Abstract
An electrochemical method based on a directly electrochemically reduced graphene oxide (ERGO) film coated on a glassy carbon electrode (GCE) was developed for the rapid and convenient determination of rutin in plasma. ERGO was modified on the surface of GCE by one-step electro-deposition method. Electrochemical behavior of rutin on ERGO/GCE indicated that rutin underwent a surface-controlled quasi-reversible process and the electrochemical parameters such as charge transfer coefficient (α), electron transfer number (n) and electrode reaction standard rate constant (ks) were 0.53, 2 and 3.4 s−1, respectively. The electrochemical sensor for rutin in plasma provided a wide linear response range of 4.70×10−7−1.25×10−5 M with the detection limit (s/n=3) of 1.84×10−8 M. The assay was successfully used to the pharmacokinetic study of rutin. The pharmacokinetic parameters such as elimination rate half-life (t1/2), area under curve (AUC), and plasma clearance (CL) were calculated to be 3.345±0.647 min, 5750±656.0 µg min/mL, and 5.891±0.458 mL/min/kg, respectively. The proposed method utilized a small sample volume of 10 μL and had no complicated sample pretreatment (without deproteinization), which was simple, eco-friendly, and time- and cost-efficient for rutin pharmacokinetic studies.
Keywords: Electrochemically reduced graphene oxide, Pharmacokinetics, Rat plasma, Rutin
1. Introduction
Rutin (3′, 4′, 5, 7-tetrahydroxy-flavone-3-rutinoside) [1], the glycoside between the flavonol quercetin and the disaccharide rutinose, is a common dietary flavonoid that is consumed in fruits, vegetables and plant-derived beverages such as tea and wine [2]. Some related investigations show that rutin exhibits a broad range of physiological activities, such as anti-inflammation [3], anti-tumor [4], immunoregulation [5], antioxidation [6] and anti-depression [7]. So it is often used as a therapeutical drug. However, quercetin, the metabolite of rutin, has been identified in the blood of animals following administration of rutin [8]. Hence, it is necessary to develop some simple, economical and efficient methods for the determination of rutin and distinguish it from quercetin in biological samples.
Numerous analytical techniques have been developed to quantify rutin in plasma, including high performance liquid chromatography (HPLC) [9], liquid chromatography–mass spectrometry (LC–MS) [10], solid phase extraction-high performance liquid chromatography-diode array detection (SPE–HPLC–DAD) [11] and ultra performance liquid chromatography–electrospray ionization–tandem mass spectrometry (UPLC–ESI–MS/MS) [12], and the limit of detection (LOD) is in the range of 1–10 ng/mL. However, the drawback of these methods is that large plasma volume (0.2–1.0 mL) is required. To minimize the volume of blood sampling, drug assays requiring smaller sample volumes are essential. Moreover, these methods have another drawback of complicated sample extraction process which limits the ease of their use [13], [14]. Sample pretreatment often needs high-purity organic solvents and nitrogen evaporation step, which are time-consuming and environmental-unfriendly [15].
Compared with these methods, electrochemical methods have been proven to be a powerful tool to get rutin information in complex samples due to their high sensitivity, easy operation and low sample volume [16], [17]. Previous studies have investigated the electrochemical behavior of rutin and achieved quantitative determination of rutin using several working electrodes such as multi-wall carbonnanotube paste electrode [18], β-cyclodextrin incorporated carbon nanotube-modified electrode [19], a sensor based on β-cyclodextrin@chemically reduced graphene/nafion composite film [20], acetylene black nanoparticles-modified electrode [21], grapheme modified carbon ionic liquid electrode [22], and graphene nanosheets modified glassy carbon electrode [23]. The performance of the modified electrodes depends on the properties of the modifiers that affect the selectivity and sensitivity of these electrodes to rutin.
Graphene has attracted tremendous attention in the past several years after its first isolation [24]. It exhibits great promises in various aspects owing to its unique properties such as high electron mobility [25], excellent mechanical strength [26], outstanding thermal conductivity [27] and large specific surface area [28], [29]. Nowadays, a novel method, preparing graphene by electrochemical reduction of the exfoliated graphene oxide (GO), has and garnered particular attention. The electrochemically reduced graphene oxide (ERGO) to reduced graphene oxide (RGO) [30], [31], as an alternative to chemical, photocatalytic, and thermal reduction techniques, can be a simple, economic, and environmentally friendly method. And graphene films with desirable thickness can be fabricated on electrode substrates by adjusting the electrode potential [3], [32].
