Abstract
Deoxyglycychloxazol (TY501) is a glycyrrhetinic acid derivative which exhibits high anti-inflammatory activity and reduced pseudoaldosteronism compared to glycyrrhetinic acid. In this study, a sensitive and rapid liquid chromatography–tandem mass spectrometry (LC–MS/MS) method was established for the quantitation of TY501 in rat plasma. Plasma samples were treated by precipitating protein with methanol and supernatants were separated by a Symmetry C8 column with the mobile phase consisting of methanol and 10 mM ammonium formate (containing 0.1% of formic acid) (90:10, v/v). The selected reaction monitoring (SRM) transitions were performed at m/z 647.4→191.2 for TY501 and m/z 473.3→143.3 for astragaloside aglycone (IS) in the positive ion mode with atmospheric pressure chemical ionization (APCI) source. Calibration curve was linear over the concentration range of 5–5000 ng/mL. The lower limit of quantification was 5 ng/mL. The mean recovery was over 88%. The intra- and inter-day precisions were lower than 6.0% and 12.8%, respectively, and the accuracy was within ±1.3%. TY501 was stable under usual storage conditions and handling procedure. The validated method has been successfully applied to a pharmacokinetic study after oral administration of TY501 to rats at a dosage of 10 mg/kg.
Keywords: Deoxyglycychloxazol (TY501), LC–MS/MS, APCI, Pharmacokinetics, Rat plasma
1. Introduction
Liquorice, the root of glycyrrhiza spp., has been used clinically over centuries in China and proved to have antibacterial, cardiac, anti-inflammatory and tonic activities [1], [2], [3]. Glycyrrhetinic acid is the sapogenin of glycyrrhizic acid, a major bioactive component of liquorice, which can relieve peptic ulcer, enhance immunity, and present anti-inflammatory, hepatoprotective and other activities [1], [3], [4]. However, it has been found that pseudoaldosteronism is usually induced by glycyrrhetinic acid. In this case, the effect of sodium retention and potassium excretion can be enhanced dramatically, leading to edema, hypertension and hypopotassemia. [1], [5], [6], [7].
Deoxyglycychloxazol (TY501, Fig. 1A) is a glycyrrhetinic acid derivative which is designed and synthesized to reduce the pseudoaldosteronism by destructing the 11-oxo system in ring C [8], [9]. On the other hand, therapeutic activities are retained and enhanced by modification of the terminal carboxyl attached to ring E with isoxazole of glycyrrhetinic acid [10], [11], [12]. According to the pharmacodynamic studies [11], [12], [13], [14], [15], promising anti-inflammatory, anti-fibrotic, anti-gastric ulcer and other activities as well as limited hepatorenal damage and gastrointestinal reaction were observed. It is suggested that TY501 is much safer than other existing steroid and non-steroid anti-inflammatory drug entities; it, therefore, has the potential to be a good drug candidate. To evaluate and understand the pharmacokinetic properties of TY501, we developed a simple and rapid bioanalytical method for quantification of TY501 in biological samples for preclinical and clinical pharmacokinetics studies.
Fig. 1.
Structures of (A) deoxyglycychloxazol and (B) astragaloside aglycone.
Compared with other detection techniques, mass spectrometry is considered as the most specific and sensitive approach for most chemicals, and becomes the first choice of quantitation for low molecular weight drugs in biological samples. To the best of our knowledge, the pharmacokinetics of TY501 has not been studied by liquid chromatography–tandem mass spectrometry (LC–MS/MS). In this study, a rapid, simple and sensitive LC–MS/MS method for the quantitation of TY501 in rat plasma has been developed and validated. The method has several advantages, including small volume plasma sample (50 μL), simple sample preparation (protein precipitation), good chromatographic resolution, specific and sensitive mass spectrometric conditions and the broad concentration range of 5–5000 ng/mL. The method established was then successfully applied to determine the plasma concentration of TY501 for a pharmacokinetic study after oral administration to rats at a dosage of 10 mg/kg.
2. Experimental
2.1. Chemicals and reagents
TY501 (Purity 99.6%) and astragaloside aglycone (Purity 99.5%) (IS, Fig. 1B) were both kindly provided by the Laboratory of Medicinal Chemistry, Tianjin Institute of Pharmaceutical Research (Tianjin, China). Methanol of HPLC grade was purchased from Tianjin Concord Tech. Reagent Co., Ltd. (Tianjin, China). Ammonium formate and other reagents were of analytical grade. Deionized water was used throughout the experiment.
