Abstract
Background
The natural course of atopic dermatitis (AD) in infancy, childhood, and adolescence is not yet completely known.
Objective
To investigate the prevalence and risk factors of atopic dermatits among 19-year-old Korean male subjects.
Methods
All 19-year-old Korean males must undergo medical examination for conscription. We precisely evaluated the prevalence of AD in three Korean provinces using the information from this physical checkup. AD was diagnosed by experienced dermatologists according to the Hanifin and Rajka criteria. The disease severity was assessed by the scoring of atopic dermatitis (SCORAD) index. In order to investigate the risk factors for AD, a questionnaire was administered to all subjects regarding parental atopic history, geographical characteristics of past habitation, past economic status, number of siblings, parental occupation, etc.
Results
The point prevalence in the Korean provinces ranged from 1.15% to 1.44%. In multivariable analysis, a parental history of AD was a significant risk factor in all 3 disease-onset groups (infancy, childhood, and adolescent onset). In the infancy-onset group, low economic status was also a significant risk factor for AD. The SCORAD index was significantly higher in AD subjects with early onset and those living in small-sized habitations. Moreover, erythema, edema, lichenification, dryness of skin, and sleep loss appeared to be more severe in early-onset cases.
Conclusion
The younger the age of disease onset, the more severe the clinical outcomes in 19-year-old male subjects. In addition, active AD treatment at younger ages might affect the prevalence and the severity of AD in adulthood.
Keywords: Adolescent, Atopic dermatitis, Prevalence, Risk factors
INTRODUCTION
The natural course of atopic dermatitis (AD) has not yet been completely described. Few studies have been conducted on the changing features of AD clinical characteristics through the adolescence and adulthood, or the final prognosis of AD with onset in infancy and childhood1,2. At the same time, few studies have investigated the epidemiological characteristics of AD in adulthood. When the prevalence of AD has been reported, the method used by the survey of the International Study of Asthma and Allergies in Childhood (ISSAC) is commonly adopted3,4,5. However, a direct skin examination of large numbers of people by doctors, especially dermatologists, is rarely performed. Furthermore, some studies concerning the prevalence of AD found in the literature do not include a sample size sufficient to accurately estimate the real prevalence. Few studies have been performed on the factors that could influence the prevalence of AD and its clinical severity during adolescence and adulthood6,7. One of the reasons is that the wide range of ages of the subjects makes it difficult to find a matching control group. In Korea, in accordance with the conscription system, all 19-year-old male individuals are mandated to receive a physical and medical examination in the local Military Manpower Administration (MMA). Medical specialists from all medical departments including dermatologists take part in the examination, and every examinee undergoes a thorough skin inspection. Therefore, it is possible to precisely diagnose AD by using the Hanifin and Rajka criteria during the inspection course. Moreover, many normal control subjects are available, providing the ability to matching AD and control groups. Using this data system, we investigated the point prevalence of AD in a cohort of 19-year-old Korean men. In addition, we investigated the risk factors for AD in these study subjects by comparison with a normal control population. The disease severity in each subject diagnosed with AD was individually assessed by experienced dermatologists in accordance with the severity scoring of atopic dermatitis (SCORAD) index. We also analyzed the factors that influence the clinical severity of AD and the differences in its clinical characteristics according to the age of onset.
MATERIALS AND METHODS
Subject recruitment
Korean male subjects of 19-year of age who were required to undergo a medical examination within the conscription system were included in this study. About 400,000 boys were born in Korea in 1989 and underwent the conscription examination on different dates throughout 2008 at a local provincial MMA. We enrolled the young men who visited the following four MMAs from June to October 2008: Seoul North Province and Seoul South Province, Gyeonggi-do North Province (located in Uijeongbu) and Gangwon-do West Province (located in Chuncheon). A total population of 19,048 subjects was included in this study. In the course of the physical and medical examinations at the MMAs, the dermatologists performed a detailed skin examination and collected the relevant histories. Subsequently, the criteria of Hanifin and Rajika8 were used to diagnose AD.
