Abstract
The objective of this study was to evaluate the free radical scavenging potential and high performance thin layer chromatography (HPTLC) fingerprinting of Indigofera tinctoria (I. tinctoria). Phytochemical analysis was carried out using standard methods, and free radical scavenging activity of the plant was determined using 2,2-diphenyl-1-picrylhydrazy (DPPH), nitric oxide (NO) and superoxide anion () radical scavenging capacities. HPTLC plate was kept in CAMAG TLC Scanner 3 and the Rf values at fingerprint data were recorded by WINCATS software. Aqueous extract of I. tinctoria reliably showed the total phenolics (267.2±2.42 mg/g), flavonoids (75.43±3.36 mg/g) and antioxidants (349.11±8.04 mg/g). The extract was found to have DPPH (52.08%), NO (23.12%) and (26.79%) scavenging activities at the concentration of 250 μg/mL and the results were statistically significant compared with ascorbic acid standard (p<0.05). HPTLC results confirmed that the extract contained several potential active components such as phenols, flavonoids, saponins and terpenoids as the slides revealed multi-colored bands of varying intensities. This study confirmed that the plant had multipotential antioxidant and free radicals scavenging activities.
Keywords: Medicinal plants, Indigofera tinctoria, Antioxidants, HPTLC
1. Introduction
Oxidative damage is a critical etiological factor implicated in several chronic human diseases including cardiovascular dysfunction, inflammation, atherosclerosis, carcinogenesis, drug poisonousness, reperfusion damage and neurodegenerative diseases. Antioxidants play an important role in the prevention of chronic ailments such as heart diseases, cancer, diabetes, hypertension, stroke and Alzheimer׳s disease by protecting the cells from damage caused by free radicals, highly reactive oxygen compounds [1]. Thus, antioxidant compounds can be used to counteract oxidative damage by reacting with free radicals, chelating free catalytic metals and also by acting as oxygen scavengers [2]. Antioxidants play an important role in inhibiting and scavenging free radicals, thus providing protection against infections and degenerative diseases [3]. Plants contain several phytochemicals which possess strong antioxidant activities [4]. Medicinal herbs have a long history in improving human health and curing various diseases. India has an extensive rich heritage of herbal medicine since the time of Ayurveda with medicinal properties. Historically, the medicinal values of plants were tested by trial and error, as in the Doctrine of Signatures [5]. Herbal medicines recently have attracted much attention as alternative medicines. Various medicinal properties have been attributed to natural herbs. Due to enormous propensities of plants which synthesize a variety of structurally various bioactive compounds, plant kingdom is a potential source of chemical constituents with antitumor and cytotoxic activities [6]. Plants have been found useful in medicine in three ways. First, they may be used directly as teas or in other extracted forms for their natural chemical constituents. Second, they may be used as agents in the synthesis of drugs. Third, the organic molecules found in plants may be used as models for synthetic drugs. Extraction of several active phytocompounds from green factories has given birth to some high activity profile drugs [7] and the use of traditional medicine is widely spread in India [8].
The plant Indigofera tinctoria (I. tinctoria) belongs to the family, Fabaceae, popularly known as Neeli in Tamil and found throughout India. I. tinctoria is a shrub, 1.2–1.8 m high and widely cultivated in many parts of the country. I. tinctoria is useful in the treatment of cancer, epilepsy, neuropathy, chronic bronchitis, asthma, ulcers and skin diseases, and is used as a hepatoprotective, diuretic and hair growth-promoting agent [9]. I. tinctoria leaves extract contains indirubin and indigtone, which were used in the treatment of hydrophobia [10]. Medicinal plants possess immunomodulatory and antioxidant activities [4]. I. tinctoria dry powder is used in the treatment of asthma [11]. Decoction of the leaves is used in bites and stings by venomous insects and reptiles to relieve the pain [12]. The present study was to evaluate the free radical scavenging activity and make a high performance thin layer chromatography (HPTLC) analysis of aqueous leaf extract of I. tinctoria.
2. Experimental
2.1. Drugs and chemicals
Gallic acid, quercetin, ascorbic acid, sodium nitroprusside, phenazonium methosulphate, reduced form of nicotinamide-adenine dinucleotid (NADH) and DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) were purchased from Sigma Aldrich, Co. (St. Louis, USA). All the other chemicals, which were obtained from Sisco Research Laboratory (Mumbai, India), were of analytical grade.
2.2. Collection and identification
The plant I. tinctoria was collected (May to November 2013) from the KSG Enterprises (Tindivanam, Tamil Nadu, India) and authenticated by Dr. D. Aravind (Department of Medical Botany, and National Institute of Siddha, Chennai, India). Voucher specimens were deposited at the Herbarium of National institute of Siddha, Reg no: NIS/MB/83/2013. The collected plants were separated from unwanted materials and dried in shade. The leaves were ground to coarse powder with the help of a suitable grinder. The powder was then stored in an airtight container, kept in a cool, dark and dry place until the analysis.
