Abstract
ADP-ribosylation factors (ARFs) are members of the Ras-related small GTPase family involved in the vesicular trafficking regulation. Immunomodulatory effects of these proteinson host cell arenot being addressed yet. H. contortus small GTPase ADP-ribosylation 1 gene (HcARF1) was cloned and recombinant protein of HcARF1 (rHcARF1) was successfully expressed in Escherichia coli. Binding activity of rHcARF1 to goat PBMCs was confirmed by immunofluorescence assay (IFA) and its immunomudulatory effects on cytokine secretion, cell proliferation, cell migration and nitric oxide production (NO) were observed by co-incubation of rHcARF1. IFA results revealed that rHcARF1 could bind to the PBMCs. The interaction of rHcARF1 modulated the cytokine production, the production of IL-4, IL-10 and IL-17 was increased in a dose dependent manner, however, the IFN-γ production was significantly decreased. Cell migration and NO production were significantly increased by rHcARF1, whereas, rHcARF1 treatment significantly suppressed the proliferation of the PBMC in a dose dependent manner. Our findings showed that the rHcARF1 play important roles on the goat PBMCs.
Keywords: H. contortus, ADP-ribosylation factor, PBMCs, cytokines, proliferation
INTRODUCTION
ADP-ribosylation factors (ARFs) are member of the Ras-related small GTPases family also known as low molecular weight guanine-nucleotide-binding (G) proteins [1] and their involvement in the vesicular trafficking regulation has been well characterized [2]. ARF1 is characteristically related with the golgi and in some cell types also be found related with the plasma membrane and is an important regulator of the biological process induced by epidermal growth factor [3–7]. Involvement of the ARF1 in the activation of signaling molecules, such as phospholipase D, PI3K and type I phosphatidylinositol 4-phosphate 5-kinase has been reported, these data suggests that in addition of trafficking regulation, ARF1 GTPase also act as a signal transducer [5, 8–10].
ARF1 is involved in membrane affinity and it actively involve in the formation of non-clatherin/clatherin coated vesicles which helps in the transportation of vesicles to carry important cellular components required for biological processes such as cell signaling [11]. ARF1 also involved in the activation of PLD enzyme which cleaves phophatidylcholine to generate phophatidic acid (PA) and choline. PA could modulate many cellular events like DNA synthesis, cell proliferation, and secretory responses [12]. Characterization of the ARF proteins has been performed in various parasites included Caenorhabditis elegans [13, 14], Entamoeba histolytica [15], Plasmodium falciparum [16, 17] and Leishmania [18].
Haemonchus contortus is an abomasal nematode parasite, it is the most important parasitic problem on a global basis [19]. H. contortus is responsible for the decline of rural economy due to weight loss and anemia resulted in decreased meat and milk production and H. contortus is one of the comprehensively used parasitic nematode for drug discovery, vaccine development and drug resistance [20–23]. The animal's body triggers several defense mechanisms during H. contortus infection to control the infection in different ways, such as reactive oxygen species production by immune cells. This protection may leads to damages of various host cells and tissues by oxidative stress [24, 25].
Previously, we identified that ARF1 protein was one of the interacting protein with goat PBMCs at multiple developmental stages in vivo [26]. Molecular cloning and functional characterization of H. contortus ARF1 has not being addressed yet. In the current study, recombinant protein of HcARF1 (rHcARF1) was constructed and its immunomodulatory effects on the goat PBMCs was evaluated.
RESULTS
Sequence and phylogenetic analysis
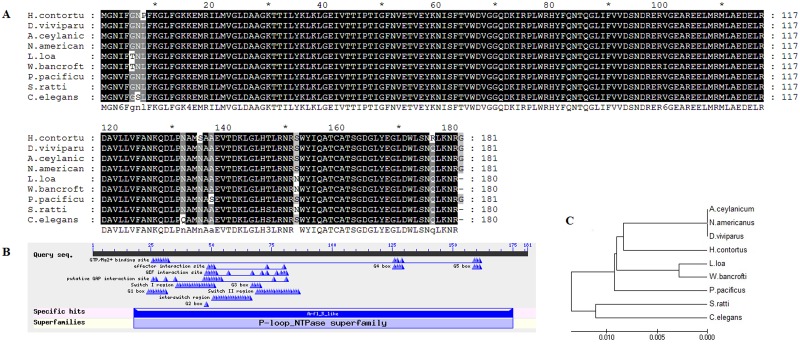
The recombinant plasmid pET32a-HcARF1 was confirmed by restriction enzyme digestion and sequencing. The results of the BLASTx revealed that, ORF contains 546 bp encodes 181 amino acids. The deduced protein sequence of HcARF1 was used for multiple sequence alignment (Figure 1A). The results of the multiple alignments showed that HcARF1 is very close to the ADP-ribosylation factor family protein of Ancylostoma ceylanicum (98%), Dictyocaulus viviparous (98%), Necator americanus (98%), Loa loa (97%), Strongyloides ratti (97%), Wuchereria bancrofti (97%), Pristionchus pacificus (97%) Caenorhabditis elegans (96%), The typical characteristics of the HcARF1 were confirmed as ARF 1-5 by their GTP/Mg2 binding and putative GAP interaction sites (Figure 1B). These findings confirmed that, the cloned ORF belongs to the H. contortus ARF1 family. The phylogenic tree analysis indicated that HcARF1 was closely related to ARF of homologous protein sequence obtained from NCBI data base (Figure 1C).
Figure 1. Multiple alignment of amino acid sequence of HcARF1.
A. The amino acid sequence of HcARF1 aligned with other organism ARF family reported in the NCBI database of Ancylostoma ceylanicum (98%), Dictyocaulus viviparous (98%), Necator americanus (98%), Loa loa (97%), Strongyloides ratti (97%), Wuchereria bancrofti (97%), Pristionchus pacificus (97%), Caenorhabditis elegans (96%). B. Putative conserved domain. C. Phylogenetic analysis of the relationships among the amino acid sequences of HcARF1 and known similar sequences by minimum evolution.