2.2. Chromatography conditions
Chromatographic separation was carried out on a Symmetry C8 column (50 mm×4.6 mm, 5 µm; Waters Corp., Milford, MA, USA) using a Shimadzu UFLC system equipped with a binary pump, a vacuum degasser and an autosampler (Shimadzu Corporation, Kyoto, Japan). The column oven was maintained at 40 °C. The mobile phase consisting of methanol and 10 mM ammonium formate (containing 0.1% of formic acid) (90:10, v/v) was performed at a flow rate of 0.5 mL/min. The samples were kept at 4 °C in the autosampler.
2.3. Mass spectrometry conditions
The mass spectrometry was performed on an API 4000 QTrap® mass spectrometer (Applied Biosystems Sciex, Toronto, Canada). Analyst 1.5 software was used for data acquisition and analysis (Applied Biosystems Sciex, Toronto, Canada). Ionization was operated using an atmospheric pressure chemical ionization (APCI) source in positive ion mode. The APCI source was operated with an ionspray voltage of 5000 V. The nebulizer gas was nitrogen delivered at 30 psi. The capillary temperature was 320 °C. The curtain gas and collision gas were 10 psi and 6 psi, respectively. The value of corona discharge needle was 5 μA. The collision cell exit potential was 10 V. The MS recordings were carried out in selected reaction monitoring (SRM) mode with specific ion transitions of precursor ion to product ion at m/z 647.4→191.2 with collision energy (CE) of 38 eV, declustering potential (DP) of 85 V and entrance potential (EP) of 15 V for TY501, and at m/z 473.3→143.3 with CE of 23 eV, DP of 55 V and EP of 10 V for IS. The total analytical time was 5 min.
2.4. Calibration standard and quality control samples preparation
The stock solution of TY501 (1.0 mg/mL) was prepared in methanol, and further diluted with methanol to obtain the working solutions at the concentrations of 5, 10, 50, 200, 1000, 2000 and 5000 ng/mL. The IS stock solution (1.0 mg/mL) was diluted with methanol to get the concentration of 0.96 μg/mL. All the solutions were kept at 4 °C and brought to room temperature before use.
The 50 μL of working solution of TY501 was evaporated to dryness at 40 °C under a gentle stream of nitrogen, and then 50 μL of blank rat plasma was added, which was vigorously vortex-mixed for 30 s. The concentration levels of TY501 in plasma ranged from 5 to 5000 ng/mL. Quality control (QC) samples were prepared in a similar manner at low, medium and high levels (10, 200, 4000 ng/mL). Both the calibration standard and QC samples were prepared from the same stock solution. All the spiked plasma samples were then treated according to sample preparation procedure. Both the calibration standard samples and the QC samples were used in the method validation and the pharmacokinetic study.
2.5. Sample preparation
A total of 50 μL of plasma sample was added with 250 μL of IS working solution (0.96 μg/mL) in polypropylene tube. Subsequently, the tube was vigorously vortex-mixed for 30 s to precipitate plasma proteins and centrifuged (12,000 rpm, Refrigerated centrifuge SIGMA 3K15, Osterode am Harz, Germany) for 10 min. The supernatant was transferred to an injection vial and then an aliquot of 20 μL of the supernatant was injected for LC–MS/MS analysis.
2.6. Method validation
2.6.1. Selectivity
Six different rat blank plasma samples were prepared and analyzed to investigate potential interferences of endogenous compounds. The chromatogram of blank plasma sample was compared with those of plasma samples spiked with the analyte and IS and incurred plasma sample after oral administration of TY501.
2.6.2. Linearity
To evaluate linearity of this method, calibration standards were prepared and analyzed in triplicate on three consecutive days. Calibration curves were constructed by plotting the peak area ratio (analyte/IS) versus the spiked concentrations of TY501 by least square linear regression analysis with a weighting factor of 1/x2. Deviations of these back-calculated concentrations from calibration standard samples were set within ±15% of nominal concentrations (±20% for the lower limit of quantitation (LLOQ)).
2.6.3. Precision and accuracy
Precision and accuracy were assessed by determining six replicates of the low, medium and high QC samples (10, 200, 4000 ng/mL) on three consecutive days. The precision was expressed as the relative standard deviation (RSD) and the accuracy was described as the relative error (RE). The acceptable intra- and inter-day precision is required to be less than 15% and the acceptable accuracy is required to be within ±15% for all QC samples.