2.3. Extraction procedure
A total of 30 g of the freshly powdered leaves were extracted with 250 mL of sterile distilled water using the Soxhlet apparatus at 100 °C. The aqueous extracts were filtered with Whatman No. 1 filter paper and then freeze dried and stored at 4 °C for further investigation. The extraction efficiency was quantified by determining the weight of the extract and the percentage yield was calculated to be 16%.
2.4. Determination of total phenolic content
Total phenolic content of the extract was determined by the Folin–Ciocalteu reagent method [13]. One milliliter of the plant extracts/standard solutions with different concentrations was mixed with 5 mL of Folin–Ciocalteu reagent (previously diluted with water (1:10, v/v)) and 4 mL of sodium carbonate (7.5%). The mixtures were vortexed for a few seconds and allowed to stand for 30 min at 20 °C for color development. Absorbance of samples and standard was measured at 765 nm using a spectrophotometer against blank. The total phenolic content of the plant extract was calculated as the gallic acid equivalent.
2.5. Determination of total flavonoid content
Total flavonoid content was determined by aluminum chloride method [14]. One milliliter of the plant extract was mixed with 3 mL of methanol, 0.2 mL of aluminum chloride, 0.2 mL of 1 M potassium acetate and 5.6 mL of distilled water. The mixture was placed at room temperature for 30 min, and the absorbance of the reaction mixture was measured at 415 nm with a spectrophotometer against blank. The total flavonoid content of the plant extract was calculated as the quercetin equivalent.
2.6. Total antioxidant capacity
The total antioxidant capacity of the plant extract was evaluated as per the method described by Prieto et al. [15]. The extract was dissolved in a mixture of 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) and incubated at 95 °C for 90 min. After cooled to ambient temperature, the absorbance of the solution was measured at 695 nm against reagent blank containing only the respective solvents. The total antioxidant content of the plant extract was calculated as the ascorbic acid equivalent.
2.7. DPPH free radical scavenging assay
The free radical scavenging capacity of the extract was determined using DPPH [16]. The mixture of DPPH (0.1 mM) in methanol was prepared and 4 mL of this solution was added to 1 mL of sample solution in methanol at different concentrations (50 to 250 μg/mL). Thirty minutes later, the absorbance was measured at 517 nm at room temperature using a spectrophotometer Shimadzu UV-1800. Lower absorbance of the reaction mixture indicates a higher free radical scavenging activity. Ascorbic acid was used as a standard.
where Abs control is the absorbance of the control reaction, and Abs test is the absorbance of the extract/standard.
2.8. NO scavenging assay
The NO scavenging assay was carried out according to the method reported by Sreejayan et al. [17]. In this assay, 200 μL of 10 mM sodium nitroprusside was mixed with 50–250 µg of the plant extract in 1 mL of water and incubated at room temperature for 150 min. Following incubation, 500 μL of Griess reagent (1% sulfanilamide in 5% orthophosphoric acid) and 0.1% N-(1-napthyl ethylenediaminedihydrochloride) in a ratio of 1:1 were added and incubated for 10 min at room temperature. Ascorbic acid was used as a standard. The absorbance was measured at 546 nm. Controls were run devoid of samples and the inhibition rate is calculated as follows:
2.9. Superoxide anion scavenging assay
Superoxide anion scavenging was carried out according to the method described by Liu et al. [18]. In this method, superoxide radicals were generated in 3 mL of Tris HCl buffer (16 mM, pH 8.0) containing 1 mL of nitroblue tetrazolium (NBT; 50 μM) solution and 1 mL of nictonamide adenine dihydrogen salts (NADH; 78 μM) solution and 50–250 µg of the plant extract in 1 mL of water. The reaction started by adding 1 mL of phenazonium methosulphate (PMS) solution (10 μM) to the mixture. The reaction mixture was incubated at 25 °C for 5 min and the absorbance at 560 nm was measured against blank samples. Decreased absorbance of the reaction mixture indicates increased superoxide anion scavenging activity.
2.10. Reducing power assessment
Reducing power was carried out using the method reported by Yildirim et al. [19]. One milliliter of the extract and its sub-fractions (final concentration 50–250 μg/mL) were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide. The mixture was then incubated at 50 °C for 20 min. To this mixture, 2.5 mL of trichloroacetic acid was added, and centrifuged at 3000 rpm for 30 min. Finally, 2.5 mL of the supernatant solution was collected and mixed with 2.5 mL of distilled water and 0.5 mL of ferric chloride, and the absorbance was measured at 700 nm. Ascorbic acid was used as a standard and phosphate buffer as the blank solution.