Expression and purification of rHcARF1
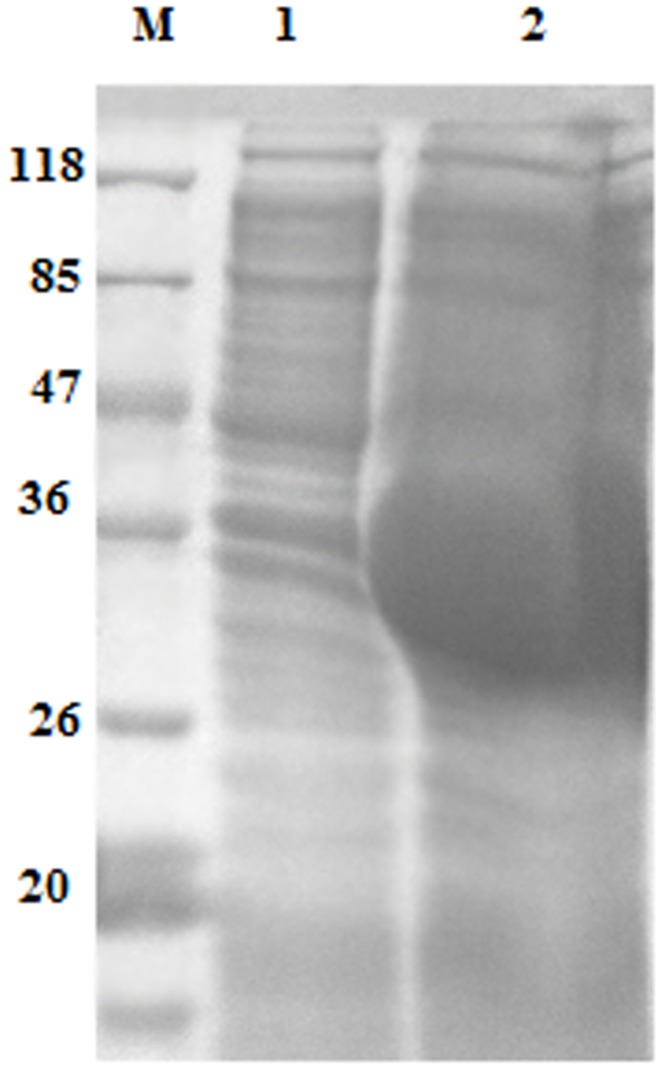
The recombinant protein of HcARF1 was expressed and purified as a His tagged fusion protein. The expressed protein was detected at 38 kDa, it is higher than the calculated mass of 20 kDa of HcARF1 because of extra molecular mass of pET 32a expression vector (Figure 2).
Figure 2. Expression of rHcARF1 protein after induction with 1mM IPTG.
Lane M: standard protein molecular weight marker, 1: recombinant expression vector before induction, Lane 2 expression after induction.
Detection of recombinant HcARF1 protein by immunoblotting
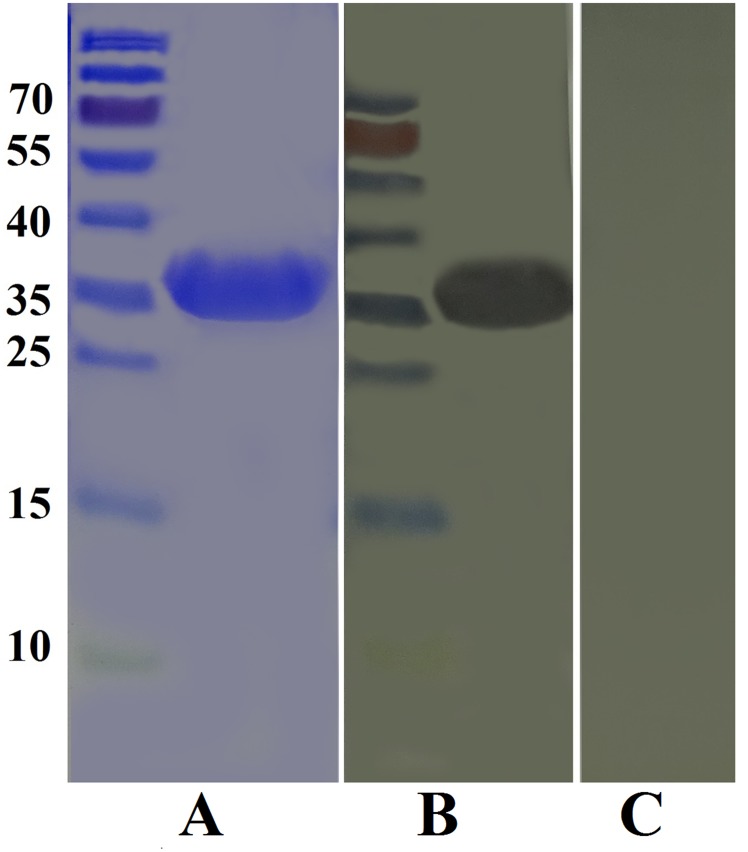
The results of the immunoblot indicated that the rHcARF1 was detected by rat anti rHcARF1, but in negative control no protein was identified by normal rat sera (Figure 3).
Figure 3. Western blot analysis of rHcARF1.
A. Purified rHcARF1 was electrophoresed in SDS-PAGE and stained with Coomassie blue, B. then transferred to a membrane for western blot analysis with rat anti-rHcARF1 sera and C. normal rat sera as control.
Interaction of rHcARF1 with goat PBMCs
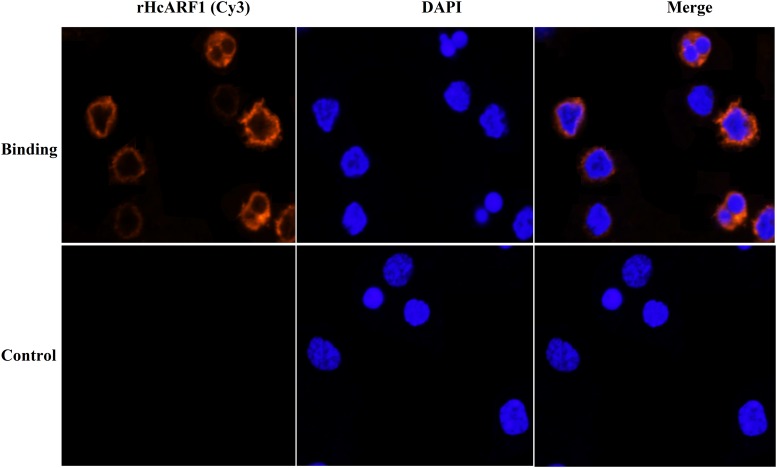
Immunofluorescence assay (IFA) was used to confirm the interaction of rHcARF1 with host PBMCs. Confocal microscopy indicated that rHcARF1 was interacted with the cell surface (red fluorescence). In the control group, no binding was observed (Figure 4).