Diagnosis of AD, data collection, and physical examination
To assess the risk factors for AD, two control subjects were selected for each AD patient from the same day of examination (a 1:2 matching) at each MMA. A survey of the various risk factors for AD was conducted for both the patient and the control group including age of onset, parental history of AD, asthma, and allergic rhinitis, population of residential region, housing type, economic status, number of siblings, family size, parental educational status and professions. Moreover, the body mass index (BMI), calculated by using heights and weights measured during the physical examination, was also collected with the above risk factors.
For the purposes of our current analyses, it was most practical to select subjects who underwent their MMA examinations on a single day between June and October 2008, making it not possible to apply a period prevalence rate. Instead, the point prevalence was used as follows:
| Pt=Ct÷Nt×100 (%) |
where Pt represents the prevalence rate of t-point, Ct is the number of patients diagnosed with AD, Nt is the total subjects in the study at t-point, which is also the total number of 19-year-old male subjects who received the examination from June to October. In addition, the AD prevalence values from the four different MMAs were compared using the chi-square test for homogeneity.
After diagnosing AD during the physical examination, the dermatologists estimated its clinical severity using the SCORAD index to measure the extent of AD as a percentage (0%~100%). These clinicians also calculated and recorded the intensity score (0~18 points) by evaluating the following items: erythema (0~3 points), edema (0~3 points), oozing (0~3 points), excoriation (0~3 points), lichenification (0~3 points), and dryness of uninvolved skin (0~3 points). The subjective symptoms of the patients were also recorded with intensity scores: pruritus (0~10) and sleep loss (0~10). The SCORAD index (0~103 points) was calculated as follows:
| SCORAD index=A/5+7B/2+C (A=total body surface area involved in %, B=intensity score, C=subjective symptoms score) |
Moreover, the objective findings of the skin lesions as well as the subjective symptoms reported by the subjects were recorded.
Statistical analysis
Statistical analyses were performed to investigate the factors affecting the clinical characteristics. First, univariable conditional logistic regression analysis of the risk factors for AD was conducted. Second, multivariable conditional logistic regression analysis was conducted to adjust for any confounding effects among the various factors. To identify factors affecting AD clinical manifestations, the correlation between disease severity and different variables was estimated by multiple linear regression analysis. The eight factors composing the SCORAD index were analyzed by one-way analysis of variance (ANOVA) to identify significant differences according to the age of AD onset (infancy-, childhood-, or adolescent onset).
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki (2008). The study protocol was approved by the institutional review board of Seoul National University Hospital (IRB no. 0807-064-251). Statistical analysis was performed using the PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA). A p-value<0.05 was considered statistically significant.
RESULTS
Prevalence rate of AD in 19-year-old Korean male subjects
The point prevalence rates in each province ranged from 1.15% to 1.44%, as indicated in Table 1. The average prevalence in the four provinces was 1.29%; 245 examinees out of 19,048 19-year-old male subjects were diagnosed with AD according to the Hanifin and Raijka criteria. The chi-square test did not show any statistically significant difference (p=0.519) between the prevalence rates in each province.
Table 1. Prevalence rates in each province of atopic dermatitis in the 19-year-old Korean male subjects.
| Province | Atopic dermatitis patients | Total subjects | Prevalence rate (%) |
|---|---|---|---|
| South part of Seoul city | 76 | 5,547 | 1.37 |
| North part of Seoul city | 81 | 7,043 | 1.15 |
| North part of Gyeonggi Province | 63 | 4,375 | 1.44 |
| West part of Gangwon Province | 25 | 2,083 | 1.20 |
| Total | 245 | 19,048 | 1.29 |
Risk factors for AD
The results of our univariate analysis of the risk factors for AD are presented in Table 2. The significant risk factors included a low economic status in infancy, parental history of AD, and allergic rhinitis in the infancy-onset group, low economic status in infancy and parental AD history in the childhood-onset group, and BMI ≥25 kg/m2 and parental AD history in the adolescent onset group.