2.11. HPTLC
For HPTLC fingerprinting analysis, 3, 6 and 9 µL of the plant samples were loaded in pre-coated HPTLC plates [silica gel 60F 254 (E. Merck KGaA) and plate size 5cm×10 cm]. The samples loaded plate was kept in TLC twin trough a developing chamber (after saturated with solvent vapor) with respective mobile phases (flavonoids and phenols), solvent system [chloroform:methanol:formic acid:acetic acid (80:15:2.5:2.5, v/v/v/v)], solvent front position 50.0 mm and volume 10 mL. The developed plate was dried by hot air at 60 °C to evaporate solvents from the plate. The plate was kept in a photodocumentation chamber (CAMAG TLC Scanner 3) and the images were captured at UV 254 nm and UV 366 nm. The retention factor (Rf) values at fingerprint data were recorded by WINCATS software.
2.12. Statistical analysis
Statistical analysis was carried out in triplicates (n=3) and standard error (SE) was calculated. All the data were analyzed using analysis of variance (ANOVA) with the statistical software Prism 5.0 version. The analyses were made with 95% confidence. The significance of differences (p<0.05) between mean values obtained from the experiments was determined by Tukey test.
3. Results
3.1. Total phenolic content
The total phenolic content of the aqueous extract of I. tinctoria was determined using the Folin–Ciocalteu reagent and expressed as gallic acid equivalent per gram of plant extract. The total phenolic content of the test fractions was calculated using the standard curve of gallic acid (y=0.454x+0.139; R2=0.0997). Aqueous extract of I. tinctoria was found to have 267.2±2.4 mg/g phenolic content.
3.2. Total flavonoid content
Aluminum chloride colorimetric methods were used to determine the total flavonoid content of the aqueous extract of I. tinctoria. Total flavonoid content was calculated using the standard curve of quercetin (y=0.494x−0.038; R2=0.977) and expressed as quercetin equivalent per gram of the plant extract. Aqueous extract of I. tinctoria was found to have 75.43±3.3 mg/g flavonoid.
3.3. Total antioxidant capacity
The total antioxidant capacity of aqueous extract of I. tinctoria was evaluated by the phosphomolybdenum method and expressed as ascorbic acid equivalent per gram of plant extract. The total antioxidant content of the test samples was calculated using the standard curve of ascorbic acid (y=0.0014x+0.0114; R2= 0.9926), and found to be 349.11±8.0 mg/g.
3.4. DPPH free radical scavenging activities
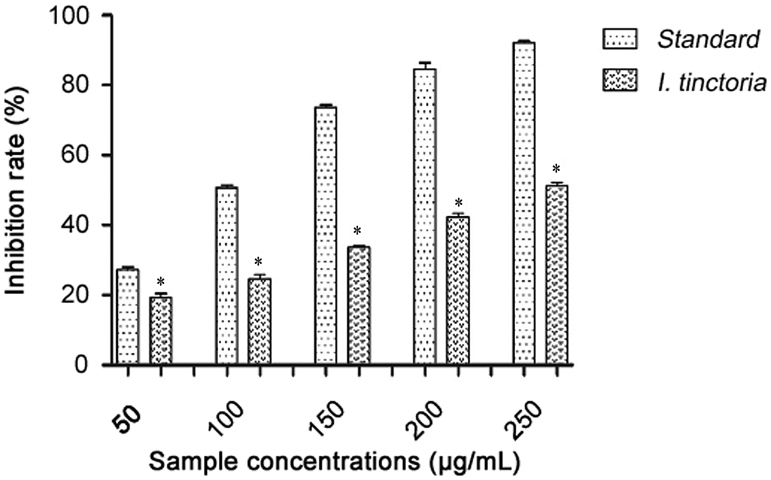
The free radical scavenging activities of aqueous extract of I. tinctoria were studied by its ability to reduce DPPH, a stable free radical; and any molecule that can donate an electron or hydrogen to DPPH can react with it and thereby bleach DPPH absorption. DPPH is a purple dye with absorption maxima at 517 nm, and upon reaction with a hydrogen donor the purple color fades or disappears due to conversion of it to 2,2diphenyl-1-picryl hydrazine, resulting in decrease in absorbance. The DPPH scavenging effect was found to increase with increased concentrations. At 250 μg/mL, aqueous extracts showed 51.08% inhibition and ascorbic acid showed 92.02% inhibition (Fig. 1). IC50 values were calculated and expressed (Table 1).
Fig. 1.
DPPH scavenging activities of Indigofera tinctoria extract and ascorbic acid. *p<0.05.
Table 1.