2.6.4. LLOQ
The LLOQ of the assay was assessed as the lowest concentration of the calibration curve that can be quantitatively determined with acceptable precision less than 20% and accuracy within ±20%. The LLOQ was established based on six replicates on three consecutive days.
2.6.5. Matrix effect
The matrix effect was defined as the ion suppression/enhancement on the ionization of the analytes, which was evaluated by comparing the corresponding peak areas of the post-extraction spiked samples to those of the standard solutions evaporated directly and reconstituted in mobile phase. Experiments were performed at the three QC levels, in six replicates.
2.6.6. Recovery
The extraction recoveries of TY501 and IS were estimated by comparing the peak areas obtained from these prepared plasma samples described above with those obtained from direct injection of standard solutions without preparation at the same concentrations. Experiments were performed at three QC concentration levels, in six replicates.
2.6.7. Stability
The stability of TY501 in rat plasma was investigated by comparing the measured concentrations of low, medium and high QC samples (in triplicate) with the spiked concentrations under the following four storage conditions: (i) stability of the analyte in plasma was assessed by placing QC samples for 24 h at room temperature and then extracted for analysis; (ii) for freeze–thaw stability, QC samples were determined through three freeze (−20 °C)–thaw (room temperature) cycles; (iii) to evaluate the stability of the treated plasma samples in the autosampler, QC samples were prepared and placed in the autosampler at 4 °C for a period of 24 h, and then injected for analysis; (iv) for long-term storage stability, QC samples were prepared and stored at −20 °C for 30 days. The analyte was considered stable when the percent deviation was within ±15%.
2.7. Pharmacokinetic study
Six Wistar rats (male and female, weighting 200±20 g, Certificate No.: SCXK (jing) 2006–0009) were purchased from Beijing Marshall Biotechnology Co., Ltd. (Beijing, China). The rats were housed under controlled environmental conditions (temperature 20–22 °C; humidity 45%–65%) and had ad libitum access to water and to a standard laboratory diet. The experimental protocols concerning the animal care and use for this study were approved by Institutional Animal Care and Use Committee of Tianjin Institutes (China). Before oral administration, the rats were fasted for 12 h but with access to water, and further fasted for 2 h after administered. TY501 was suspended in 0.5% carboxymethyl cullulose sodium (CMC-Na) solution and orally administrated to each rat at a dosage of 10 mg/kg. 0.1 mL of blood samples were collected in heparinized tubes pre-dose and at 0.5, 1, 2, 3, 5, 8, 12, 24, 32, 48, 56, 72 and 96 h post-dose. Plasma samples were obtained by centrifugation at 3000 rpm for 10 min. All plasma samples were stored at −20 °C and analyzed within a month.
Non-compartment model was used to calculate the pharmacokinetic parameters with DAS 2.0 software. The area under the curve from zero to the last sampling time (AUC0−t) was calculated by trapezoidal method. The terminal elimination half-life (t1/2) was then calculated as 0.693/ke. Plasma clearance (Cl) was calculated as Dose/AUC.
3. Results and discussion
3.1. Chromatographic conditions
Multiple chromatographic conditions were explored in order to have appropriate retention time, and better resolution and sensitivity. Symmetry C18 column (50 mm×4.6 mm) and C8 column (50 mm×4.6 mm) were evaluated to attain better separation. It was found that the binding of TY501 on C18 column was quite strong; therefore it could not be eluted easily. In comparison, C8 column showed shorter retention time and better peak shape than C18 column and was finally chosen for the chromatographic separation. The mobile phase consisting of methanol and 10 mM ammonium formate (containing 0.1% of formic acid) (90:10, v/v) was found to be optimal for this study. Better chromatographic separation and higher response were obtained using methanol than acetonitrile. The addition of 10 mM ammonium formate and formic acid was believed to be able to have better peak shape; thus, the sensitivity can be enhanced. In addition, under the optimized conditions, no significant endogenous interference was found.