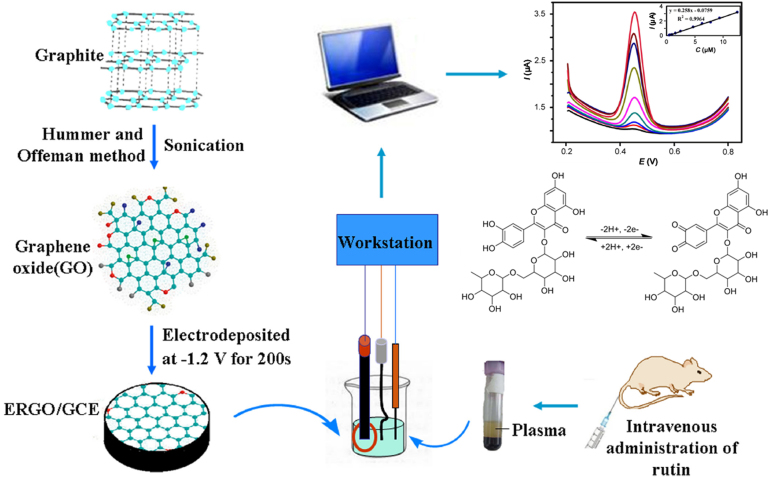
Based on these, this study developed an electrochemical method based on ERGO to facilitate pharmacokinetic studies of rutin. The electrochemical behavior of rutin on ERGO/GCE was investigated and the values of pH and accumulation time were optimized. Then ERGO/GCE was applied to determine rutin in 10 μL of plasma obtained from rats after intravenous administration of rutin. The pharmacokinetic parameters were calculated. For comparison, these results were validated by HPLC. The procedure is shown in Scheme 1.
Scheme 1.
The preparation of ERGO/GCE and its application to the pharmacokinetic study of rutin in rats.
2. Materials and methods
2.1. Reagents
Graphite powder (KS-10, 99.5%) was from Sigma (USA). 0.2 M phosphate buffer solution (PBS) was prepared by Na2HPO4 and NaH2PO4. Acetonitrile of HPLC grade was purchased from Oceanpak (Sweden). Ultrapure water was generated from the Milli-Q system (Millipore, Bedford, MA, USA). All other reagents were of analytical grade.
2.2. Apparatus
All electrochemical measurements were carried out on a CHI 1220A electrochemical workstation (Shanghai CH Instrument, China). A three-electrode system was employed with a bare or modified GCE as the working electrode, a platinum wire (Pt) as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The pH measurement was performed via a pHs-3B digital pH-meter (Shanghai Precision Scientific Instrument, China).
2.3. Preparation of graphene oxide and fabrication of the modified electrodes
Graphite oxide (GO) was prepared from graphite powder by a modified method reported by Hummers and Offeman [33]. Preparation of graphene oxide and fabrication of the modified electrodes were performed as described in our previous paper [34]. In brief, before modification, the bare GCE was first polished to a mirror-like surface with 0.3 μm and 0.05 μm of alumina slurry and then sonicated for 1 min in methanol and water successively. After the surface was dried by nitrogen, exfoliated GO (3 mg/mL) was electrodeposited at room temperature at a constant potential of −1.2 V for 200 s by a CHI 1220A electrochemical workstation under computer control to obtain the ERGO/GCE.
2.4. Plasma samples preparation
Six male Wistar rats weighing 220–260 g were acquired from the Experimental Animal Center of Lanzhou University (Lanzhou, China). Following intravenous administration of rutin via caudal vein at the dose of 26.67 mg/kg, blood samples were collected via the fossa vein orbitalis into the heparinized microcentrifuge tubes at 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 20.0, 30.0, 60.0, and 120.0 min, and centrifuged. The supernatant was collected and stored at −20 °C until analysis.
A series of rutin alcohol solutions were added to the blank plasma, and the mixed samples were stirred and vibrated for 2 min, and then centrifuged. The supernatant was collected and stored at −20 °C for establishing the calibration curve.
2.5. Electrochemical measurements
Electrochemical behavior of rutin on ERGO/GCE was recorded by cyclic voltammetry (CV) from −0.2 V to 0.8 V in PBS at a scan rate of 100 mV/s. Under the optimal conditions, the prepared electrodes were first immersed in the PBS containing rutin plasma for 9 min. Next, differential pulse voltammetry (DPV) was recorded from 0.2 V to 0.8 V at a scan rate of 100 mV/s.
2.6. Validation by HPLC
HPLC measurements were carried out on a Waters Alliance 2695 HPLC system (America) equipped with a 2998 Photo-Diode Array. Chromatographic separations were performed using a Diamonsil C18 (250 mm×4.6 mm, 5 μm), connected with a C18 guard column (10 mm×4.6 mm, 5 μm). A linear gradient elution of eluent A (acetonitrile) and B (0.5% acetic acid; v/v) was used for the separation. The elution program was performed as follows: 19% A at 0–10 min, 19%–50% A at 10–20 min, 50%–80% A at 20–30 min, and 80%–100% A at 30–40 min.