IC50 values of aqueous extracts of Indigofera tinctoria in DPPH, nitric oxide and superoxide anion scavenging assay.
| Extract | IC50 (μg/mL) |
||
|---|---|---|---|
| DPPH | NO | ||
| Aqueous extract | 244.71 | 540.65 | 466.59 |
| Ascorbic acid | 135.14 | 136.37 | 140.38 |
3.5. NO radical scavenging activities
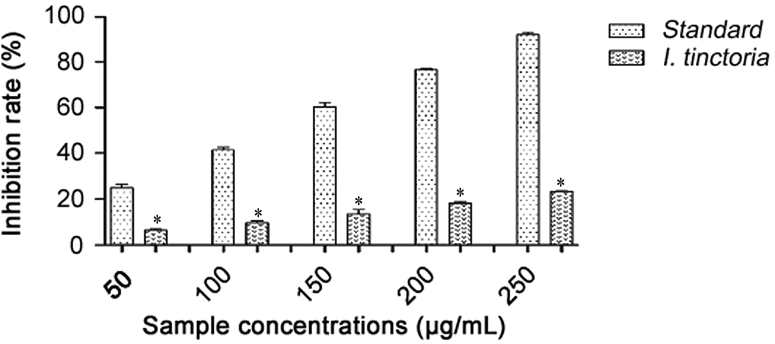
The extents of NO radical scavenging of I. tincoria aqueous extract at concentrations (50–250 μg/mL) were measured, with ascorbic acid as a standard. The radical scavenging effect was found to increase with increased concentrations. At 250 μg/mL, aqueous extract showed 23.12% inhibition and ascorbic acid showed 91.66% inhibition (Fig. 2).
Fig. 2.
Nitric oxide scavenging activities of Indigofera tinctoria extract and ascorbic acid. *p<0.05.
3.6. Superoxide anion scavenging activities
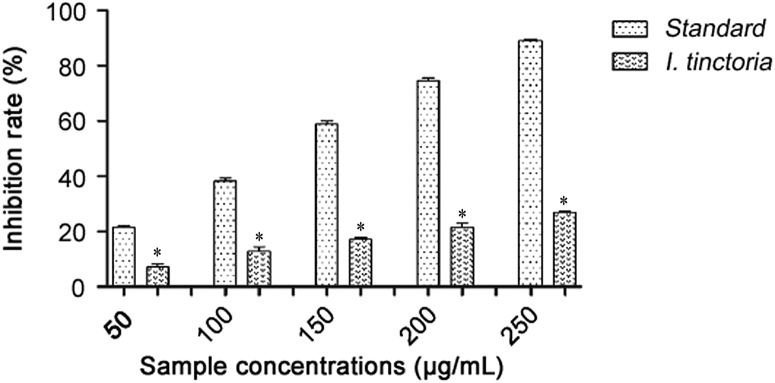
The extents of superoxide anion scavenging of I. tincoria aqueous extract at concentrations (50–250 μg/mL) were measured, with ascorbic acid as a standard. The radical scavenging effect was found to increase with increased concentrations. At 250 μg/mL, aqueous extract showed 26.79% inhibition and ascorbic acid showed 89.04% inhibition (Fig. 3).
Fig. 3.
Superoxide anion scavenging activities of Indigofera tinctoria of extract and ascorbic acid. *p<0.05.
3.7. Reducing power
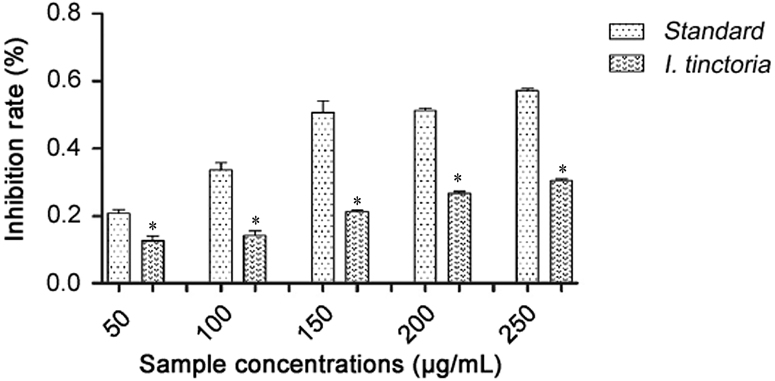
Reducing power of the fractions can be assessed by using ferric to ferrous reducing activity as determined spectrophotometrically from the formation of Perl׳s prussian blue color complex. In the presence of antioxidants in the sample, the absorbance was measured at 700 nm. The reducing power of aqueous extract (0.305) and ascorbic acid (0.572) at 250 μg/mL is shown in Fig. 4. Higher absorbance indicates more reducing power.
Fig. 4.
Comparison of reducing power of Indigofera tinctoria extract and ascorbic acid. *p<0.05.