Figure 4. Confirmation of binding of rHcARF1 to goat PBMCs by IFA.
The nuclei of the corresponding cells were visualized by DAPI (blue) staining. Staining of the target proteins (red) were visualized by Cy3-conjugated secondary antibody. Merge, overlap of red and blue channels. No red fluorescence was observed in control group.
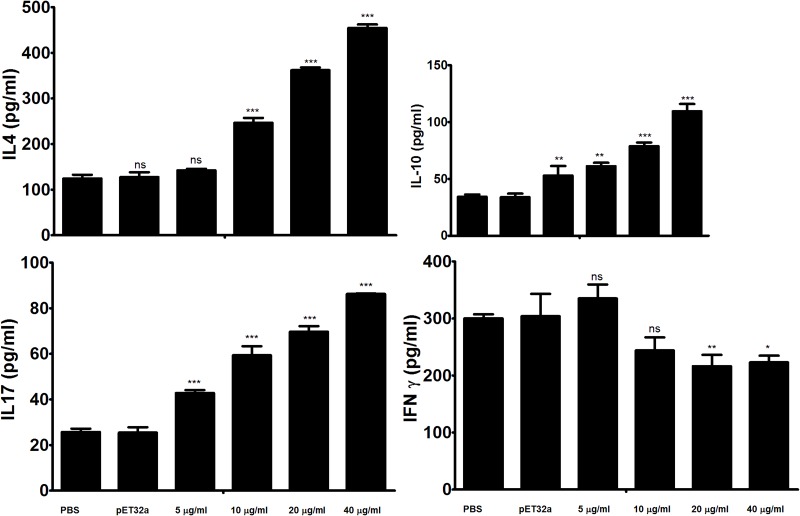
The binding of rHcARF1 to goat PMBCs increased IL-4, IL-10 and IL-17 and suppressed IFN-γ
In the present study ELISA was used to analyze the impacts of the rHcARF1 on the cytokine production. Our findings indicated that rHcARF1 modulating the cytokine production (Figure 5). Secretion of IL-4, IL-10 and IL-17 was significantly increased whereas, the production of the IFN-γ was decreased in PBMCs incubated with different concentration of rHcARF1.
Figure 5. Analysis of the level of multiple cytokine production by PBMCs in vitro.
PBMCs were stimulated with ConA (10 μg/ml) for 24 h in the presence or absence of various concentrations of rHcARF1 and pET32a. Cytokine secretion in the supernatant of cell cultures was quantified by ELISA. The data are representative of three independent experiments (*p <0.01, **p < 0.001, ns non significant).
Interaction of rHcARF1 with goat PBMCs significantly increased the production of cytokine IL-4, IL-10 and IL-17 in a dose dependent manner. On the contrary, type II interferon (IFN-γ) was decreased by the interaction of rHcARF1 (Figure 5).
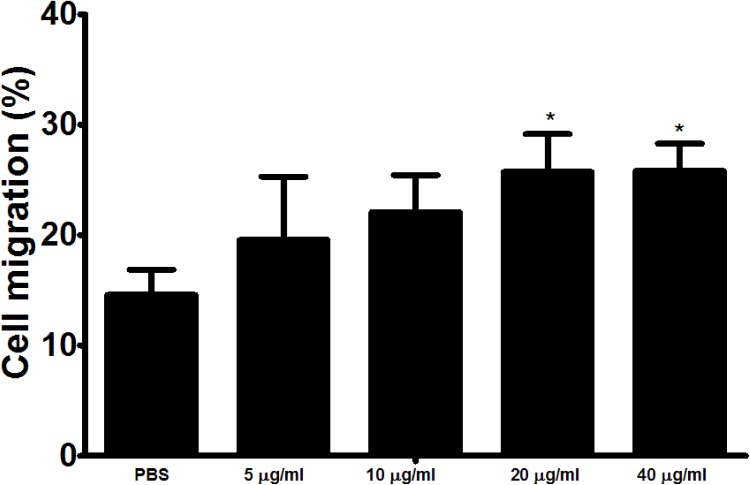
The interaction of rHcARF1 with goat PMBCs increased cell migration
In the current study, cell migration assay was performed to appraise the effect of rHcARF1 on cell migration (Figure 6). Our findings showed that cell migration was significantly increased in cells incubated with 20 and 40 μg/ml of rHcARF1.
Figure 6. Impact of the various concentration of rHcARF1 on PBMC migration.
PBMC were treated with control buffer and different concentrations of rHcARF1, Then the random migration was determined. The difference between the mean values was calculated using ANOVA. Data are representative of 3 independent experiments; *p < 0.01versus the control.
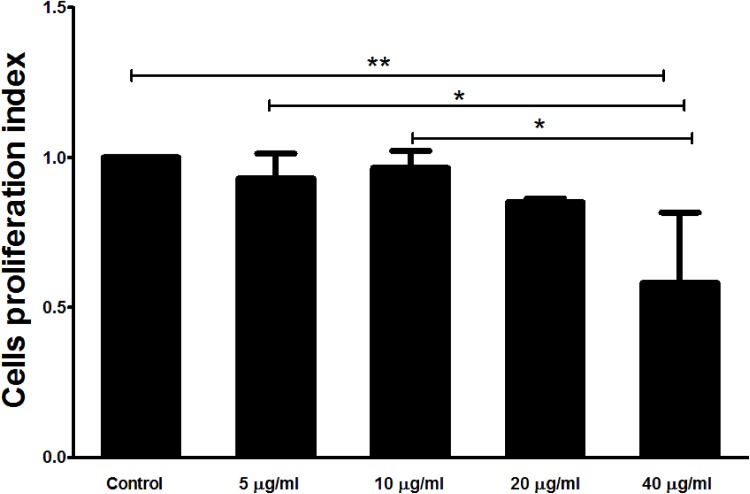
The interaction of rHcARF1 with goat PMBCs decreased cell proliferation
The treatment of rHcARF1 significantly decreased the multiplication of the PBMC at the concentration 40 μg/ml as compared to the control group (Figure 7).
Figure 7. Effects of rHcARF1 on PBMCs proliferation.
Cells were activated with ConA and incubated at the same time with serial concentrations of rHcARF1 at 37°C and 5% CO2. The proliferation was measured by CCK-8 incorporation after 72 h. Cell proliferation index was calculated considering the OD450 values in controls as 100%. The data were representative of three independent experiments (*p < 0.01 and **p < 0.001).