3.2. Mass spectrometric conditions
Electron spray ionization (ESI) and APCI are the most commonly used soft ionization sources in mass spectrometry. Although the product ion mass spectra of TY501 using ESI- and APCI-MS were similar in terms of full-scan and fragmentation, a higher response and less susceptibility to the matrix can be achieved by APCI. Accordingly, APCI, rather than ESI, as the ionization source, was chosen in this study. By investigating the full-scan mass spectra of TY501, it was found that the signal intensity in the positive mode was much higher than that in the negative ion mode. Thus, all detections were carried out using the predominantly positive ion mode. The full-scan positive ion mass spectrum showed that TY501 and IS were protonated molecular ion ([M+H]+) of m/z 647.4 and dehydrated protonated molecular ion ([M+H-H2O]+) of m/z 473.3, respectively. After fragmentation in the collision cell, the most abundant and stable product ions (Fig. 2) were at m/z 191.2 for TY501 and at m/z 143.3 for IS, respectively. The collision-induced dissociation (CID) parameters were optimized to enhance the response and specificity using the SRM mode comprising the precursor and product ions. The most suitable mass spectrometric conditions were determined by optimizing all the parameters of the mass spectrometer such as collision energy, nebulizer gas, curtain gas, collision gas, spray voltage and capillary temperature to obtain much higher and more stable response.
Fig. 2.
Product ion mass spectra of (A) deoxyglycychloxazol and (B) astragaloside aglycone.
3.3. Sample preparation
A number of samples need to be analyzed in pharmacokinetic study. Thus, a simple, rapid and convenient sample preparation is necessary and critical. Several liquid chromatography-mass spectrometry (LC–MS) or LC–MS/MS studies on the quantitation of glycyrrhetinic acid in human plasma samples have been reported in literature [16], [17], [18], [19]. Most of the methods used liquid-liquid extraction (LLE) and solid phase extraction (SPE) steps for sample processing. Protein precipitation was chosen for the sample preparation in this study, since it was much easier to handle and less time-consuming; most importantly, the extraction recoveries of TY501 were very close in the three sample pretreatment processes.
3.4. Method validation
3.4.1. Selectivity and specificity
The selectivity of the method was investigated by comparing chromatograms of blank plasma, spiked plasma and rat plasma after oral administration of TY501. The retention time was 3.0 and 1.7 min for TY501 and IS, respectively (Fig. 3). As shown in the figures, there were no significant endogenous peaks that could interfere with the analyte and IS. The results indicated that the method exhibited good specificity and selectivity and was applicable to plasma samples for the pharmacokinetic study.
Fig. 3.
SRM chromatograms of (A) blank rat plasma; (B) blank rat plasma spiked with deoxyglycychloxazol (LLOQ, 5 ng/mL) and IS (0.96 µg/mL); (C) blank rat plasma spiked with deoxyglycychloxazol (1000 ng/mL) and IS (0.96 µg/mL); (D) a rat plasma sample at 5 h after oral administration of 10 mg/kg of deoxyglycychloxazol.
3.4.2. LLOQ
Calibration curves were linear over the concentration range of 5–5000 ng/mL. The typical regression equation obtained by least square regression was y=0.0315x+0.0007, where y is the peak area ratio of the analyte to IS, and x is the concentration of the analyte. The correlation coefficient (r2) was ≥0.99 for all calibration curves, and the observed deviation was within ±15% for all calibration concentrations. The LLOQ of TY501 was measured to be 5 ng/mL. The intra- and inter-day precisions of the LLOQ were within ±5.2% and ±11.6%, and the accuracy was within ±4.8% (Table 1), which was sufficient for pharmacokinetic studies of TY501 in rats.
Table 1.
Precision and accuracy of deoxyglycychloxazol in rat plasma.
| Concentration (ng/mL) |
Precision (RSD (%)) |
Accuracy (RE (%)) | ||
|---|---|---|---|---|
| Spiked | Measured (Mean±SD) | Intra-day | Inter-day | |
| 5 (LLOQ) | 4.7±0.3 | 5.2 | 11.6 | −4.8 |
| 10 | 10.0±0.6 | 6.0 | 3.1 | −0.0 |
| 200 | 200±8.7 | 3.8 | 7.3 | −0.1 |
| 4000 | 4050±225 | 3.6 | 12.8 | 1.3 |
3.4.3. Precision and accuracy
QC samples at three concentrations were analyzed in six replicates in order to determine the assay accuracy and precision. As shown in Table 1, the intra- and inter-day precisions were within ±6.0% and ±12.8%, and the accuracy was within ±1.3% for TY501, which indicated that the present method has a satisfactory accuracy and precision.
3.4.4. Matrix effect
The ion suppression/enhancement of TY501 in signal ranged from 6.5% to 12.4% for three QC levels, indicating that the matrix effect on the ionization of the analyte is not obvious under these conditions.