Table 2. Univariate conditional logistic regression analysis of the risk factors for AD in the 19-year-old Korean male study subjects.
| Variable | Infancy-onset (n=103/206) (case/control) | Childhood-onset (n=89/178) (case/control) | Adolescent onset (n=53/106) (case/control) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| High body mass index (≥25 kg/m2) | 1.027 (0.590~1.788) | 0.925 | 1.265 (0.647~2.470) | 0.492 | 2.610 (1.289~5.286) | 0.008 |
| Population size of city lived in infancy | ||||||
| ≤50,000 | 1 | 1 | 1 | |||
| 50,000~100,000 | 1.651 (0.152~17.902) | 0.680 | 3.921 (0.255~60.268) | 0.327 | 0.000 | 0.982 |
| 100,000~1,000,000 | 2.042 (0.228~18.311) | 0.524 | 2.024 (0.182~22.535) | 0.567 | 0.346 (0.039~3.043) | 0.338 |
| ≥1,000,000 | 1.731 (0.160~18.702) | 0.651 | 4.187 (0.338~51.912) | 0.265 | 0.633 (0.087~4.593) | 0.651 |
| Population size of city lived in childhood | ||||||
| ≤50,000 | 1 | 1 | ||||
| 50,000~100,000 | 2.647 (0.180~38.963) | 0.478 | 1.041 (0.046~23.576) | 0.980 | ||
| 100,000~1,000,000 | 1.315 (0.134~12.879) | 0.814 | 0.259 (0.028~2.387) | 0.233 | ||
| ≥1,000,000 | 2.377 (0.219~25.813) | 0.477 | 0.475 (0.062~3.648) | 0.474 | ||
| Population size of city lived in adolescence | ||||||
| ≤50,000 | ||||||
| 50,000~100,000 | 3.019 (0.146~62.508) | 0.475 | ||||
| 100,000~1,000,000 | 0.631 (0.083~4.802) | 0.656 | ||||
| ≥1,000,000 | 1.105 (0.197~6.202) | 0.910 | ||||
| Housing type in infancy | ||||||
| Rural single family residencies | 1 | 1 | 1 | |||
| Urban single family residencies | 2.081 (0.659~6.574) | 0.212 | 1.120 (0.479~2.619) | 0.793 | 1.437 (0.457~4.517) | 0.535 |
| Low-rise multi-family residencies | 1.588 (0.432~5.846) | 0.487 | 1.198 (0.408~3.519) | 0.742 | 1.662 (0.473~5.838) | 0.428 |
| High-rise multi-family residencies | 1.588 (0.432~5.846) | 0.199 | 0.842 (0.351~2.016) | 0.699 | 1.108 (0.354~3.467) | 0.861 |
| Housing type in childhood | ||||||
| Rural single family residencies | 1 | 1 | ||||
| Urban single family residencies | 1.388 (0.534~3.603) | 0.501 | 0.991 (0.274~3.588) | 0.989 | ||
| Low-rise multi-family residencies | 0.949 (0.291~3.102) | 0.931 | 1.171 (0.304~4.513) | 0.818 | ||
| High-rise multi-family residencies | 0.785 (0.308~1.999) | 0.611 | 0.929 (0.263~3.281) | 0.909 | ||
| Housing type in adolescence | ||||||
| Rural single family residencies | 1 | |||||
| Urban single family residencies | 0.763 (0.164~3.559) | 0.731 | ||||
| Low-rise multi-family residencies | 1.133 (0.251~5.119) | 0.871 | ||||
| High-rise multi-family residencies | 1.051 (0.256~4.318) | 0.945 | ||||
| Economic status in infancy | ||||||
| High | 1 | 1 | 1 | |||
| Middle | 1.433 (0.520~3.955) | 0.487 | 1.981 (0.793-4.946) | 0.143 | 2.546 (0.806~8.048) | 0.111 |