3. Results and discussion
3.1. Characterization of ERGO/GCE
The morphology and microstructure of the modified electrodes were characterized by scanning electron microscopy (SEM) and Raman spectroscopy. The SEM image of ERGO/GCE showed that a few layers of ERGO were partially packed with their basal planes, intensely crumpled, and folded into a typical wrinkled structure after electrochemical reduction occurred. The corrugations in ERGO can cause electron transfer rates 10-fold faster than those at the basal plane of graphite. Raman spectra showed that the bands of ERGO were strongly strengthened compared with those of GO, indicating the restored ordered crystal structure of ERGO and the smaller sp2 domains in ERGO sheets. As a result, the communication between the ERGO-active electrode and the redox species was improved [34].
3.2. Electrochemical behavior of rutin
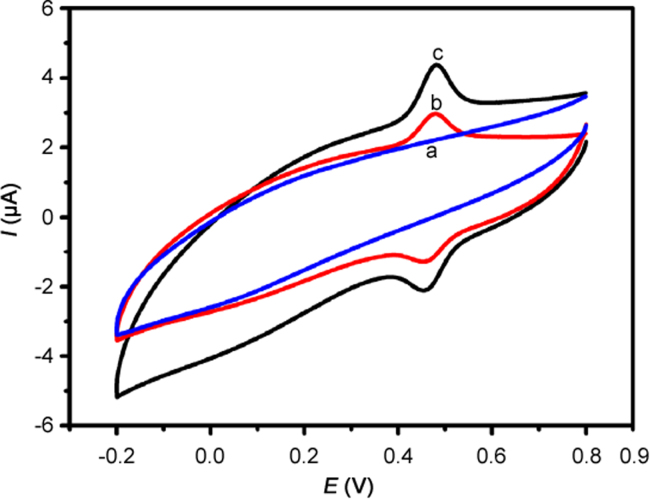
The electrochemical responses of rutin on bare GCE and ERGO/GCE were examined using CV in PBS at pH 3.0. As shown in Fig. 1, no redox peaks were observed on ERGO/GCE in the blank PBS (curve a), indicating that the proposed sensor was non-electroactive in the selected scanning potential region. After a certain amount of rutin was added into the blank PBS, the bare GCE (curve b) showed a pair of relatively poor redox peaks at 0.466 V and 0.482 V while the redox peaks currents of ERGO/GCE significantly increased (curve c). These results may be attributed to ERGO, as ERGO can provide a large specific surface area to increase the loading amount of rutin on the electrode surface. Meanwhile, ERGO can also accelerate the electron transfer rate on electrode surface and amplify the electrochemical signals as a result of its effective electrical conductivity.
Fig. 1.
CVs of ERGO/GCE (a) without rutin, and bare GCE (b), and ERGO/GCE (c) with 2.0×10−5 M rutin in 0.2 M PBS (pH 3.0) at 100 mV/s.
3.3. Optimization of experimental conditions
3.3.1. Influence of pH
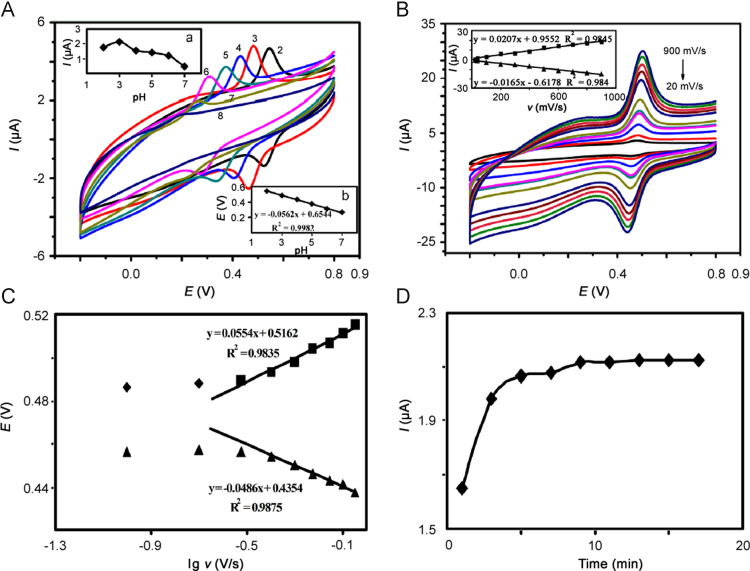
The influence of pH on the cyclic voltammetric responses of 1.0×10−5 M rutin on ERGO/GCE was investigated in the pH range from 2.0 to 8.0 with the voltammograms (Fig. 2A). It could be seen that a pair of well-defined redox peaks appeared and the redox peaks potentials shifted to the negative direction with the increase of buffer pH, indicating that protons took part in the electrode reaction. The redox peaks potentials (E0′) changed linearly with pH values from 2.0 to 8.0 (inset of Fig. 2A), and the linear regression equation was E0′ (V)=− 0.0562 pH+0.6544 with R2=0.9982. The slope of −0.0562 V/pH was close to theoretical value of −0.059 V/pH, indicating that the ratio of electrons and protons involved in the electrochemical reaction was 1:1. The peaks currents increased until pH 3.0 and then decreased. However, when the pH was over 7.0, the redox peaks became very small. These experimental phenomena were related to the protons involved in the electrochemical reaction. Therefore, pH 3.0 was selected as the optimal pH for detection in the following experiments.