3.8. HPTLC
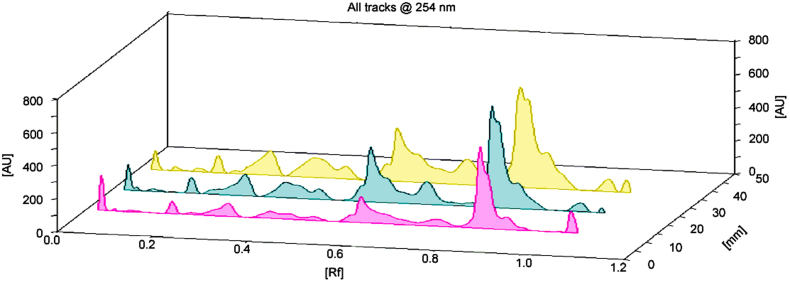
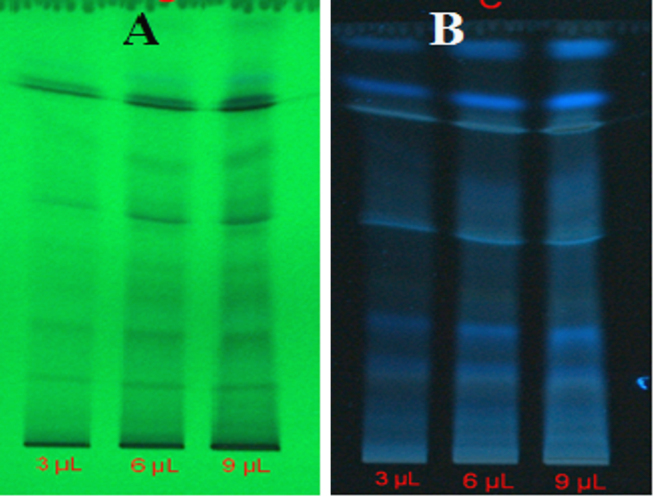
Various solvent compositions of the mobile phase for HPTLC analysis were examined in order to achieve high resolution and reproducible peaks. Chromatogram was developed in I. tinctoria aqueous extract under chamber saturation conditions using chloroform:methanol:formic acid:acetic acid (80:15:2.5:2.5, v/v/v/v) as the mobile phase or solvent system. Aqueous extract of I. tinctoria was subjected to chromatographic fingerprinting. After chromatographic deprivation with anisaldehyde H2SO4, I. tinctoria aqueous extract at different concentrations displayed the chromatogram in 3D at 254 nm (Fig. 5). The HPTLC fingerprinting of I. tinctoria revealed several peaks, 16 spots at 3 µL and 6 µL of plant samples (Fig. 6A and B) and 17 spots at 9 µL of plant samples (Fig. 6C). There were 17 polyvalent phytoconstituents and the corresponding ascending order of Rf values ranged from 0.03 to 1.02 (Table 2). The highest area (%) of the phytoconstituents was found to be 19.60% and its corresponding Rf value was 0.53. Exposure of the spotted and developed HPTLC plate to UV 254 nm showed the presence of numerous organic compounds as dark and light bands in a green background (Fig. 7A). HPTLC slides developed at UV 366 nm exposure revealed multi-colored bands with varying intensities (Fig. 7B). It corresponded to several polar and non-polar compounds, which showed light and dark blue, light yellow, bluish purple and fluorescent sky blue color bands, respectively. In the present study, dark blue color bands were observed, which confirmed the presence of phenolic compounds in the plant extract. Light yellow zones were detected from the chromatogram after derivatization, which confirmed the presence of flavanoids and saponins. Bluish purple color indicated the presence of terpenoids.
Fig. 5.
3D Chromatogram of Indigofera tinctoria extract at different concentrations at 254 nm.
Fig. 6.
HPTLC chromatogram of aqueous extract of Indigofera tinctoria.
Table 2.
Rf values of the peak and peak height.