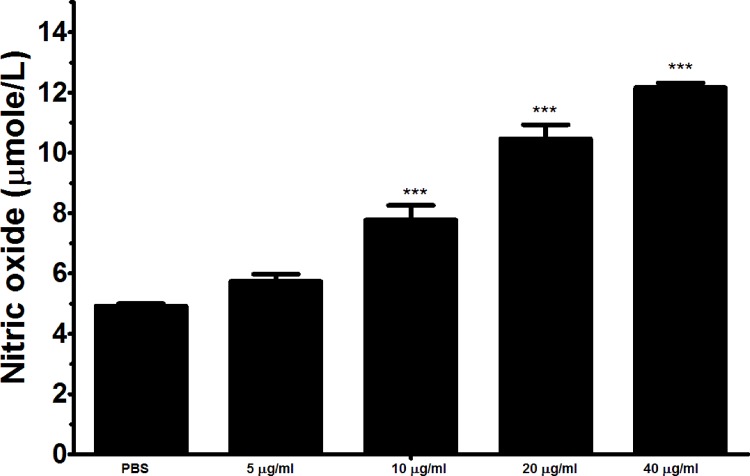
The binding of rHcARF1 to goat PMBCs increased nitric oxide production
Total nitric oxide assay kit was used to measure the Nitric oxide (NO) production by PBMCs incubated with various concentration of rHcARF1. Our findings showed that, rHcARF1 significantly increased the NO production at 10, 20 and 40μg/ml (Figure 8).
Figure 8. Effects of rHARF1 on nitric oxide production by PBMCs in vitro.
Cells were activated with ConA and incubated at the same time with serial concentrations of rHcARF1 at 37°C and 5% CO2. The nitrite concentration in the PBMCs was measured by using the Griess assay and used as an indicator of nitric oxide production by the PBMCs. The data were representative of three independent experiments (***p < 0.0001).
DISCUSSION
A GTP-binding protein has been concerned in the regulation of membrane traffic by the secretory pathway and ARFs are highly conserved family of eukaryotic small GTP-binding proteins with roles in membrane dynamics and vesicle trafficking [27, 28]. ARFs proteins also being identified in different parasites [13, 15, 29–32].
To date, little information is available on the ARFs in the parasitic nematode, in case of H. contortus molecular and functional characterization has not been done. In the current research we have cloned a small GTPase ARF1 gene from the Haemonchus contortus cDNA. The multiple sequence alignment of HcARF1 with other organism indicated that the phylogenetic relationship of H. contortus was closely related with the nematodes (Ancylostoma ceylanicum, Angiostrongylus cantonensis, Dictyocaulus viviparous, Dirofilaria immitis and Loa loa). The multiple sequence alignment of HcARF1 indicated that cloned gene belongs to the ARF protein family.
Cytokine produced by the immune cells are responsible for communication as well as regulation of the immune system. First line of the defense against various organisms including parasites provided by the innate immune system by toll-like receptors (TLRs) family of pattern recognition receptors (PRRs) that recognize the broad range of pathogen-associated molecular patterns (PAMPs) [33, 34].
In the present study, rHcARF1 increased cytokines IL-4, IL-10 and IL-17 secretion by PBMCs but decreased the secretion of Type II interferon (IFN-γ). Previously, it was suggested that an ADP ribosylation factor-GTPase activating protein 1 was negatively regulated LPS-induced pro-inflammatory mediators production by down-regulation of LPS signaling [35]. Wu et. al reported that, class III PI3K/ARF6-dependent pathway was involved in regulating cellular subsequently modulates CpGODN/TLR9-signaling cascades such as TLR9 trafficking and cytokine production [36]. In our study, cytokine secretion modulated by rHcARF1 strongly indicated the multiple and distinct regulatory effects of ARF on goat immune cells.
It is generally considered that, type 2 immunity (Th2) associated with secretion of IL-4 and IL-5 is the main immune mechanism against helminths including H. contortus [37–39]. In our previous study we described that interaction of HcESPs with goat PBMCs decreased the production of IL-4 in vitro [40]. The findings of our study conflicting the above results. Here, we found that the rHcARF1 could increase the production of IL-4. These results directed that rHcARF1 might be played a part in the initiation of the Th2 immunity. However, HcESPs are the group of various proteins, thus proteins involved in decline of IL-4 are worthy of further investigations.
IL-17 a cytokine is strong inducer of inflammation produced by Th17 cells [41] and it is concerned with pathogenesis [42–48]. In our previous studies we found that HcARF1 could interact with host PBMCs at various developmental stages of H. contortus [26] and the secretion of IL-17 was increased by the interaction of HcESPs [40]. Here, we reported that rHcARF1 enhanced the production of IL-17, which indicated that rHcARF1 could part play some roles in the HcESPs on IL-17 production.
It has been recognized that immune suppressive cytokine IL-10 secreted by inducible Treg cells (iTReg) suppress the IFN-γ production [49, 50]. The generation of immune suppressive cytokine IL-10 might be an important tactic by which parasites could suppress the IFN-γ-dependent, cell-mediated immunity [51, 52]. In our previous research we found that rHcESPs suppressed the immune response by increasing the IL-10 and decreasing IFN-γ production [40]. Here we found that interaction of rHcARF1 with goat PBMCs could increase the IL-10 and decrease the IFN-γ production. Therefore, we suggested that, rHcARF1 is very important protein of HcESPs that could suppress the Th1 immune response and might be beneficial for H. contortus evading from the host immunity at early stage.
Previous studies demonstrated that ARF could be regulating the cell cycle progression by the suppression of transcription factor E2F1 activity [53, 54]. Currently, the effects of the rHcARF1 on the cell proliferation was evaluated and results indicated that rHcARF1 significantly inhibited the cell proliferation. Complex regulatory activities was ultimately linked with each other, such as cell activation, cytokine secretion and cell cycling leading to cell proliferation [55].
NO has been reported as an important immune-mediator and play important role in immunoregulation in various infections including H. contortus by mediating host protection by parasite killing or by suppressing the growth [56–58]. Previously reported that, endogenous IL-17 was involved in T cell-mediated NO production [59, 60]. In the present study immune-modulating effects of the rHcARF1 on the NO production by goat PBMCs was evaluated. Cells incubated with rHcARF1 significantly increased the NO production in a dose dependent manner. This up-regulation might be associated with the increased level of IL-17. Our results indicated that, up-regulation of IL-17 as well as NO production might involve in the TH17/NO inflammatory response and pathogenesis.