Fig. 2.
(A) CVs of ERGO/GCE in PBS containing 1.0×10−5 M of rutin with different pH values (2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0). (a) Plot of peaks currents of rutin (Ip) vs pH. (b) The linear relationship between the anodic peaks potentials and pH. (B) CVs of 1.0×10−5 M rutin on ERGO/GCE with different scan rates (20, 40, 100, 200, 300, 400, 500, 600, 700, 800, 900 mV/s, respectively) in PBS. Inset shows linear relationship between scan rates (v) and redox peaks currents (Ip). (C) Linear relationship between the redox peaks potentials (Ep) and lgv. (D) The relationship between accumulation time (t) and peaks currents (Ip).
3.3.2. Influence of scan rate
Useful information usually can be acquired from the relationship between the peaks currents and scan rates. CVs of ERGO/GCE were applied to study the electrochemical mechanism in PBS containing 1.0×10−5 M rutin at different scan rates. The redox peaks currents increased with the increase of scan rates in the range of 20–900 mV/s (Fig. 2B), accompanied with the shift of the redox peaks potentials. Both the cathodic and anodic peaks currents of rutin were linearly proportional to the scan rates ranging from 20 mV/s to 900 mV/s. The regression equations were Ipa (μA)=0.0207 v (mV/s)+0.9552 and Ipc (μA)=−0.0165 v (mV/s) − 0.6178 with R2=0.9845 and 0.9840, respectively, which indicated an adsorption controlled electrode process.
In addition, as shown in Fig. 2C, at the high scan rates, the redox peaks potentials and lgv exhibited a good linear relationship with the linear regression equations as follows: Epa (V)=0.0554lgν (V/s)+0.5162 and Epc (V)=−0.0486lgν (V/s)+0.4354 with R2=0.9835 and 0.9875, respectively. According to Laviron’s equations [35],
| (1) |
| (2) |
| (3) |
where α is the charge transfer coefficient, n is the electron transfer number, ks is the electrode reaction standard rate constant, R is the gas constant, T is the absolute temperature, and ΔEp is the peak-to-peak separation. The value of α, n and ks were calculated as 0.53, 2 and 3.4 s−1, respectively. Hence, the electro-oxidation reaction of rutin on the ERGO/GCE was a two-electron two-proton process, which was in agreement with Ref. [20].
3.3.3. Influence of accumulation time
Accumulation can improve the amount of rutin adsorbed on the electrode surface, and then improve determination sensitivity and decrease detection limit. Thus, the effect of the accumulation time on the proposed electrode response was investigated for 1.0×10−5 M rutin in 0.2 M PBS at pH 3.0. As shown in Fig. 2D, the peaks currents increased gradually from 0 min to 9 min, and then were almost constant. The peaks currents increased with the accumulation time because more rutin was adsorbed on the electrode surface. The peaks currents kept constant after 9 min, which could be explained by the saturated adsorption of rutin on the electrode surface. Therefore, 9 min was employed as the optimal accumulation time.
3.4. Method validation
3.4.1. Matrix effect
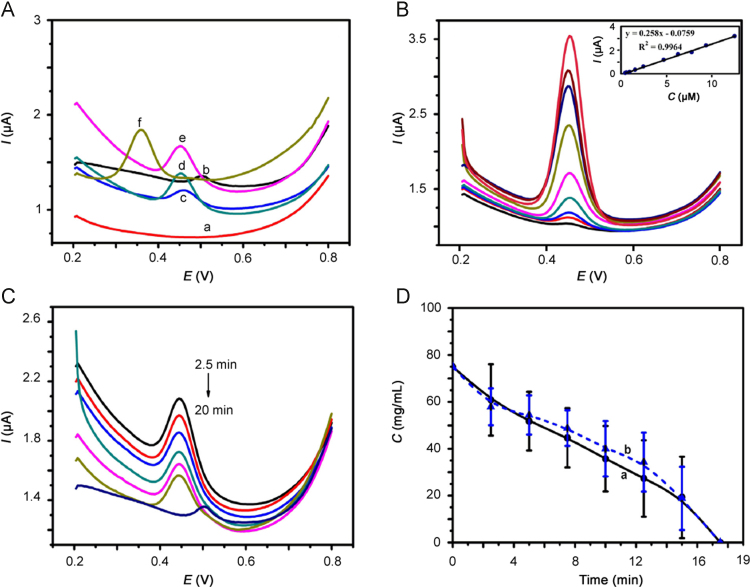
The feasibility of the method was evaluated by comparing the DPV curves of different plasma samples. As depicted in Fig. 3A, in the absence of rutin, no current peaks could be observed in PBS (curve a) while a current peak appeared at 0.50 V in the potential window after the addition of the blank plasma (curve b). In the presence of rutin standard solution (curve c), plasma spiked with rutin (curve d) or actual plasma sample (curve e), well-defined peaks at the peak potentials of 0.45 V were observed, which was ascribed to the redox reaction of rutin. From the results above, we concluded that there was a weak endogenous peak at 0.50 V in blank plasma, but it did not interfere the determination of rutin. In order to further verify whether the sensor could distinguish rutin from quercetin (the metabolism of rutin) or not, the spiked quercetin plasma (curve f) was investigated and the current peak appeared at 0.36 V, which was different from that of rutin. Thus, the proposed sensor could be applied to the pharmacokinetic study of rutin in plasma.