| Peak | Start Rf. | Start height | Max Rf. | Max (%) | End Rf. | Max height | End height | Area | Area (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.03 | 41.7 | 0.04 | 4.05 | 0.05 | 112.6 | 0.05 | 937.9 | 1.36 |
| 2 | 0.07 | 1.2 | 0.08 | 0.87 | 0.10 | 24.2 | 0.10 | 275.2 | 0.40 |
| 3 | 0.11 | 0.6 | 0.12 | 0.78 | 0.15 | 21.6 | 0.15 | 349.9 | 0.51 |
| 4 | 0.15 | 1.1 | 0.17 | 3.67 | 0.19 | 102.3 | 0.19 | 1305.2 | 1.89 |
| 5 | 0.19 | 0.0 | 0.21 | 0.57 | 0.22 | 15.8 | 0.22 | 230.6 | 0.33 |
| 6 | 0.22 | 12.7 | 0.28 | 5.21 | 0.31 | 145.1 | 0.31 | 5199.4 | 7.53 |
| 7 | 0.31 | 0.1 | 0.37 | 4.20 | 0.42 | 117.0 | 0.42 | 6022.5 | 8.73 |
| 8 | 0.42 | 50.4 | 0.44 | 2.69 | 0.48 | 75.0 | 0.48 | 1781.7 | 2.58 |
| 9 | 0.48 | 0.2 | 0.53 | 3.69 | 0.53 | 102.7 | 0.53 | 1704.3 | 2.47 |
| 10 | 0.53 | 99.2 | 0.55 | 11.59 | 0.63 | 322.9 | 0.63 | 13526.3 | 19.60 |
| 11 | 0.63 | 88.4 | 0.63 | 3.19 | 0.66 | 88.7 | 0.66 | 1739.2 | 2.52 |
| 12 | 0.66 | 69.2 | 0.70 | 5.46 | 0.75 | 151.9 | 0.75 | 5806.2 | 8.41 |
| 13 | 0.75 | 13.6 | 0.81 | 21.65 | 0.82 | 602.9 | 0.82 | 12082.9 | 17.51 |
| 14 | 0.82 | 530.8 | 0.82 | 19.28 | 0.85 | 537.0 | 0.85 | 9466.5 | 13.72 |
| 15 | 0.85 | 207.3 | 0.86 | 7.76 | 0.93 | 216.2 | 0.93 | 6296.7 | 9.12 |
| 16 | 0.96 | 0.6 | 0.99 | 2.63 | 1.01 | 73.2 | 10.1 | 1505.3 | 2.18 |
| 17 | 1.02 | 1.02 | 1.03 | 2.71 | 1.04 | 75.6 | 1.04 | 781.7 | 1.13 |
Fig. 7.
Photo-documentation of aqueous extract of Indigofera tinctoria.
4. Discussion
In the present study, the total phenolic and flavonoid contents of aqueous extract of I. tinctoria leaves were estimated. The total phenolic content of aqueous extract was 267.6±2.4 mg/g extract. The result reported by Nagarajan et al. [2] was in agreement that the total phenolic and flavonoid contents of different solvent extracts of I. tinctoria leaves were maximum in water extract. The higher content of total phenolic in the highly polar solvent (ethyl acetate, methanol and water) extract of I. tinctoria might contribute to the presence of furano-flavones, flavonol glycosides and high molecular tannins [20]. Flavonoid is also known to have a wide array of therapeutic activities as antihypertensive, anti-rheumatism, antimicrobial, diuretic, antioxidative [21] and chemopreventive agents [22]. Phenolic compounds are well known as antioxidative and scavenging agents against oxidative damage associated with free radicals [23].
The aqueous extract of I. tinctoria plant exhibited some degree of reducing power. The reducing power increased with an increasing sample concentration. The proton-radical scavenging action has been known as an important mechanism of anti-oxidation. DPPH radical is a stable organic free radical which has been extensively used for evaluating the free radical scavenging potential of natural antioxidants. Aqueous extract of I.tinctoria showed IC50 of 244.71 μg/mL and ascorbic acid showed IC50 of 135.14 μg/mL. The aqueous extract could scavenge more DPPH radicals as compared with methanolic extract [24]. Nagarajan et al. [2] reported free radical scavenging potential of different solvent extracts of I. tinctoria leaves. Bakasso et al. [1] reported that I. tinctoria showed a good antioxidant activity. The DPPH scavenging activity can also be attributed to the electron transfer or the hydrogen donating nature of the polyphenols. Free radical scavenging is the accepted mechanism for antioxidants׳ inhibiting lipid oxidation [25].
The nitric oxide scavenging assay is based on the principle that sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions that can be estimated using Griess reagent. In the present study, aqueous extract of I. tinctoria showed NO scavenging activity with IC50 of 540.65 μg/mL and ascorbic acid showed IC50 of 136.37 μg/mL. The nitric acid scavenging activity of aqueous plant extract against NO formation was comparable to that of the standard drugs [26]. The aqueous extract of I. tinctoria leaves had greater NO scavenging activity. The polyphenolic compounds in I. tinctoria might be responsible for the observed scavenging activity. NO scavenging capacities of flavonoid and phenolic compounds are already well known [2].
Aqueous extract of I. tinctoria had superoxide anion scavenging activity with IC50 of 466.59 μg/mL and ascorbic acid showed IC50 of 140.38 (Table 1). This statement proved Manju׳s [24] result that aqueous extract had the superoxide radical scavenging ability. Superoxide anion radical is one of the strongest ROSs among the free radicals and becomes converted to other harmful reactive oxygen species such as hydrogen peroxide and hydroxyl radical, damaging biomolecules which result in chronic diseases [27]. Antioxidants are substances that delay the oxidation process by inhibiting the polymerization chain initiated by free radicals and other subsequent oxidizing reactions [28].
The reducing power of aqueous extract of I. tinctoria showed significant changes compared with ascorbic acid (Fig. 4). The aqueous extract of I. tinctoria had a free radical scavenging potential and its reducing power was increased with increased concentration [2].