3.4.5. Recovery
Under the given set of operating condition, the extraction recoveries of TY501 in rat plasma were (88.9±4.6)%, (89.6±2.5)% and (91.0±2.3)% at concentrations of 10, 200 and 4000 ng/mL. The mean absolute recovery of the IS was 96.1%.
3.4.6. Stability
Results of the stability tests for TY501 are shown in Table 2. It can be seen that TY501 was stable in rat plasma samples under various storage and analysis conditions, including being placed at room temperature for 24 h and being stored at –20 °C for 30 days or through three freeze-thaw cycles. On the other hand, processed samples were found to be stable at 4 °C in the autosampler for a period of 24 h, indicating that a large number of samples could be processed in each analytical run.
Table 2.
Stability of deoxyglycychloxazol in rat plasma (n=3).
| Storage conditions | Concentration (ng/mL) |
RE (%) | |
|---|---|---|---|
| Spiked | Measured (Mean±SD) | ||
| At room temperature for 24 h | 10 | 9.9±0.2 | −0.6 |
| 200 | 194.0±4.0 | −3.0 | |
| 4000 | 4070.0±125.8 | 1.8 | |
| Three freeze-thaw cycles | 10 | 10.3±0.9 | 3.0 |
| 200 | 191.0±7.8 | −4.5 | |
| 4000 | 3890.0±141.8 | −2.8 | |
| At 4 °C in the autosampler for 24 h | 10 | 9.8±0.6 | −2.1 |
| 200 | 195.0±7.0 | −2.5 | |
| 4000 | 4030±143.0 | −0.8 | |
| At −20 °C for 30 days | 10 | 9.5±0.9 | −4.9 |
| 200 | 189.0±7.0 | −5.5 | |
| 4000 | 3935.7±87.9 | −1.6 | |
3.5. Pharmacokinetic study
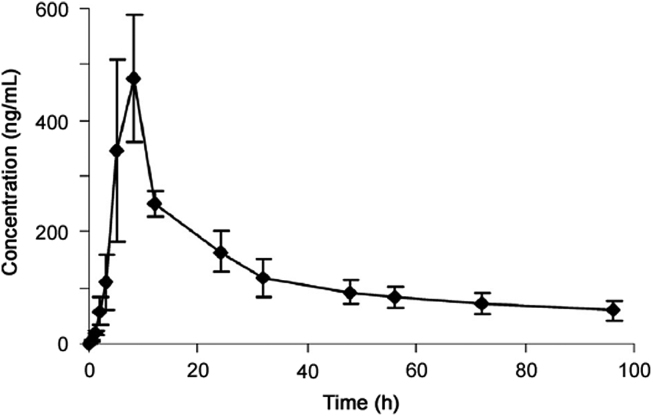
The mean plasma concentration-time profile following oral administration of TY501 to rats is shown in Fig. 4. The main pharmacokinetic parameters of TY501 are calculated and demonstrated in Table 3. The Cmax and t1/2 were different between male and female rats, which suggests that the absorption and metabolism of deoxyglycychloxazol may be affected by genders.
Fig. 4.
Mean plasma concentration–time profile of deoxyglycychloxazol after a single oral administration of 10 mg/kg to rats (n=6, Mean±SD).
Table 3.
Main pharmacokinetic parameters of deoxyglycychloxazol after oral administration of 10 mg/kg to rats (Female/male: n=3, Total: n=6, Mean±SD).
| Pharmacokinetic parameters | Deoxyglycychloxazol |
||
|---|---|---|---|
| Female | Male | Total | |
| Cmax (ng/mL) | 563±93.3 | 388±36.2 | 475±115 |
| Tmax (h) | 8±0 | 8±0 | 8±0 |
| t1/2 (h) | 35.2±20.1 | 82.0±17.0 | 58.6±30.6 |
| CL (L/kg h) | 585±78.1 | 565±199 | 575±136 |
| AUC0−t (ng h/mL) | 11,985±2573 | 11,668±2636 | 11,827±2336 |
| AUC0−∞ (ng h/mL) | 15,135±4976 | 19,344±6140 | 17,239±5505 |
Cmax: the maximum plasma concentration.
Tmax: the time to reach peak concentration.
t1/2: the terminal elimination half-life.
CL: the plasma clearance.
AUC0−t: the area under the curve from zero to the last sampling time.
AUC0−∞: the AUC0−t extrapolated to infinity.
