2 h at room temperature (25 °C) as well as after three freeze/thaw cycles. The stability of the analytes and IS in the mobile phase stored in autosampler vials at room temperature for 4 h was also evaluated. The result shows that the stability of the analytes is excellent and the data are summarized in Table 3.
Table 3.
Stability of VPA, 2-ene VPA and 4-ene VPA in human plasma under different storage conditions.
| Analytes | Concentration (μg/mL) | Long-term |
Post-preparation |
Freeze–thaw |
Bench-top |
||||
|---|---|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| VPA | 30 | 4.2 | 5.5 | 3.8 | 9.8 | 6.2 | 8.7 | 3.9 | 9.9 |
| 100 | 3.8 | 0.9 | 2.6 | 7.0 | 3.2 | 4.1 | 7.6 | 8.8 | |
| 2-ene VPA | 1.5 | 2.3 | 8.0 | 2.4 | 9.4 | 4.3 | 8.8 | 5.3 | 10.0 |
| 5.0 | 1.6 | 7.6 | 5.4 | 5.6 | 4.8 | 9.0 | 5.8 | 11.0 | |
| 4-ene VPA | 0.03 | 4.6 | 4.3 | 4.3 | 7.0 | 8.9 | 7.8 | 6.0 | 10.9 |
| 0.1 | 3.7 | 5.1 | 6.7 | 8.0 | 6.9 | 9.9 | 7.3 | 8.4 | |
3.4. Application to therapeutic drug monitoring
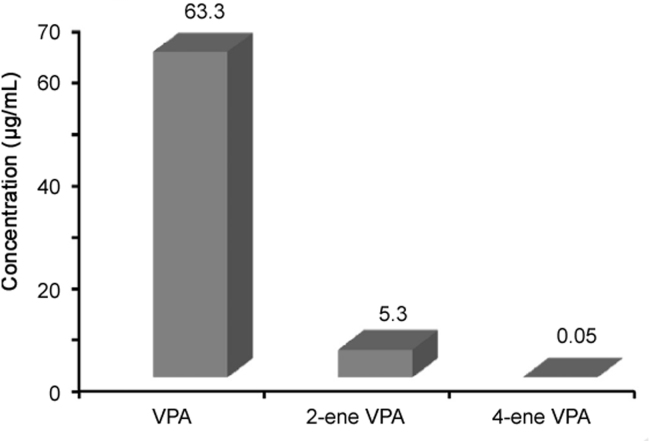
The full validated LC–MS/MS method was applied to determine VPA, 2-ene VPA and 4-ene VPA concentration in epilepsy patient plasma. The study was approved by the Ethical Committee of the First Hospital of Jilin University. Venous blood samples (4 mL) were collected into EDTA tubes at 8:00 a.m. before oral administration of Debakin (sodium valproate sustained release tablets, 0.5 g) from 60 epilepsy patients (mean age was 30 years old and mean body weight was 60 kg) with continuous oral administration of Debakin twice daily for three months. Blood samples were centrifuged at 3500 rpm for 5 min at 4 °C after collection, and the plasma was then transferred into 2 polypropylene tubes and stored at −20 °C until analysis. The average trough concentration in plasma was determined as 63.3±5.7, 5.3±0.4, 0.05±0.004 μg/mL, respectively (Fig. 3). The retention time of VPA, 2-ene VPA, 4-ene VPA and IS was 4.46, 4.38, 4.08 and 4.66 min, respectively.
Fig. 3.
Mean plasma trough concentrations of VPA (63.3±5.7 μg/mL), 2-ene-VPA (5.3±0.4 μg/mL) and 4-ene-VPA (0.050±0.0035 μg/mL) in epilepsy patients (n=60) after oral administration of Debakin at 0.5 g twice daily.
4. Conclusions
A rapid and sensitive LC–MS/MS assay for the simultaneous determination of VPA, 2-ene VPA and 4-ene VPA in epilepsy patient plasma after oral administration of a single dose of 0.5 g Debakin has been developed and validated. The major advantages of the method are low matrix effect, high recovery, small sample volume (20 μL) and the relative short run time for high throughput analysis for therapeutic drug monitoring. The developed LC–MS/MS method can be successfully applied to routine therapeutic monitoring for VPA, 2-ene VPA and 4-ene VPA in epilepsy patients.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Jingkai Gu, Email: gujk@mail.jlu.edu.cn.