In the present study, phytochemical screening results of I. tinctoria leaves revealed the presence of phenols, flavonoids, saponins and terpenoids in aqueous extract, which was confirmed by performing HPTLC separation technique with H2SO4 spraying reagents. HPTLC showed clear separation of components in the aqueous extract of I. tinctoria. This method was applied to identify the plant of I. tinctoria from other species. HPTLC fingerprint enables a particular plant to be identified and distinguished from closely related species [29]. The presence or absence of chemical constituent may be useful for identifying the taxonomy of the plant. The Rf values of aqueous extract of I. tinctoria were 0.03–1.02 in the absorbance of 254 nm and 366 nm. The highest area (%) of the phytoconstituents was found to be 19.60% and its corresponding Rf value was 0.53 (Table 2). It might be an indigo compound. These results are supported by Francesca et al. [30] who reported that I. tinctoria plant had henna commercial product collected from different places and Rf value was 0.55. The developed HPTLC at UV 366 nm exposure of slides revealed multi-colored bands with varying intensities (Fig. 7B). HPTLC developed plates at 366 nm exposure display light and dark blue, light yellow, bluish purple and fluorescent sky blue color bands. HPTLC fingerprint analysis of aqueous extract of I. tinctoria leaves revealed the presence of phenols, flavonoids, saponins and terpenoids. It corresponds to several polar and non-polar compounds. I. tinctoria methanol extract contained flavonoids, saponins, tannins, steroidal terpens, phenols and anthroquinonesence [31]. Blue and light brown color zone detected in UV after derivatization in the chromatogram confirmed the presence of polyphenols [32]. Light yellow colored fluorescent zone peak observed in the chromatogram after derivatization confirmed the presence of flavonoid and saponin [31]. Blue-violet colored zones were detected from the chromatogram after derivatization, which confirmed the presence of flavanoids [32]. Purple and bluish purple observed in the chromatogram after derivatization confirmed the presence of terpenoids [33]. More components were identified from aqueous extract of I. tinctoria [34]. The results obtained in this study suggest that the identified phytochemical compounds might be the bioactive constituents responsible for the efficacy of the leaves of the plants studied. Plant extract could be a source for the industrial manufacture of drugs useful in the chemotherapy of some microbial infections.
5. Conclusion
In the present study, aqueous extract of I. tinctoria showed free radical scavenging activity on NO, and DPPH models. The preliminary study showed the presence of phenols and flavonoids, indicating its potent antioxidant activity, and HPTLC analysis confirmed the presence of phenols, flavonoids, saponins and terpenoids. This investigation on I. tinctoria could serve as a primary basis for further pharmacological and drug designing studies.
Acknowledgments
This work was financially supported by the UGC-UPE-Phase II (No: 2013/PFEP/C3/280) from University of Madras, India.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Bakasso S.A., Lamien-Meda C.E., M. Lamien J. Polyphyenol contents and antixoidant activities of five Indigofera species (Fabaceae) from Burkina Faso. Pak. J. Biol. Sci. 2008;11:1429–1435. doi: 10.3923/pjbs.2008.1429.1435. [DOI] [PubMed] [Google Scholar]
- 2.Nagarajan A., Sellamuthu M. Antioxidant and free radical scavenging potential of different solvent extracts of Indigofera tinctoria L. Leaves. Int. J. Pharm. Pharmacol. Sci. 2013;5:142–147. [Google Scholar]
- 3.Annie Felicia F., Muthulingam M. Antihepatotoxic efficacy of methanolic extract of Indigofera tinctoria (Linn.) on paracetamol induced liver damage in rats. J. Nat. Prod. Plant Resour. 2012;2:244–250. [Google Scholar]
- 4.Senthilkumar A., Venkatesalu V. Photochemical analysis and antibacterial activity of the essential oil of Clausenaanisata (Willd.) hook. f. ex benth. Int. J. Integr. Biol. 2009;5:116–120. [Google Scholar]
- 5.Gayathri P., Uma D. Plants tested by trial and error, as in the doctrine of signatures. Mad. Agric. J. 2009;96:50–54. [Google Scholar]
- 6.Kim J.B., Koo H.N., Joeng H.J. Introduction of apoptosis by Korean medicine Gagam-whanglyun-haedoktang through activation of capase-3 in human leukemia cell line, HL-60 cells. J. Pharmacol. Sci. 2005;97:138–145. doi: 10.1254/jphs.fpj04021x. [DOI] [PubMed] [Google Scholar]
- 7.Mandal V., Mohan Y., Hemalatha S. Microwaves assisted extraction an innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007;1:7–18. [Google Scholar]