In Conclusion, we firstly cloned the HcARF1 and demonstrated that HcARF1 is one of the active protein of HcESPs that might be involved in the immune modulation. These findings indicated that, the interaction of rHcARF1 with host cells increased the production of IL-4, IL-10, IL-17, NO and cell migration. However, the secretion of IFN-γ and proliferation of PBMCs was significantly decreased. Our results might help to understand the mechanism involved in host parasite interaction. The immune system has different cell populations including T and B lymphocytes, macrophages, antigen presenting cells, NK cells, etc. How does HcARF1 activate immune and cellular response and which immune cells have been actively involved during the infection need to be further researched.
MATERIALS AND METHODS
Ethics statement
Animal experiments were conducted following the guidelines of the Animal Ethics Committee, Nanjing Agricultural University, China. All experimental protocols were approved by the Science and Technology Agency of Jiangsu Province. The approval ID is SYXK (SU) 2010-0005.
Synthesis of Haemonchus contortus cDNA
Total RNA was isolated from adult worms of H. contortus collected from the abomasums of donor goats as described previously [61]. The worms were ground using a pre-chilled mortor and pestle. One ml of Trizol (Invitrogen) was added and homogenized for 30 minutes. Then 200μl of Tri-chloromethane was added and the mixture was spun at 12,000 rpm for 15 min at 4°C. After that, RNA was precipitated from the supernatant by the addition of 0.25 volumes of isopropyl alcohol per each milliliter of Trizol and incubated at -20°C for 30 min. The RNA was pelleted at 12,000 rpm at 4°C for 10 min. Hereafter, RNA pellets were washed by 70% ethanol and dried. The Pellets were resuspended in DEPC-treated water and the RNA solution was used in subsequent cDNA preparation immediately. The cDNA was synthesized by reverse transcription reaction using cDNA Kit (TaKaRa Biotechnology) according to the manufacturer's instructions.
Molecular cloning of HcARF1 and expression of recombinant HcARF1 protein (rHcARF1)
The complete open reading frame (ORF) of HcARF1 was amplified by reverse transcription-polymerase chain reaction (RT-PCR) using the designed primers of H. contortus ARF gene (GI: 533372025), gene bank accession number HF964523.1. The sense and antisense primer sequences are as the following: 5’- GGATCCATGGGTAACATTTTCGG -3’ and 5’- CTCGAGTTATCCTCTGTTTTTCA -3.
The PCR products were purified by using E.Z.N.A. Gel Extraction Kit (Omega bio-tech, USA) and ligated into pMD19-T cloning vector (TaKaRa Biotechnology, China) and then transformed into E. coli DH5α strain. The positive clones were confirmed by double digestion with BamHI/Xho1 enzymes, and the selected positive recombinant clones were sequenced by Invitrogen Bio-tech (Shanghai, China). The sequence data was assembled and analyzed by DNAssist software version 2.2. The HcARF1 gene was then cloned into BamHI/Xho1 sites of expression plasmid pET32a (+) vector (Novagen, USA). The recombinant plasmid was sequenced to confirm the correct insertion of HcARF1 gene in the proper reading frame.
The expression of the recombinant fusion protein in E. coli BL-2 1 cells (DE3) was induced by isopropy- ß D -thiogala ctoside (IPTG ) at a final concentration of 1mM for 4 h at 37°C in Luria-Bertini (LB) medium with ampicillin (100 μg/ml). The histidine-tagged fusion protein was purified from the supernatant of bacterial lysates using the His·Bind®Resin Chromatography kit (Novagen) and dialyzed in phosphate buffered saline (PBS, pH 7.4) to remove imidazole. Endotoxins were removed from the recombinant proteins using ToxinEraserTM Endotoxin Removal kit (GeneScript, USA). The purity and concentration of the purified rHcARF1 was analyzed by 12% sodium dodecyl sulfate polyacrylamide gelelectrophoresis (SDS- PAGE ) followed by Coomassie blue staining.
Sequence alignments and phylogenetic analysis of HcARF1
Sequence similarity was assessed using protein-protein basic local alignment search tools BLASTp and BLASTx sequences (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi). HcARF1 sequences were aligned using ClustalX 1.83 program (http://www.clustal.org/). The phylogenetic tree was constructed by aligning the amino acid sequences using the Neighbor-Joining method and plotted and visualized using the Molecular Evolutionary Genetics Analysis 5.1 program (http://www.megasoftware.net/).
Generation of polyclonal antibodies
To generate polyclonal antibodies against rHcARF1 , 0.4 mg of rHcARF1 was mixed with Freund's complete adjuvant (1:1) and injected subcutaneously into 3 female Sprague Dawley (SD) rats [62, 63]. Rats received four doses of injection with the same proteins at 2-week intervals. Ten days after the last injection, the rats were anesthetized with diethyl ether, and sera containing specific anti-rHcARF1 antibodies were collected. The concentration of antibodies was determined by ELISA. The specific reactivity with rHcARF1 was confirmed by western blot analysis.
Immuno-blot for the rHcARF1
Purified rHcARF1 were resolved by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) Membrane (Millipore, USA). Non-specific binding was blocked by incubating the membranes in 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature. The membranes were then washed 5 times (5 min each) with TBST, followed by incubation with the primary antibodies (anti-rHcARF1) for 1 h at 37 °C (1:100 dilution in TBST). After washing 5 times with TBST, the membranes were incubated with HRP-conjugated rabbit anti-rat IgG (Sigma, USA) for 1 h at 37 °C (diluted 1:2000 in TBST). Finally, the bound antibody was detected using 3,3-diaminobenzidine tetra hydrochloride (DAB) kit (Boster Bio-technology) according to manufacturer's instructions.