Xiaohui Chen, Email: cxh_syphu@hotmail.com.
References
- 1.Glauser T., Menachem E.B., Bourgeois B. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094–1120. doi: 10.1111/j.1528-1167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 2.Glauser T., Menachem E.B., Bourgeois B. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54:551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- 3.Rettenmeier A.W., Gordon W.P., Barnes H. Studies on the metabolic fate of valproic acid in the rat using stable isotope techniques. Xenobiotica. 1987;17:1147–1157. doi: 10.3109/00498258709167407. [DOI] [PubMed] [Google Scholar]
- 4.Nau H., Löscher W. Valproic acid and metabolites: pharmacological and toxicological studies. Epilepsia. 1984;25:14–22. doi: 10.1111/j.1528-1157.1984.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 5.Kesterson J.W., Granneman G.R., Machinist J.M. The hepatotoxicity of valproic acid and its metabolites in rats.I. Toxicologic, biochemical and histopathologic studies. Hepatology. 1984;4:1143–1152. doi: 10.1002/hep.1840040609. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y., Feng Y., Jiang W. Determination of valproates in serum by TLC after derivatization. Chin. J. Hosp. Pharm. 2001;21:475–477. [Google Scholar]
- 7.Jing Y. Determination of CAR VAP by rat nephelometric inhibition immunoassay. Chin. J. Hosp. Pharm. 2001;21:519–520. [Google Scholar]
- 8.Tang F., Wang W., Li Y. Simultaneous determination of the concentrations of valproate and topiramate in human plasma by GC method. Chin. J. Hosp. Pharm. 2010;30:674–702. [Google Scholar]
- 9.Zhan X., Xu M. Determination of sodium valproate in human serum by HPLC. Chin. Pharm. 2009;18:25–26. [Google Scholar]
- 10.Li Z., Su X., Zheng F. Simultaneous determination of carbamazepineand sodium valproate in serum by RP-HPLC. Eval. Anal. Drug. Use. Hosp. Chin. 2010;10:150–151. [Google Scholar]
- 11.Zhang Z., Lei L., Li D. Effects of influence factors on derivation of serum concentration of sodium valproat by HPLC. Chin. Pharm. 2010;21:3204–3206. [Google Scholar]
- 12.Li S., Mao M., Li G. Determination of sodium valproate in serum by HPLC after precolumn derivatization. Chin. J. Hosp. Pharm. 2004;13:27–30. [Google Scholar]
- 13.He L., Yu J., Wu Z. Determination of the concentration of valproic acid in serum with RP-HPLC. Chin. Pharm. 2002;13:32–33. [Google Scholar]
- 14.Chen J., Fang W., Wu F. Determination of valproic acid in serum by high performance liquid chromatography. Chin. J. Clin. Med. 2000;9:296–298. [Google Scholar]
- 15.Zhu Y., Li H., Wang F. HPLC–MS/ESI determination of valproic acid in human serum. Chin. J. Pharm. Anal. 2005;25:817–819. [Google Scholar]
- 16.Z. Chen, Z. Fang, X. Wang, et al., The establishment of analytical methods to determinate sodium valproate in human plasma and its toxic metabolite by HPLC, The 12th National Congress of Clinical Pharmacology, Wuhan, 2010
- 17.Yang X., Chen G., Wang X. LC–ESI–MS determination of sodium valproate in human plasma. Chin. J. Health Lab. Technol. 2010;20:1356–1451. [Google Scholar]
- 18.S. Gao, H. Miao, W. Chen, LC–MS/MS method for simultaneous determination of valproic acid and majaor metabolites in human plasma, The 2011 Congress of Chinese pharmaceutical and 11th Chinese Medicine week, Yantai, 2011, pp. 1–17
- 19.Dziadosz M., Klintschar M., Teske J. Small molecule adduct formation with the components of the mobile phase as a way to analyse valproic acid in human serum with liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2014;959:36–41. doi: 10.1016/j.jchromb.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Wilimowska J. Analysis of variability of concentrations of valproic acid (VPA) and its selected metabolites in the blood serum of patients treated with VPA and patients hospitalized because of VPA poisoning. Arch. Med. Sadowej Kryminol. 2014;64:212–229. doi: 10.5114/amsik.2014.50527. [DOI] [PubMed] [Google Scholar]
- 21.FDA, Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CV), 2001.