| Low | 7.307 (1.908~27.989) | 0.004 | 3.347 (1.091~10.269) | 0.035 | 1.441 (0.329~6.316) | 0.628 |
| Economic status in childhood | ||||||
| High | 1 | 1 | ||||
| Middle | 2.260 (0.871~5.862) | 0.094 | 1.989 (0.641~6.174) | 0.234 | ||
| Low | 2.885 (0.893~9.321) | 0.077 | 1.541 (0.357~6.653) | 0.563 | ||
| Economic status in adolescence | ||||||
| High | 1 | |||||
| Middle | 1.396 (0.465~4.196) | 0.552 | ||||
| Low | 1.115 (0.282~4.408) | 0.877 | ||||
| Nuclear family in infancy | 0.835 (0.441~1.583) | 0.581 | 1.349 (0.661~2.756) | 0.411 | 1.177 (0.531~2.611) | 0.689 |
| Nuclear family in childhood | 0.958 (0.476~1.930) | 0.905 | 1.342 (0.583~3.090) | 0.489 | ||
| Nuclear family in adolescence | 1.135 (0.480~2.684) | 0.773 | ||||
| Parental history of AD | 10.342 (3.977~26.893) | 0.000 | 8.000 (3.003~21.315) | 0.000 | 13.277 (3.007~58.628) | 0.001 |
| Parental history of asthma | 4.000 (1.000~15.994) | 0.050 | 3.000 (0.501~17.954) | 0.229 | 1.000 (0.091~11.028) | 1.000 |
| Parental history of allergic rhinitis | 7.165 (2.912~17.626) | 0.000 | 1.547 (0.738~3.242) | 0.248 | 1.480 (0.630~3.476) | 0.368 |
| Mother's educational status | ||||||
| College graduate or above | 1 | 1 | 1 | |||
| High school graduate | 0.938 (0.583~1.508) | 0.791 | 1.082 (0.608~1.927) | 0.788 | 0.958 (0.473~1.939) | 0.904 |
| Middle school graduate or below | 3.381 (0.978~11.686) | 0.054 | 1.798 (0.734~4.406) | 0.200 | 0.712 (0.212~2.390) | 0.582 |
| Mother's profession as housewife | 0.958 (0.581~1.579) | 0.865 | 1.151 (0.684~1.937) | 0.596 | 0.922 (0.467~1.822) | 0.816 |
| Father's educational status | ||||||
| Middle school graduate or below | 1 | 1 | 1 | |||
| High school graduate | 2.640 (0.536~13.000) | 0.233 | 0.894 (0.329~2.425) | 0.826 | 1.799 (0.471~6.872) | 0.390 |
| College graduate or above | 1.681 (0.350~8.064) | 0.516 | 0.600 (0.244~1.477) | 0.266 | 1.616 (0.441~5.921) | 0.469 |
| Father's profession as a white collar worker | 1.025 (0.601~1.747) | 0.928 | 0.977 (0.583~1.637) | 0.930 | 1.845 (0.903~3.769) | 0.093 |
| Number of siblings | ||||||
| Only child | 1 | 1 | 1 | |||
| Two | 1.321 (0.794~2.196) | 0.284 | 1.256 (0.702~2.246) | 0.442 | 0.597 (0.290~1.229) | 0.161 |
| Three or above | 1.531 (0.630~3.722) | 0.347 | 0.719 (0.228~2.263) | 0.572 | 0.791 (0.247~2.538) | 0.693 |
AD: atopic dermatitis, OR: odds ratio, CI: confidence interval.
The results of our multivariate analysis are presented in Table 3. In all the three disease-onset groups, the parental AD history was a significant risk factor for AD in 19-year-old male subjects (p<0.001, p<0.0001, and p=0.001 for the infancy-, childhood-, and adolescent onset group, respectively). In addition, a low economic status (p=0.018) was a significant risk factor in the infancy-onset group.