Fig. 3.
(A) DPV of rutin in different matrices on ERGO/GCE (a. PBS. b. blank plasma. c. rutin standard solution. d. spiked rutin plasma. e. actual plasma sample obtained from rat after intravenous administration of rutin. f. spiked quercetin plasma). (B) DPV of ERGO/GCE in PBS (0.2 M, pH 3.0) with various concentrations of spiked rutin plasma solutions. Inset: concentration calibration curve of the DPV currents response for rutin. (C) DPV of ERGO/GCE in PBS (0.2 M, pH 3.0) containing plasma samples that were obtained at different time (2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 20.0 min) from rats after intravenous administration of rutin. (D) Mean plasma concentration–time curves of rutin in rats determined by ERGO/GCE (curve a) and HPLC (curve b) (n=6).
3.4.2. Calibration curve
To study the analytical performance of the obtained electrochemical sensor, DPV was explored for the amperometric responses of spiked rutin standard plasma solutions. Fig. 3B shows the typical DPV obtained from different rutin concentrations. Under the optimal conditions, the peaks currents had a good linear relationship with rutin concentrations in the range of 4.70×10−7–1.25×10−5 M, as shown in the inset of Fig. 3B. The linear regression equation was I (μA)=0.258 c (μM)−0.0759 with R2=0.9964. The detection limit (s/n=3) was estimated at approximately 1.84×10−8 M. The comparison of the analytical performance of ERGO/GCE with other electrochemical methods [18], [19], [20], [21], [22], [23], [36], [37], [38] for rutin determination is summarized and listed in Table 1.
Table 1.
Comparison of electrochemical methods for rutin detection.
| Electrodes | Linear range (M) | Detection limit (M) | Refs. |
|---|---|---|---|
| MWNTs/β-CD | 4.0×10−7–1.0×10−3 | 2.0×10−7 | [19] |
| CNTPE | 1.0×10−7–1.0×10−8 | 4.0×l0−8 | [18] |
| AB nanoparticle/GCE | 3.1×10−8–7.8×10−6 | 1.5×10−8 | [21] |
| GR/CILE | 7.0×10−8–1.0×10−4 | 2.4×10−8 | [22] |
| β-CD@CRG/Nafion/GCE | 6.0×10−9–1.0×10−5 | 2.0×10−9 | [20] |
| GNs/GCE | 1.0×10−7–1.0×10−5 | 2.1×10−8 | [23] |
| GR-MnO2/CILE | 1.0×10−8–5.0×10−4 | 2.7×10−9 | [36] |
| SWCNTs/Au electrode | 2.0×10−8–1.0×10−6 | 1.0×10−8 | [37] |
| SWCNTs/CILE | 1.0×10−7–1.7×10−6 | 7.0×10−8 | [38] |
| ERGO/GCE | 4.7×10−7–1.2×10−5 | 1.8×10−8 | This work |
MWNTs: multi-walled carbon nanotubes. β-CD: β-cyclodextrin. CNTPE: multi-wall carbonnanotube paste electrode. AB nanoparticle: acetylene black nanoparticle-modified electrode. GCE: glassy carbon electrode. GR: grapheme. CILE: carbon ionic liquid electrode. β-CD@CRG: β-cyclodextrin@chemically reduced grapheme. GNs: graphene nanosheets. SWCNTs: single-walled carbon nanotube. ERGO: electrochemically reduced graphene oxide.
3.4.3. Reproducibility and specificity study
Six modified electrodes were fabricated independently, which gave a satisfactory average relative standard deviation (RSD) value of 3.90% for the detection of 1.0×10−5 M rutin solution, indicating the good reproducibility of the electrode fabrication. The specificity of the sensor was studied through the interference of some foreign species on the determination of rutin under the optimized conditions. PBS containing 1.0×10−6 M rutin and different quantities of interferents were prepared to record the DPV. The results are shown in Table 2. Under the existence of those interferents, the recoveries of rutin changed between 95.14% and 104.76%, which displayed the species caused no interference at high concentrations.
Table 2.