Binding of rHcARF1 to goat PBMC
Freshly isolated PBMCs were incubated in the presence and absence (control) of rHcARF1 (5μg/ml) for 1 h at 37°C. Confirmation of binding was determined by an immunofluorescence assay (IFA) as described by Yuan et al. [56]. Briefly, washed cells (105 / ml) were fixed with 4% paraformaldehyde on a poly-L-lysine-coated glass slide. The cells were then treated with blocking solution (4% BSA in PBS) for 30 min to minimize background staining. After sequential incubation with rat anti-rHcARF1 IgG (1:100) for 2 h and a secondary antibody (1:300) coupled to the fluorescent dye Cy3 (Beyotime, Jiangsu, China) for 1 h, nuclear staining with 2-(4-amidinophenyl)-6-indole carbamidinedihydrochloride (DAPI, 1.5 μM; Sigma, MO, USA) was performed for 6 min. Then, protein localization was determined by observing the staining patterns with a 100× oil objective lens on a laser scanning confocal microscope (L SM710, Zeiss, Jena, Germany). Digital images were captured using the Zeiss microscope software package ZEN 2012 (Zeiss, Jena, Germany).
Detection of the cytokine levels by ELISA of PBMCs treated with rHcARF1
The freshly isolated PBMCs were re-suspended to a final density of 5 × 106 /ml in complete medium (RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin,2 mM L-glutamine, 10% FCS). In the test groups, cells were treated with ConA (10μg/ml) and different concentration of the rHcARF1 (5, 10, 20, and 40μg/ml). The control groups were treated with ConA in equal volume of PBS or ConA and recombinant protein of empty pET32a. Then, the cells were seeded into 24-well plates (1ml/well) and cultured for 24h in 5% CO2 atmosphere at 37 °C. The plates were then centrifuged at 200 × g for 15 min and the supernatants were collected. The levels of IL-4, IL-10, IL-17 and IFN-γ in supernatants were determined using commercially available goat ELISA kits (Jian cheng Biotech, China). The cell viability was assessed by means of the trypan blue exclusion test before the incubation of PBMCs with rHcARF1. Three individual experiments were performed.
Cell migration assay
The cell migration assay was performed using a Transwell system (Corning, USA), this allowed cells to migrate throughout an 8 μm pore size polycarbonate membrane [27]. The treatment group was incubated with different concentrations of rHcARF1 (5, 10, 20, and 40μg/ml) and the control group was treated with an equal volume of PBS. Each experiment was performed in triplicate.
Cell proliferation assay
Cell proliferation assay was performed as previously described [64]. Briefly, 100 μl of cell suspension (1 × 106 cells/ml) were activated with ConA (10 μg/ml) and a serial concentrations of rHcARF1 (5, 10, 20, and 40μg/ml). The control group was treated with ConA in equal volume of PBS. The plate was cultured at 37°C and 5% CO2 for 72 h. Then 10 μl of CCK-8 solutions (Beyotime Biotechnology, China) were added to each well of the plates 4 h before harvesting and the absorbance values at 450 nm (OD450) were measured using a microplate reader (Thermo Scientific, USA). The OD450 of controls were set as 100%. Cell proliferation index was calculated by the formula: OD450 rHcARF1 /OD450 control. Each experiment was performed in triplicate.
Nitric oxide production assay
The goat PBMCs were harvested and washed twice with PBS. Then, 100 μl of cells (1 × 106 cells/ml) were incubated either with PBS and a serial concentrations of rHcARF1 (5, 10, 20, and 40μg/ml) in 96-well plates in DMEM medium. Production of nitric oxide by PBMCs was determine by measurement of intracellular nitrite in the PBMC by using the Griess assay [65] according to the instruction of Total Nitric Oxide Assay Kit (Beyotime Biotechnology, China). Absorbance of the colored solution at 540 nm (OD540) in each well was measured using a plate reader (Bio-Rad Laboratories, USA). Absorbance values were converted to micromoles per liter (μmol/L) using a standard curve that was generated by addition of 0 to 80 μmol/L sodium nitrite to fresh culture media. Three individual experiments were performed.
Footnotes
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
The project supports were provided by the “National Key Basic Research Program (973 program) of China” (Grant No. 2015CB150300) and by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
REFERENCES
- 1.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–46. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 2.Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–11. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- 3.Balch WE, Kahn RA, Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992;267:13053–61. [PubMed] [Google Scholar]
- 4.Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Dong C, Davis JE, Wu WH, Surrao K, Wu G. The mechanism and function of mitogen-activated protein kinase activation by ARF1. Cell Signal. 2015;27:2035–44. doi: 10.1016/j.cellsig.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulay PL, Cotton M, Melançon P, Claing A. ADP-ribosylation factor 1 controls the activation of the phosphatidylinositol 3-kinase pathway to regulate epidermal growth factor-dependent growth and migration of breast cancer cells. J Biol Chem. 2008;283:36425–34. doi: 10.1074/jbc.M803603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlienger S, Ramirez RA, Claing A. ARF1 regulates adhesion of MDA-MB-231 invasive breast cancer cells through formation of focal adhesions. Cell Signal. 2015;27:403–15. doi: 10.1016/j.cellsig.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Zhang GF, Patton WA, Lee FJ, Liyanage M, Han JS, Rhee SG, Moss J, Vaughan M. Different ARF domains are required for the activation of cholera toxin and phospholipase D. J Biol Chem. 1995;270:21–24. doi: 10.1074/jbc.270.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the golgi compartment. J Biol Chem. 2000;275:13962–66. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- 10.Cherfils J. Arf GTPases and their effectors: assembling multivalent membrane-binding platforms. Curr Opin Struct Biol. 2014;29:67–76. doi: 10.1016/j.sbi.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Boulakirba S, Macia E, Partisani M, Lacas-Gervais S, Brau F, Luton F, Franco M. Arf6 exchange factor EFA6 and endophilin directly interact at the plasma membrane to control clathrin-mediated endocytosis. Proc Natl Acad Sci USA. 2014;111:9473–78. doi: 10.1073/pnas.1401186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruntz RC, Lindsley CW, Brown HA. Phospholipase D signaling pathways and phosphatidic acid as therapeutic targets in cancer. Pharmacol Rev. 2014;66:1033–79. doi: 10.1124/pr.114.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackema KB, Hench J, Böckler S, Wang SC, Sauder U, Mergentaler H, Westermann B, Bard F, Frank S, Spang A. The small GTPase Arf1 modulates mitochondrial morphology and function. EMBO J. 2014;33:2659–75. doi: 10.15252/embj.201489039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Kelly WG, Logsdon JM, Jr, Schurko AM, Harfe BD, Hill-Harfe KL, Kahn RA. Functional genomic analysis of the ADP-ribosylation factor family of GTPases: phylogeny among diverse eukaryotes and function in C. elegans. FASEB J. 2004;18:1834–50. doi: 10.1096/fj.04-2273com. [DOI] [PubMed] [Google Scholar]