Table 3. Multivariate conditional logistic regression analyses of the risk factors for AD in the 19-year-old Korean male study subjects.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Infancy-onset (n=103/206) (case/control) | ||
| Economic status (0~2 yr) | ||
| High | 1 | |
| Middle | 1.285 (0.423~3.903) | 0.658 |
| Low | 5.789 (1.357~24.704) | 0.018 |
| Parental history of AD | 6.967 (2.551~19.030) | <0.001 |
| Parental history of allergic rhinitis | 5.326 (1.996~14.209) | <0.001 |
| Childhood-onset (n=89/178) (case/control) | ||
| Parental history of AD | 8.000 (3.003~21.315) | <0.0001 |
| Adolescent onset (n=53/106) (case/control) | ||
| Parental history of AD | 13.277 (3.007~58.628) | 0.001 |
AD: atopic dermatitis, OR: odds ratio, CI: confidence interval. Multivariate conditional logistic regression analysis with forward conditional variable selection was performed with p<0.05 as the criterion for model inclusion using PASW Statistics ver. 18.0 software.
Factors affecting the clinical severity of AD
Table 4 presents our analysis of the factors affecting the clinical severity (SCORAD index) of AD in the study subjects. The SCORAD index was progressively higher in the groups with an earlier disease onset (adolescence<childhood<infancy, p=0.002), and in those with smaller habitation size during adolescence (from above 1,000,000 to under 50,000), implying that the clinical severity of AD was higher in these groups (p<0.00001).
Table 4. Association of the severity scoring of atopic dermatitis index with clinical variables by multiple linear regression analysis.
| Clinical variable | β±standard error | p-value |
|---|---|---|
| Body mass index | 0.697±2.200 | 0.752 |
| Age of onset | −3.880±1.256 | 0.002 |
| Population of residential region in adolescence | −9.724±1.475 | <0.00001 |
| Economic status in adolescence | −0.284±2.210 | 0.898 |
| Nuclear Family in adolescence | 0.236±2.858 | 0.934 |
| Housing type in adolescence | 0.945±1.021 | 0.356 |
| Parental history of atopic dermatitis | −3.172±2.165 | 0.144 |
| Parental history of asthma | 5.514±4.963 | 0.268 |
| Parental history of allergic rhinitis | −4.28±2.415 | 0.078 |
| Number of siblings | −0.873±1.652 | 0.598 |
| Mother's educational status | 0.039±1.769 | 0.983 |
| Mother's profession as housewife | 3.099±1.902 | 0.105 |
| Father's educational status | −0.893±1.91 | 0.641 |
| Father's profession as a white collar worker | −1.094±2.024 | 0.589 |
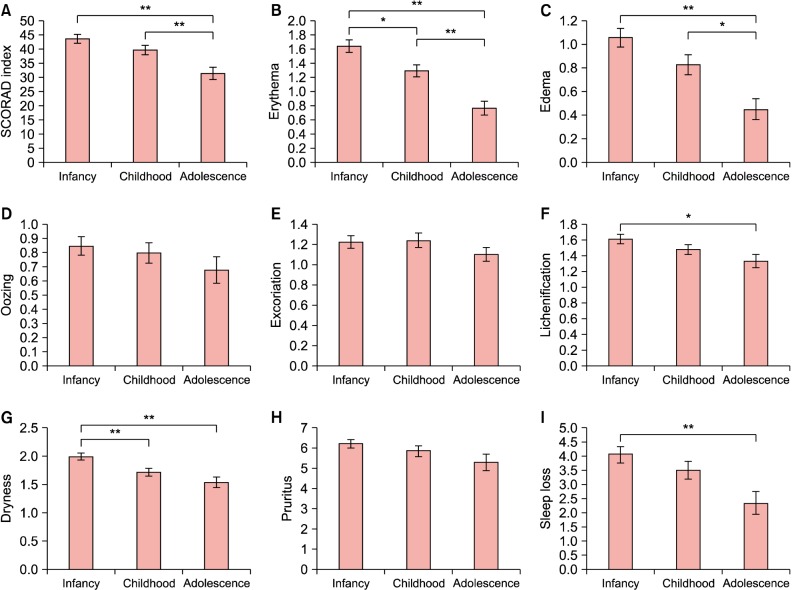
Differences in the clinical characteristics of the AD patients in the study series according to the age of disease onset
Fig. 1. presents the one-way ANOVA results for the SCORAD index and for each individual factor included in this index according to the age of disease onset. The overall SCORAD index and the individual symptoms of erythema, edema, lichenification, dryness of skin, and sleep loss were progressively more severe in the disease-onset groups from adolescence to infancy, and these differences were statistically significant (p<0.05 or p<0.01).