Interference of different species on rutin determination by ERGO/GCE.
| Interferents | Concentration (M) | Recovery (%) |
|---|---|---|
| Na+ | 1.005×10−4 | 104.76 |
| K+ | 1.005×10−4 | 99.15 |
| Cl− | 1.005×10−4 | 99.15 |
| SO42− | 1.005×10−4 | 95.38 |
| NO3− | 1.005×10−4 | 95.14 |
| Acetyl aminophenol | 1.005×10−4 | 97.45 |
| Citric acid | 1.005×10−4 | 103.87 |
| Glucose | 1.005×10−4 | 99.23 |
| L-glutamic acid | 5.025×10−5 | 96.31 |
| L-glycine | 5.025×10−5 | 95.65 |
| Ascorbic acid | 5.025×10−5 | 102.49 |
| Quercetin | 1.507×10−5 | 101.75 |
| Puerarin | 1.507×10−5 | 102.13 |
| Baicalin | 1.507×10−5 | 104.52 |
| Dopamine | 1.507×10−5 | 104.21 |
| Folic acid | 1.507×10−5 | 97.76 |
3.4.4. Accuracy, inter-day and intra-day precision
The accuracy of this proposed method was evaluated by determination of three parallel spiked rutin plasma solutions (38.20 μg/mL). As shown in Table 3, the results were satisfactory with the recovery varying from 97.98% to 103.69%, showing that the proposed sensor could be efficiently applied to the detection of rutin with good accuracy. The intra-day precision was estimated by determining three spiked rutin plasma solutions (5.73, 22.92 and 42.02 μg/mL) with three replicate measurements in the same run. The RSDs of the measurements were 5.96%, 2.20% and 2.11%, respectively. Similarly, the RSDs of the inter-day precision were 6.50%, 5.04% and 4.59%, respectively (Table 4). Both the RSDs of inter-day and intra-day precisions were less than 15%, indicating that the prepared sensor was applied to determine rutin with a sufficient precision [12], [39].
Table 3.
Recovery of rutin detection in plasma using ERGO/GCE (n=3).
| No. | Spiked amount (μg/mL) | Detected amount (μg/mL) | Recovery (%) |
|---|---|---|---|
| 1 | 38.20 | 39.00 | 102.09 |
| 2 | 38.20 | 37.43 | 97.98 |
| 3 | 38.20 | 39.61 | 103.69 |
| Average | 38.20 | 38.68 | 101.25 |
Table 4.
Intra-day and inter-day precision for rutin determination in plasma using ERGO/GCE (Mean±SD, n=3).
| Added conc. (μg/mL) | Intra-day |
Inter-day |
||
|---|---|---|---|---|
| Detected conc. (μg/mL) | RSD (%) | Detected conc. (μg/mL) | RSD (%) | |
| 5.73 | 5.95±0.35 | 5.96 | 5.58±0.36 | 6.50 |
| 22.92 | 21.00±0.46 | 2.20 | 20.19±1.02 | 5.04 |
| 42.02 | 42.72±0.90 | 2.11 | 44.50±2.04 | 4.59 |
RSD: relative standard deviation.
3.5. Pharmacokinetic study of rutin in rats
On the optimization of electrochemical parameters, determination of rutin in rat plasma samples (10 μL) was performed according to the procedures for treatment of plasma samples and electrochemistry measurement described in Sections 2.4 and 2.5. As depicted in Fig. 3C, there were current peaks of rutin at 0.45 V only in the plasma samples that were collected at 0–15 min after injection. In comparison, HPLC was employed to the determination of rutin in actual plasma samples. The mean plasma concentration–time curves of rutin in plasma determined by ERGO/GCE (curve a) and HPLC (curve b) are shown in Fig. 3D. The pharmacokinetic parameters were calculated using PKSOLN 2.0 and are summarized in Table 5. There was no significant difference between the results given by the two methods, that was, the proposed method could be potentially useful for determination of rutin in biological samples. Urine and feces samples were also determined by ERGO/GCE or HPLC, but there was no rutin in these samples.
Table 5.
Pharmacokinetic parameters of rutin in rats after intravenous administration of rutin determined by ERGO/GCE and HPLC (n=6).
| Pharmacokinetic parameter | Methods |
|
|---|---|---|
| ERGO/GCE | HPLC | |
| Ke (min−1) | 0.223±0.083 | 0.240±0.062 |
| t1/2 (min) | 3.345±0.647 | 2.881±0.351 |
| AUC (µg min/mL) | 5750±656.0 | 6294±52.01 |
| MRT (min) | 1.367±0.098 | 0.934±0.082 |
| Vd (mL/kg) | 31.90±0.864 | 17.61±0.923 |
| CL (mL/min/kg) | 5.891±0.458 | 4.230±0.676 |
Ke: elimination rate constant.
t1/2: half-life of drug elimination during the terminal phase.
AUC: area under the plasma mean concentrations–time curve.
MRT: mean retention time.