- 8.Jeyachandran R., Mahesh A. Enumeration of antidiabetic herbal flora of Tamil Nadu. Res. J. Med. Plant. 2007;1:144–148. [Google Scholar]
- 9.Asuntha G., Prasannaraju Y., Prasad K. Effect of ethanol extract of Indigofera tinctoria L (Fabaceae) on lithium/pilocarpine-induced status epilepticus and oxidative stress in Wistar rats. Trop. J. Pharm. Res. 2010;9:149–156. [Google Scholar]
- 10.Muthulingam M., Mohandoss P., Indra N. Antihepatotoxic efficacy of Indigofera tinctoria L on paracetamolinduced liver damage in rats. Ind. J. Pharm. Biol. Res. 2010;1:13–18. [Google Scholar]
- 11.Savithramma N.C.H., Rao S.K.N. Treatment in asthma of dry powder of Indigofera tinctoria L. Ethanopharmacology. 2007;113:54–61. [Google Scholar]
- 12.DeFeudis F.V., Papadopoulos V., Drieu K. Ginkgo biloba extracts and cancer: a research area in its infancy. Fundam. Clin. Pharmacol. 2003;17:405–417. doi: 10.1046/j.1472-8206.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 13.Demiray S., Pintado M.E., Castro P.M.L. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad. Sci. Eng. Technol. 2009;54:312–317. [Google Scholar]
- 14.Jiao H., Wang S.Y. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J. Agric. Food Chem. 2000;48:5672–5676. doi: 10.1021/jf000765q. [DOI] [PubMed] [Google Scholar]
- 15.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 16.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 17.Sreejayan, Rao M.N. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu F., Ooi V.E.C., Chang S.T. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 1997;60:763–771. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 19.Yildirim A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 20.Narender T., Khaliq T., Puri A. Antidyslipidemicactivity of furano-flavonoids isolated from Indigofera tinctoria. Bioorg. Med. Chem. Lett. 2006;16:3411–3414. doi: 10.1016/j.bmcl.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Burkill H.M. 2nd edition. Vol. 1. Royal Botanical Garden; Kew: 1988. The Useful Plants of West Tropical Africa. [Google Scholar]
- 22.Kameswaran R., Ramanibai R. Protective effect of flavonoidal fraction of Indigofera tinctoria on benzo (alpha) pyrene induced lung carcinogenicity in Swiss albino mice. Int. J. Cancer Res. 2008;4:71–80. [Google Scholar]
- 23.Ferguson L.R., Philpott M., Karunasinghe N. Oxidative DNA damage and repair: significance and biomarker. J. Nutr. 2006;136:2687–2689. doi: 10.1093/jn/136.10.2687S. [DOI] [PubMed] [Google Scholar]
- 24.Manju S., Pratap C.R., Shaji P.K. In vitro free radical scavenging potential of Acorus calamus L. rhizome from Kuttanad Wetlands, Kerala, India. Int. J. Pharm. Pharm. Sci. 2013;5:376–380. [Google Scholar]
- 25.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. Technol. 1995;28:25–30. [Google Scholar]
- 26.Mbaebie B.O., Edeoga H.O., Afolayan A.J. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pac. J. Trop. Med. 2012;2:118–124. doi: 10.1016/S2221-1691(11)60204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Mamun M., Yamaki K., Masumizuet T. Superoxide anion radical scavenging activities of herbs and pastures in Northern Japan determined using electron spin resonance spectrometry. Int. J. Biol. Sci. 2007;3:349–355. doi: 10.7150/ijbs.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliwell B., Aruoma O.I. DNA damage by oxygen-derived species, its mechanism and measurement in mammalian systems. FEBS. Lett. 1991;9:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 29.John Houghton P. Establishing and identification criteria for botanicals. Drug Inf. J. 1998;32:461–469. [Google Scholar]
- 30.Francesca R.G., Multaria G., Palazzino G. Henna through the centuries: a quick HPTLC analysis proposal to check henna identity. Rev. Bras. Farmacogn. 2014;24:133–140. [Google Scholar]
- 31.Renukadevi K.P., Suhani Sultana S. Determination of antibacterial, antioxidant and cytotoxicity effect of Indigofera tinctoria on lung cancer cell line NCI-h69. Int. J. Pharmacol. 2011;7:356–362. [Google Scholar]
- 32.Wettasinghe M., Shahidi F. Evening primrose meal, a source of natural antioxidants and scavenger of hydrogen peroxide and oxygen-derived free radicals. J. Agric. Food Chem. 1999;47:1801–1812. doi: 10.1021/jf9810416. [DOI] [PubMed] [Google Scholar]
- 33.Taganna J.C., Quanico J.P., Perono R.M.G. Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. Ethnopharmacology. 2011;134:865–871. doi: 10.1016/j.jep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Rashmi T., Maushumi K., Kiran B. Preliminary phytochemical screening and HPTLC fingerprinting of bark extracts of Symplocos racemosa. J. Pharm. Phytochem. 2013;2:45–49. [Google Scholar]