4. Conclusion
A simple, sensitive, selective and novel LC–MS/MS method was established for the determination of TY501 in rat plasma. The present method employed a simple and rapid protein precipitation procedure for sample preparation, and a high sensitivity could be achieved, with the LLOQ of 5 ng/mL for TY501. Furthermore, only a limited amount of plasma sample (50 μL) was needed for the analysis. The method has been successfully validated via an oral pharmacokinetic study of TY501 in rats.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 2.Fiore C., Eisenhut M., Ragazzi E. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005;99:317–324. doi: 10.1016/j.jep.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asl M.N., Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza Sp. and its bioactive compounds. Phytother. Res. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X., Wang W., Wei S. Review of pharmacological effects of Glycyrrhiza radix and its bioactive compounds. China J. Chin. Mater. Med. 2009;34:2695–2700. [PubMed] [Google Scholar]
- 5.Stormer F.C., Reistad R., Alexander J. Glycyrrhizic acid in liquorice – evaluation of health hazard. Food Chem. Toxicol. 1993;31:303–312. doi: 10.1016/0278-6915(93)90080-i. [DOI] [PubMed] [Google Scholar]
- 6.Wu P., Zhang Y., Liu Y. Effects of glycyrrhizin on production of vascular aldosterone and corticosterone. Horm. Res. 1999;51:189–192. doi: 10.1159/000023356. [DOI] [PubMed] [Google Scholar]
- 7.Liu R. Licorice and glycyrrhizinic acid preparation-induced pseudohypera-ldosteronism: prevention and treatment. Adverse Drug React. J. 2009;11:416–419. [Google Scholar]
- 8.Shibata S., Takahashi K., Yano S. Chemical modification of glycyrrhetinic acid in relation to the biological activities. Chem. Pharm. Bull. 1987;35:1910–1918. doi: 10.1248/cpb.35.1910. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y.X., Fan X.S., Peng Y. Phase transfer catalysis for synthesizing 11-deoxyglycyrrhetinic acid-3-esters derivates. Hua Xue Shi Jie. 2001;42 533–524. [Google Scholar]
- 10.Liu L.J., Yong P.J., Dai X.J. Synthesis of novel Isoxazole contained glycyrrhetinic acid derivatives. Chem. J. Chin. Univ. 2006;27:1669–1672. [Google Scholar]
- 11.Tang L.D., Wang J.W., Yong J.P. Novel 11-deoxylglycyrrhetinic acid-30-acylamide derivatives. Chin. Tradit. Herb. Drugs. 2006;37:20–25. [Google Scholar]
- 12.J.W. Wang, W.R. Xu, J.P. Wong, et al., Glycyrrhetinic Acid-30-Amide Dedivatives and Their Use, U.S. Patent 7790759, 2010.
- 13.Jin R.F., Liu J., Zhang J.X. Effect of glycyrrhetinic acid and its derivative TY501 on the proliferation of murine macrophage RAW264.7. Drug Eval. Res. 2011;34:255–257. [Google Scholar]
- 14.Geng Y.Y., Yu B., Zhou Z.X. Antifibrotic effect of TY501 on bleomycin-induced pulmonary fibrosis in rats and related mechanism. Chin. Pharmacol. Bull. 2015;31:210–215. [Google Scholar]
- 15.Cui L.P., Wang Y.L., Zhang S.J. Effects of glycyrrhetic acid derivative on renal injury of rats. Chin. Trandit. Herb. Drugs. 2011;42:1177–1179. [Google Scholar]
- 16.Lin Z.J., Qiu S.X., Wufuer A. Simultaneous determination of glycyrrhizin, a marker component in radix Glycyrrhizae, and its major metabolite glycyrrhetic acid in human plasma by LC–MS/MS. J. Chromatogr. B. 2005;814:201–207. doi: 10.1016/j.jchromb.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Ding L., Huang X., Yang J. Determination of glycyrrhetic acid in human plasma by LC-ESI-MS. J. Pharm. Biomed. Anal. 2006;40:758–762. doi: 10.1016/j.jpba.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W.J., Wang B.J., Wei C.M. Determination of glycyrrhetic acid in human plasma by HPLC–MS method and investigation of its pharmacokinetics. J. Clin. Pharm. Ther. 2008;33:289–294. doi: 10.1111/j.1365-2710.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 19.Zou Q., Wei P., Li J. Simultaneous determination of 18alpha- and 18beta-glycyrrhetic acid in human plasma by LC-ESI-MS and its application to pharmacokinetics. Biomed. Chromatogr. 2009;23:54–62. doi: 10.1002/bmc.1082. [DOI] [PubMed] [Google Scholar]