Fig. 1. Comparison of the severity of clinical manifestations according to the age of onset in patients with atopic dermatitis (AD), using analysis of variance (ANOVA). (A) Severity scoring of atopic dermatitis (SCORAD) index, (B) erythema, (C) edema, (D) oozing, (E) excoriation, (F) lichenification, (G) dryness, (H) pruritus, (I) sleep loss. *p<0.05, **p<0.01.
DISCUSSION
The point prevalence of adulthood AD is known to range from 1% to 3%, yet few real epidemiological studies of this disease can be found in the literature9,10,11,12. In our current study, the prevalence rates in adults over 19 years old were relatively similar to the 1%~3% rates described previously. The National Health Insurance Corporation in Korea analyzed a large body of national statistical data in this regard in 2008 and reported a prevalence rate in 18-year-old male subjects of 1.9%10. The AD prevalence in our present study was lower than that in the aforementioned analyses because we measured the point-prevalence instead of 1-year period prevalence. Moreover, it can be speculated that the low AD prevalence estimated in our present study (1.15% to 1.44%) could be attributed to the study design where the dermatologists involved in our series screened for AD directly and calculated the prevalence rate on the basis of the Hanifin and Rajka criteria. We are thus convinced that our study gives an accurate estimate of the actual AD prevalence in young Korean men. In addition, as we included a large number of subjects without many factors that could potentially lead to selection bias, we are confident that our study population is very representative of the actual male adolescent population in Korea.
In Korea from 1994 to 1995, some dermatologists tried to investigate the real prevalence of AD in infancy by analyzing the history of 6~8-year-old subjects on the basis of the Hanifin and Rajka criteria. They reported prevalence rates of AD in the Seoul and Chuncheon areas of 17.5%±2.9% and 9.6%±2.6%, respectively, which referred to patients born in 1989, i.e., close in age to the subjects of our current study9. Taken together, the results of that earlier study and our present analysis could help to reconstruct the natural course of AD: the prevalence rates in individuals born in 1989 in Seoul and Chuncheon was 17.5% and 9.6%, respectively, at the age of 6, but dropped to around 1% at the age of 19. In addition, the prevalence rates at 6~8 years of age were significantly different between Seoul (urban area) and Chuncheon (rural area) in the previous study, yet were not significant among North Seoul, South Seoul, and West Gangwon-do (Chuncheon) in our present analyses. This discrepancy suggests that differences between urban and rural areas affect the AD prevalence in infancy and childhood, whereas these differences might decrease during adulthood.
The strength of our present epidemiological study on AD prevalence is that dermatologists directly took part in the physical examination of a large sampling group of 19,048 individuals. Our very large sample size of individuals of the same age and sex greatly increased the accuracy of our prevalence rate calculations when compared with previous reports that used the ISSAC survey and also ensured that our study population was highly representative. An additional strength of our current study is that experienced dermatologists evaluated all the subjects according to the SCORAD index.
Another merit of our current case-controlled study was our ability to closely match subjects of the same age and sex. In order to reduce socio-economic differences, which can vary between provinces, the control group was selected via a 1:2 matching in the same MMA on the same day when the corresponding AD patient was diagnosed. Moreover, given that housing type, size of habitation, and economic status would not be consistent during 19 years of life, the collection of these various factors was stratified by infancy, childhood, and adolescence. Therefore, in the childhood-onset group, the various factors present in infancy and childhood were used for the analysis, whereas in cases of adolescent onset disease, the factors were taken into account separately in each life period.