Vd: volume of distribution.
CL: plasma clearance.
4. Conclusions
In the present study, a rapid and convenient electrochemical method based on ERGO/GCE had been developed and fully validated for the pharmacokinetic study of rutin in plasma. The proposed sensor displayed satisfactory analytical performance with wide linear range, low detection limit and excellent specificity to rutin detection.
The electrochemical method described here used a much smaller plasma volume (10 μL) and had no complicated sample extraction processes (without deproteinization), which was suitable for pharmacokinetic studies of rutin. The pharmacokinetic parameters of rutin calculated were in agreement with these of HPLC. This research provides a promising way for investigation of the pharmacokinetics of rutin and for its utilization in clinical practice.
Acknowledgments
The authors acknowledge the support of the Project of Science and Technology Agency of Gansu (No.1208RTZA211) and Lanzhou (Nos. 2012-2-67 and 2013-4-75)
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Fang-Di Hu, Email: hufd@lzu.edu.cn.
Wang-Hong Zhao, Email: wanghong_zhao@sina.com.
References
- 1.Moghbelinejad S., Nassiri-Asl M., Farivar T.N. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 2014;224:108–113. doi: 10.1016/j.toxlet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Pashikanti S., de Alba D.R., Boissonneault G.A. Rutin metabolites: novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Bio. Med. 2010;48:656–663. doi: 10.1016/j.freeradbiomed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Umar S., Mishra N.K., Kaushal P. Protective effect of rutin in attenuation of collagen-induced arthritis in Wistar rat by inhibiting inflammation and oxidative stress. Indian J. Rheumatol. 2012;7:191–198. [Google Scholar]
- 4.Ren W., Qiao Z., Wang H. Flavonoids: promising anticancer agents. Med. Res. Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 5.Xu P.X., Wang S.W., Yu X.L. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Abeta oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014;264:173–180. doi: 10.1016/j.bbr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Guo R., Wei P. Studies on the antioxidant effect of rutin in the microenvironment of cationic micelles. Microchim. Acta. 2007;161:233–239. [Google Scholar]
- 7.Daniele G.M., Luis E.B.B., Mauricio P.C. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008;587:163–168. doi: 10.1016/j.ejphar.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Miao D., Li J., Yang R. Supersensitive electrochemical sensor for the fast determination of rutin in pharmaceuticals and biological samples based on poly(diallyldimethylammonium chloride)-functionalized graphene. J. Electroanal. Chem. 2014;732:17–24. [Google Scholar]
- 9.Kuntic V., Pejic N., Ivkovic B. Isocratic RP-HPLC method for rutin determination in solid oral dosage forms. J. Pharm. Biomed. Anal. 2007;43:718–721. doi: 10.1016/j.jpba.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 10.He J., Feng Y., Ouyang H.Z. A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: application to a pharmacokinetic study of total flavonoids from mulberry leaves. J. Pharm. Biomed. Anal. 2013;84:189–195. doi: 10.1016/j.jpba.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Zeng H.J., Yang R., Guo C. Pharmacokinetic study of six flavones in rat plasma and tissues after oral administration of ‘JiangYaBiFeng’ using SPE-HPLC-DAD. J. Pharm. Biomed. Anal. 2011;56:815–819. doi: 10.1016/j.jpba.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Xu M., Yu C. Simultaneous determination of vitexin-4”-O-glucoside, vitexin-2”-O-rhamnoside, rutin and vitexin from hawthorn leaves flavonoids in rat plasma by UPLC-ESI-MS/MS. J. Chromatogr. B. 2010;878:1837–1844. doi: 10.1016/j.jchromb.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X., Bi K., Wang X. A UFLC-MS/MS method coupled with one-step protein precipitation for determination of docetaxel in rat plasma: comparative pharmacokinetic study of modified nanostructured lipid carrier. J. Pharm. Biomed. Anal. 2013;83:202–208. doi: 10.1016/j.jpba.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 14.D’Avila F.B., Pereira A.G., Salazar F.R. Determination of cocaine/crack biomarkers in colostrum by LC–MS following protein precipitation. J. Pharm. Biomed. Anal. 2014;103C:67–72. doi: 10.1016/j.jpba.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Sun J., Lian H. Determination of paclitaxel in hyaluronic acid polymeric micelles in rat blood by protein precipitation-micelle breaking method: application to a pharmacokinetic study. J. Chromatogr. B. 2013;935:10–15. doi: 10.1016/j.jchromb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Gupta V.K., Jain R., Radhapyari K. Voltammetric techniques for the assay of pharmaceuticals--a review. Anal. Biochem. 2011;408:179–196. doi: 10.1016/j.ab.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Hu X., Dou W., Fu L. A disposable immunosensor for Enterobacter sakazakii based on an electrochemically reduced graphene oxide-modified electrode. Anal. Biochem. 2013;434:218–220. doi: 10.1016/j.ab.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Lin X.Q., He J.B., Zha Z.G. Simultaneous determination of quercetin and rutin at a multi-wall carbon-nanotube paste electrodes by reversing differential pulse voltammetry. Sens. Actuators B-Chem. 2006;119:608–614. [Google Scholar]