- 15.Serbzhinskiy DA, Clifton MC, Sankaran B, Staker BL, Edwards TE, Myler PJ. Structure of an ADP-ribosylation factor, ARF1, from Entamoeba histolytica bound to Mg(2+)-GDP. Acta Crystallogr F Struct Biol Commun. 2015;71:594–99. doi: 10.1107/S2053230X15004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thavayogarajah T, Gangopadhyay P, Rahlfs S, Becker K, Lingelbach K, Przyborski JM, Holder AA. Alternative Protein Secretion in the Malaria Parasite Plasmodium falciparum. PLoS One. 2015;10:e0125191. doi: 10.1371/journal.pone.0125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook WJ, Smith CD, Senkovich O, Holder AA, Chattopadhyay D. Structure of Plasmodium falciparum ADP-ribosylation factor 1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1426–31. doi: 10.1107/S1744309110036997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin A, Espiau B, Tetaud E, Cuvillier A, Lartigue L, Ambit A, Robinson DR, Merlin G. The leishmania ARL-1 and Golgi traffic. PLoS One. 2008;3:e1620. doi: 10.1371/journal.pone.0001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peter JW, Chandrawathani P. Haemonchus contortus: parasite problem No. 1 from tropics - Polar Circle. Problems and prospects for control based on epidemiology. Trop Biomed. 2005;22:131–37. [PubMed] [Google Scholar]
- 20.Gilleard JS. Understanding anthelmintic resistance: the need for genomics and genetics. Int J Parasitol. 2006;36:1227–39. doi: 10.1016/j.ijpara.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Redman E, Sargison N, Whitelaw F, Jackson F, Morrison A, Bartley DJ, Gilleard JS. Introgression of ivermectin resistance genes into a susceptible Haemonchus contortus strain by multiple backcrossing. PLoS Pathog. 2012;8:e1002534. doi: 10.1371/journal.ppat.1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilleard JS. Haemonchus contortus as a paradigm and model to study anthelmintic drug resistance. Parasitology. 2013;140:1506–22. doi: 10.1017/S0031182013001145. [DOI] [PubMed] [Google Scholar]
- 23.Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, Weber SS, Wenger A, Wieland-Berghausen S, Goebel T, Gauvry N, Pautrat F, Skripsky T, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–80. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- 24.Kotze AC. Catalase induction protects Haemonchus contortus against hydrogen peroxide in vitro. Int J Parasitol. 2003;33:393–400. doi: 10.1016/s0020-7519(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeldt F, Wilson M, Lee G, Kure C, Ou R, Braun L, de Haan J. Oxidative stress in surgery in an ageing population: pathophysiology and therapy. Exp Gerontol. 2013;48:45–54. doi: 10.1016/j.exger.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Gadahi JA, Wang S, Bo G, Ehsan M, Yan R, Song X, Xu L, Li X. Proteomic analysis of the excretory and secretory proteins of Haemonchus contortus (HcESP) binding to goat PBMCs in vivo revealed stage-specific binding profiles. PLoS One. 2016;11:e0159796. doi: 10.1371/journal.pone.0159796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price HP, Stark M, Smith DF. Trypanosoma brucei ARF1 plays a central role in endocytosis and golgi-lysosome trafficking. Mol Biol Cell. 2007;18:864–73. doi: 10.1091/mbc.E06-08-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpicelli-Daley LA, Li Y, Zhang CJ, Kahn RA. Isoform-selective effects of the depletion of ADP-ribosylation factors 1-5 on membrane traffic. Mol Biol Cell. 2005;16:4495–508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price HP, Peltan A, Stark M, Smith DF. The small GTPase ARL2 is required for cytokinesis in Trypanosoma brucei. Mol Biochem Parasitol. 2010;173:123–31. doi: 10.1016/j.molbiopara.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senkovich O, Chattopadhyay D. Plasmodium falciparum ARFGAP: expression and crystallization of the catalytic domain. Biochim Biophys Acta. 2004;1698:127–30. doi: 10.1016/j.bbapap.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Stafford WH, Stockley RW, Ludbrook SB, Holder AA. Isolation, expression and characterization of the gene for an ADP-ribosylation factor from the human malaria parasite, Plasmodium falciparum. Eur J Biochem. 1996;242:104–13. doi: 10.1111/j.1432-1033.1996.0104r.x. [DOI] [PubMed] [Google Scholar]
- 32.Shi A, Grant BD. Interactions between Rab and Arf GTPases regulate endosomal phosphatidylinositol-4,5-bisphosphate during endocytic recycling. Small GTPases. 2013;4:106–09. doi: 10.4161/sgtp.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt AQ, Ahmed S, Maratha A, Miggin SM. 14-3-3ε and 14-3-3σ inhibit Toll-like receptor (TLR)-mediated proinflammatory cytokine induction. J Biol Chem. 2012;287:38665–79. doi: 10.1074/jbc.M112.367490. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Ben-Addi A, Mambole-Dema A, Brender C, Martin SR, Janzen J, Kjaer S, Smerdon SJ, Ley SC. IκB kinase-induced interaction of TPL-2 kinase with 14-3-3 is essential for Toll-like receptor activation of ERK-1 and -2 MAP kinases. Proc Natl Acad Sci USA. 2014;111:E2394–403. doi: 10.1073/pnas.1320440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haque A, Noman AS, Koide N, Odkhuu E, Naiki Y, Hashimoto S, Komatsu T, Yoshida T, Yokochi T. An ADP ribosylation factor-GTPase activating protein negatively regulates the production of proinflammatory mediators in response to lipopolysaccharide. Cancer Immunol Immunother. 2011;60:1439–46. doi: 10.1007/s00262-011-1048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JY, Kuo CC. Pivotal role of ADP-ribosylation factor 6 in Toll-like receptor 9-mediated immune signaling. J Biol Chem. 2012;287:4323–34. doi: 10.1074/jbc.M111.295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Rezende MC, Araújo ES, Moreira JM, Rodrigues VF, Rodrigues JL, Pereira CA, Negrão-Corrêa D. Effect of different stages of Schistosoma mansoni infection on the parasite burden and immune response to Strongyloides venezuelensis in co-infected mice. Parasitol Res. 2015;114:4601–16. doi: 10.1007/s00436-015-4706-6. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen A, Petersen HH, Kringel H, Iburg TM, Skovgaard K, Dawson H, Urban JF, Jr, Thamsborg SM. Immune and inflammatory responses in pigs infected with Trichuris suis and Oesophagostomum dentatum. Vet Parasitol. 2015;207:249–58. doi: 10.1016/j.vetpar.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Shakya KP, Miller JE, Horohov DW. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf Coast Native lambs. Vet Parasitol. 2009;163:57–66. doi: 10.1016/j.vetpar.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 40.Gadahi JA, Yongqian B, Ehsan M, Zhang ZC, Wang S, Yan RF, Song XK, Xu LX, Li XR. Haemonchus contortus excretory and secretory proteins (HcESPs) suppress functions of goat PBMCs in vitro. Oncotarget. 2016;7:35670–79. doi: 10.18632/oncotarget.9589. https://doi.org/10.18632/oncotarget.9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–12. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 42.da Matta Guedes PM, Gutierrez FR, Maia FL, Milanezi CM, Silva GK, Pavanelli WR, Silva JS. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS Negl Trop Dis. 2010;4:e604. doi: 10.1371/journal.pntd.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guiton R, Vasseur V, Charron S, Arias MT, Van Langendonck N, Buzoni-Gatel D, Ryffel B, Dimier-Poisson I. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis. 2010;202:427–35. doi: 10.1086/653738. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y, Wang W, Tong J, Pan Q, Long Y, Qian W, Hou X. Th17: a new participant in gut dysfunction in mice infected with Trichinella spiralis. Mediators Inflamm. 2009;2009:517052. doi: 10.1155/2009/517052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, Stadecker MJ. Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol. 2012;34:873–88. doi: 10.1007/s00281-012-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katawa G, Layland LE, Debrah AY, von Horn C, Batsa L, Kwarteng A, Arriens S, W Taylor D, Specht S, Hoerauf A, Adjobimey T. Hyperreactive onchocerciasis is characterized by a combination of Th17-Th2 immune responses and reduced regulatory T cells. PLoS Negl Trop Dis. 2015;9:e3414. doi: 10.1371/journal.pntd.0003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mbow M, Larkin BM, Meurs L, Wammes LJ, de Jong SE, Labuda LA, Camara M, Smits HH, Polman K, Dieye TN, Mboup S, Stadecker MJ, Yazdanbakhsh M. T-helper 17 cells are associated with pathology in human schistosomiasis. J Infect Dis. 2013;207:186–95. doi: 10.1093/infdis/jis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutitzky LI, Stadecker MJ. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):327–30. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 49.McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adalid-Peralta L, Fragoso G, Fleury A, Sciutto E. Mechanisms underlying the induction of regulatory T cells and its relevance in the adaptive immune response in parasitic infections. Int J Biol Sci. 2011;7:1412–26. doi: 10.7150/ijbs.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 52.Xu Y, Chen W, Bian M, Wang X, Sun J, Sun H, Jia F, Liang C, Li X, Zhou X, Huang Y, Yu X. Molecular characterization and immune modulation properties of Clonorchis sinensis-derived RNASET2. Parasit Vectors. 2013;6:360–360. doi: 10.1186/1756-3305-6-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell JL, Powers JT, Rounbehler RJ, Rogers PM, Conti CJ, Johnson DG. ARF differentially modulates apoptosis induced by E2F1 and Myc. Mol Cell Biol. 2002;22:1360–68. doi: 10.1128/mcb.22.5.1360-1368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, Sherr CJ, Zambetti GP. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev. 2000;14:2358–65. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter A, Löhning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J Exp Med. 1999;190:1439–50. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan C, Zhang H, Wang W, Li Y, Yan R, Xu L, Song X, Li X. Transmembrane protein 63A is a partner protein of Haemonchus contortus galectin in the regulation of goat peripheral blood mononuclear cells. Parasit Vectors. 2015;8:211. doi: 10.1186/s13071-015-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bian M, Li S, Wang X, Xu Y, Chen W, Zhou C, Chen X, He L, Xu J, Liang C, Wu Z, Huang Y, Li X, Yu X. Identification, immunolocalization, and immunological characterization of nitric oxide synthase-interacting protein from Clonorchis sinensis. Parasitol Res. 2014;113:1749–57. doi: 10.1007/s00436-014-3820-1. [DOI] [PubMed] [Google Scholar]
- 58.Nahrevanian H. Involvement of nitric oxide and its up/down stream molecules in the immunity against parasitic infections. Braz J Infect Dis. 2009;13:440–48. doi: 10.1590/s1413-86702009000600010. [DOI] [PubMed] [Google Scholar]
- 59.Miljkovic D, Cvetkovic I, Vuckovic O, Stosic-Grujicic S, Mostarica Stojkovic M, Trajkovic V. The role of interleukin-17 in inducible nitric oxide synthase-mediated nitric oxide production in endothelial cells. Cell Mol Life Sci. 2003;60:518–25. doi: 10.1007/s000180300043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zidi S, Boulaneb-Bediar F, Belkhelfa M, Belguendouz H, Medjeber O, Henchiri C, Laouar M, Chachoua L, Lankar A, Touil-Boukoffa C. ID: 27: IL-17A and Nitric Oxide production are involved in primary Pterygium pathogenesis: A study in Algerian patients. Cytokine. 2015;76:68–69. [Google Scholar]
- 61.Sun Y, Yan R, Muleke CI, Zhao G, Xu L, Li X. Recombinant Galectins of Haemonchus contortus Parasite Induces Apoptosis in the Peripheral Blood Lymphocytes of Goat. Int J Pept Res Ther. 2006;13:387–92. [Google Scholar]
- 62.Han K, Xu L, Yan R, Song X, Li X. Molecular cloning, expression and characterization of enolase from adult Haemonchus contortus. Res Vet Sci. 2012;92:259–65. doi: 10.1016/j.rvsc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Yuan C, Wang S, Song X, Xu L, Yan R, Hasson IA, Li X. Transcriptional and proteomic analysis reveal recombinant galectins of Haemonchus contortus down-regulated functions of goat PBMC and modulation of several signaling cascades in vitro. J Proteomics. 2014;98:123–37. doi: 10.1016/j.jprot.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Wang W, Wang S, Zhang H, Yuan C, Yan R, Song X, Xu L, Li X. Galectin Hco-gal-m from Haemonchus contortus modulates goat monocytes and T cell function in different patterns. Parasit Vectors. 2014;7:342. doi: 10.1186/1756-3305-7-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–38. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]