Peters et al.7 aimed in their previous study to describe the course of AD over puberty and prospectively determine the risk factors for the incidence, recurrence, and persistence of AD until adolescence in a population-based cohort study. The significant predictors of AD course in that study were found to be a high socioeconomic status, female sex, asthma symptoms, positive skin prick test response at baseline, parental history of rhinitis/AD, and having worked in a high-risk job. Although it is difficult to directly compare that study with our current analyses because our present study design was a retrospective investigation of the natural course of AD from the periods of infancy and childhood to the age of 19, our results confirmed that a parental history of AD is a risk factor in all of the disease-onset groups, and that parental history of rhinitis is a significant risk factor in the infancy-onset group.
A strong familial component has always been a feature of AD, and previous twin studies have pointed to a strong influence of genetic factors13. Our present study findings were consistent with these earlier data. A parental history of AD was found in our analysis to be a risk factor in all of the disease-onset groups, a parental history of allergic rhinitis was a risk factor only in the infancy-onset AD group, and the parental history of asthma was not a risk factor regardless of the disease-onset age. Our study findings show some support for a previous report that the portion of AD related to allergy increases with a younger age of onset. A hygiene-related hypothesis has also been proposed for AD. Factors including a high economic status, high parental education, urban living, and small number of siblings would provide an environment which prevents children from coming in contact with various antigens during their younger age, ultimately contributing to the risk of AD14,15. This hypothesis has been confirmed by many studies. However, our current case-control study led to the different conclusion that a low economic status during infancy was a risk factor for AD, and that the clinical severity of AD correlated with a smaller regional population for adolescent AD patients. In light of our present results, we hypothesize that low economic status may lead to a delay in the diagnosis of AD and low adherence to therapy, consequently resulting in a more severe clinical course. As the risk factors for AD in infancy and childhood were retrospectively investigated in 19-year-old subjects in our current analyses, it is possible that a different conclusion could be drawn because the treatment behaviors in infancy have reciprocal actions. Conversely, active treatment behavior and the use of better facilities in families with a high economic status in more populated residential regions could have a protective effect against the onset of AD and its clinical severity.
Garmhausen et al.1 have reported that adolescent and adult patients with AD can be classified into five main groups according to the clinical course. The total immunoglobulin E (IgE) number, number of sensitizations, Diepgen criteria, prevalence of flexural lichenification and eczema, and portion of allergic form are higher in early-onset disease with a chronic persisting course until adulthood than in adolescent-onset disease with a chronic persisting course until adulthood. Similarly, our present analyses indicated that the SCORAD index and the scores for erythema, edema, lichenification, dryness of skin, and sleep loss, all variables included in the index, progressively reduce from the infancy-onset through to the childhood-onset and adolescent onset group. A further study is necessary to elucidate the reasons for this finding and its relevance to the data in previous studies.
There were some limitations of our present study of note. First, the results we obtained from questionnaires were subject to a recall bias; in particular, memories of various social factors at the age of 0~2 may not be exact. Likewise, such experiences as breastfeeding or exposure to pet animals during infancy cannot be investigated. In addition, the responses to the questions regarding economic status at a certain period of the subjects' lives could be arbitrary and purely based on memory recall. For reference, the average annual household income for a family ranking in the top 30% according to annual income was US$12,400, whereas the average annual household income for a family ranking in the bottom 30% was US$5,100. The overall average annual household income was US$8,100 in Korea 1998, when the subjects were 10 years of age. Similarly in 1990, when the subjects were two years old, the annual household income for a family ranking in the top 30%, bottom 30% and the overall average income were US$5,450, US$2,570, and US$3,750, respectively. And in 2004, when the subjects were sixteen, the annual household income for a family ranking in the top 30%, bottom 30% and the overall average income were US$18,700, US$7,690, and US$12,440, respectively16.