- 19.He J.L., Yang Y., Yang X. β-Cyclodextrin incorporated carbon nanotube-modified electrode as an electrochemical sensor for rutin. Sens. Actuators B-Chem. 2006;114:94–100. [Google Scholar]
- 20.Liu K., Wei J., Wang C. Sensitive detection of rutin based on β-cyclodextrin@chemically reduced graphene/Nafion composite film. Electrochim. Acta. 2011;56:5189–5194. [Google Scholar]
- 21.Song J., Yang J., Zeng J. Acetylene black nanoparticle-modified electrode as an electrochemical sensor for rapid determination of rutin. Microchim. Acta. 2010;171:283–287. [Google Scholar]
- 22.Gao F., Qi X., Cai X. Electrochemically reduced graphene modified carbon ionic liquid electrode for the sensitive sensing of rutin. Thin Solid Films. 2012;520:5064–5069. [Google Scholar]
- 23.Du H., Ye J., Zhang J. Graphene nanosheets modified glassy carbon electrode as a highly sensitive and selective voltammetric sensor for rutin. Electroanalysis. 2010;22:2399–2406. [Google Scholar]
- 24.Novoselov K.S., Geim A.K., Morozov S.V. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 25.Nikolakopoulou A., Tasis D., Sygellou L. Dispersion of graphene in organic solvents and their use for improving efficiency of dye- and quantum dot-sensitized solar cells. Electrochim. Acta. 2014;139:54–60. [Google Scholar]
- 26.Zan W. Chemical functionalization of graphene by carbene cycloaddition: a density functional theory study. Appl. Surf. Sci. 2014;311:377–383. [Google Scholar]
- 27.Zhang Y., Ma H.L., Zhang Q. Facile synthesis of well-dispersed graphene by γ-ray induced reduction of graphene oxide. J. Mater. Chem. 2012;22:13064. [Google Scholar]
- 28.Khoh W.H., Hong J.D. Solid-state asymmetric supercapacitor based on manganese dioxide/reduced-graphene oxide and polypyrrole/reduced-graphene oxide in a gel electrolyte. Colloids Surf. A. Physicochem. Eng. Asp. 2014;456:26–34. [Google Scholar]
- 29.Zhang B., Zhang Y., Peng C. Preparation of polymer decorated graphene oxide by gamma-ray induced graft polymerization. Nanoscale. 2012;4:1742–1748. doi: 10.1039/c2nr11724j. [DOI] [PubMed] [Google Scholar]
- 30.Li M., Bo X., Mu Z. Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically reduced graphene oxide film as a nonenzymatic glucose sensor. Sens. Actuators B-Chem. 2014;192:261–268. [Google Scholar]
- 31.Peng S., Zou G., Zhang X. Nanocomposite of electrochemically reduced graphene oxide and gold nanoparticles enhanced electrochemilunescence of peroxydisulfate and its immunosensing abililty towards human IgG. J. Electroanal. Chem. 2012;686:25–31. [Google Scholar]
- 32.Ping J., Wang Y., Wu J. Development of an electrochemically reduced graphene oxide modified disposable bismuth film electrode and its application for stripping analysis of heavy metals in milk. Food Chem. 2014;151:65–71. doi: 10.1016/j.foodchem.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Hummers W.S., Offeman R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80:1339. [Google Scholar]
- 34.Liu L.J., Gou Y.Q., Gao X. Electrochemically reduced graphene oxide-based electrochemical sensor for the sensitive determination of ferulic acid in A. sinensis and biological samples. Mater. Sci. Eng. C. 2014;42:227–233. doi: 10.1016/j.msec.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 35.Laviron E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfac. Electrochem. 1979;101:19–28. [Google Scholar]
- 36.Sun W., Wang X., Zhu H. Graphene-MnO2 nanocomposite modified carbon ionic liquid electrode for the sensitive electrochemical detection of rutin. Sens. Actuators B-Chem. 2013;178:443–449. [Google Scholar]
- 37.Zeng B., Wei S., Xiao F. Voltammetric behavior and determination of rutin at a single-walled carbon nanotubes modified gold electrode. Sens. Actuators B-Chem. 2006;115:240–246. [Google Scholar]
- 38.Zhu Z., Sun X., Zhuang X. Single-walled carbon nanotubes modified carbon ionic liquid electrode for sensitive electrochemical detection of rutin. Thin Solid Films. 2010;519:928–933. [Google Scholar]
- 39.Di X., Wang X., Di X. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J. Pharm. Biomed. Anal. 2015;115:144–149. doi: 10.1016/j.jpba.2015.06.027. [DOI] [PubMed] [Google Scholar]