Additionally, the time lag between the various factors in infancy-childhood and the incidence of AD in adolescence makes a risk evaluation difficult. In addition, our study subjects comprised only male subjects, which may not give an accurate estimate of the overall AD prevalence in the entire population. Moreover, as this was an epidemiological study of physical examinations at an MMA, clinical tests such as skin prick test and serum IgE level test could not be conducted. Conditional logistic regression analysis was not possible for our whole cohort of 245 AD patients, because of possible discrepancies in the chronology of the risk factors and of the time of AD onset (for example, economic status in adolescence and infancy-onset AD).
ACKNOWLEDGMENT
We would like to thank three dermatologists (Soo-Jung Park, MD., Tach-Hun Kim, MD., Joong-Geun Kim, MD.) who contributed to this epidemiological research in 2008 at local Military Manpower Administration centers (MMA: Seoul North MMA, Seoul South MMA, Gyeonggi South MMA, and Gangwon West MMA).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506. doi: 10.1111/all.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsarou A, Armenaka M. Atopic dermatitis in older patients: particular points. J Eur Acad Dermatol Venereol. 2011;25:12–18. doi: 10.1111/j.1468-3083.2010.03737.x. [DOI] [PubMed] [Google Scholar]
- 3.Duhme H, Weiland SK, Rudolph P, Wienke A, Kramer A, Keil U. Asthma and allergies among children in West and East Germany: a comparison between Münster and Greifswald using the ISAAC phase I protocol. International Study of Asthma and Allergies in Childhood. Eur Respir J. 1998;11:840–847. doi: 10.1183/09031936.98.11040840. [DOI] [PubMed] [Google Scholar]
- 4.Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 5.Björkstén B, Dumitrascu D, Foucard T, Khetsuriani N, Khaitov R, Leja M, et al. Prevalence of childhood asthma, rhinitis and eczema in Scandinavia and Eastern Europe. Eur Respir J. 1998;12:432–437. doi: 10.1183/09031936.98.12020432. [DOI] [PubMed] [Google Scholar]
- 6.Ziyab AH, Raza A, Karmaus W, Tongue N, Zhang H, Matthews S, et al. Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy. 2010;40:1776–1784. doi: 10.1111/j.1365-2222.2010.03633.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters AS, Kellberger J, Vogelberg C, Dressel H, Windstetter D, Weinmayr G, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126:590–595. 595.e1–595.e3. doi: 10.1016/j.jaci.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Rudzki E, Samochocki Z, Rebandel P, Saciuk E, Gałecki W, Raczka A, et al. Frequency and significance of the major and minor features of Hanifin and Rajka among patients with atopic dermatitis. Dermatology. 1994;189:41–46. doi: 10.1159/000246781. [DOI] [PubMed] [Google Scholar]
- 9.Kim CW, Park CJ, Kim JW, Koo DW, Kim KW, Kim TY. Prevalence of atopic dermatitis in Korea. Acta Derm Venereol. 2000;80:353–356. doi: 10.1080/000155500459295. [DOI] [PubMed] [Google Scholar]
- 10.Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, et al. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012;27:681–685. doi: 10.3346/jkms.2012.27.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836–845. doi: 10.1111/all.12619. [DOI] [PubMed] [Google Scholar]
- 12.Roduit C, Frei R, Loss G, Büchele G, Weber J, Depner M, et al. Development of atopic dermatitis according to age of onset and association with early-life exposures. J Allergy Clin Immunol. 2012;130:130–136.e5. doi: 10.1016/j.jaci.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen SF, Ulrik CS, Kyvik KO, Hjelmborg Jv, Skadhauge LR, Steffensen I, et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy Asthma Proc. 2007;28:535–539. doi: 10.2500/aap2007.28.3041. [DOI] [PubMed] [Google Scholar]
- 14.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl 1):S2–S10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grammatikos AP. The genetic and environmental basis of atopic diseases. Ann Med. 2008;40:482–495. doi: 10.1080/07853890802082096. [DOI] [PubMed] [Google Scholar]
- 16.Korean Statistical Information Service. National statistics on household income and expenditure [Internet] Daejeon: Korean Statistical Information Service; 2015. Sep 20, [cited 2015 Sep 20]. Available from: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1L6E001&conn_path=I2. [Google Scholar]