Abstract
Kalopanax septemlobus is a medicinal woody species of the family Araliaceae, and the pharmaceutical properties of saponins obtained from K. septemlobus suggest that K. septemlobus has the potential to be a crude drug and dietary health supplement. In this study, we established cell suspension culture of K. septemlobus to develop a sustainable source of natura-ceuticals. Friable calli were used for establishing cell suspension culture. The maximum amount of total saponins (1.56 mg/60 ml suspension) was obtained during the 15th day of incubation, whereas the maximum capacity of saponin production was reached after day 6 (0.42 μg/mg of fresh weight). The total saponin production in the cell suspension of K. septemlobus was significantly increased by coronatine (COR) at 160% at a dose of 1 μM compared with the mock-treated control, whereas methyl jasmonate treated cells exhibited less increase in total saponin level as compared to the COR-treated cells. In addition, the elicitation of COR strongly induced the expression of beta-amyrin synthase, thus resulting in the accumulation of oleanolic acid (2.369 ± 0.98 μg/mg of extract), a precursor for oleanane-type triterpene saponins. These results indicate that COR is an efficient elicitor for inducing phytochemicals in cell suspension culture and that it provides the possibility for producing saponins of K. septemlobus using cell suspension culture.
Keywords: Kalopanax septemlobus, Cell suspension culture, Coronatine, Saponin, Beta-amyrin synthase
Introduction
Known as castor aralia or prickly castor oil tree, Kalopanax septemlobus (synonymous with Kalopanax pictus) is a deciduous tree used in traditional medicine (Hyun and Kim 2009; Park et al. 2016). Since the stem bark of K. septemlobus has been used as a traditional crude drug for the treatment of rheumatic arthritis and neurotic pain, current pharmaceutical studies suggest that the anti-inflammatory effect of the stem bark extract is due to the inhibition of the nuclear factor-κB (NF-κB) translocation into the nucleus by the suppression of IκB-α (inhibitor of NF-κB) phosphorylation and degradation (Bang et al. 2010). In addition, in the extract of K. septemlobus, saponins, polyacetylenes, isoquinoline alkaloid, lignans, phenylpropanoid glycosides, and phenolic glycosides have been reported to have a range of biological effects, including antioxidant, antimicrobial, anti-diabetic, anti-inflammatory, and anticancer (Hu et al. 2012; Quang et al. 2013; Salunke et al. 2014; Moon et al. 2015; Park et al. 2016). Although these findings suggest that K. septemlobus has the potential to be a crude drug and a dietary health supplement, the wild or cultivated source of this plant is not economically feasible because of several limitations, such as long germination period, propagation problem, long growth period, and variation of secondary metabolites by environmental change (Moon et al. 2005). Therefore, the development of an economically viable and environmentally sustainable source for secondary metabolite production remains an outstanding challenge in industrial crops.
The production of valuable secondary metabolites in plant cell or organ cultures should be an attractive alternative to the traditional cultivation (Murthy et al. 2014). The important advantage of plant suspension culture system is that it is a homogeneous population of nearly identical cells and that is independent of geographical, seasonal, and environmental variations (Ochoa-Villarreal et al. 2016). These advantages have made the plant suspension culture system important in the production of natural products associated with the cosmetic, food, and pharmaceutical industries (Ochoa-Villarreal et al. 2016). However, the application of this system for the production of secondary metabolites is limited by low product yields and challenges in scaling up (Wilson and Roberts 2012). To overcome these limitations, various strategies, including elicitation and metabolic engineering using genetic modification techniques, have been introduced.
Most bioactive compounds found in plants belong to a secondary metabolite, which varies because of environmental factors. In fact, various environmental stresses and chemical treatments have been shown to affect the variation in concentration and composition of secondary metabolites (Ochoa-Villarreal et al. 2016), thus suggesting that secondary metabolites play an important role in plant defense and protection against biotic and abiotic stresses. Elicitors [e.g., ultraviolet, light, chitin, salicylic acid, and methyl jasmonate (MeJA)] are used to induce a metabolic shift from growth to defense in many plant species (Wilson and Roberts 2012). The treatment of elicitors was able to increase the production yield of some natural products starting from 1.0 to a maximum of 2230-fold across plant species (Giri and Zaheer 2016). Using differentiated cells (hairy root, adventitious root, callus, and cambial meristematic cells), the differential effect of elicitors on secondary metabolites was introduced (Lee et al. 2010; Giri and Zaheer 2016; Ochoa-Villarreal et al. 2016). This finding indicates that elicitation is a useful method to increase secondary metabolite accumulation in plant cell cultures, but the optimization of elicitation condition should be required for improving production yield.
To achieve a stable production of saponins in K. septemlobus, we introduced the callus suspension culture of K. septemlobus in this study. Moreover, the effects of elicitors, MeJA, and coronatine (COR) on the production of saponins and the expression of genes involved in the biosynthesis pathway of saponins were compared to optimize the elicitation condition.
Materials and methods
Plant materials and culture conditions
Callus of K. septemlobus was induced as described by Kim and Moon (2009) using a callus induction medium containing Murashige and Skoog (MS) solid media supplemented with 1.0 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), 3% sucrose, and 0.7% plant agar. All cultures were kept in a dark condition at 24 °C. The friable callus was subcultured into callus induction medium regularly for 4 weeks and was used to establish the cell suspension cultures.
Establishment of cell suspension cultures
To establish suspension cultures, friable calli were transferred into the liquid MS medium (50 ml) containing 1.0 mg/l 2,4-D and 3% sucrose in a 300-ml Erlenmeyer flask. All culture flasks were incubated on a rotary shaker at 120 rpm and 24 °C in a dark condition. Although 2,4-D promoted the growth and biomass of suspension-cultured cells, it induced the abnormal growth of cells. Therefore, to stabilize the suspension cultures, the suspension-cultured cells were continuously subcultured in a medium containing a lower content of 2,4-D than before. Finally, the suspension cultures were established in the medium without 2,4-D. 10 ml of suspension-cultured cells was transferred to a 300-ml Erlenmeyer flask, which contained 50 ml of liquid MS medium consisting of 3% sucrose, and subcultured every week.
Analysis of cell growth and elicitor treatment
The growth of the suspension-cultured cells against different incubation periods up to 15 days was analyzed. Approximately 0.7 g fresh weight of seven-day-old cultured cells was subcultured, and the cells were harvested at different time points using vacuum filtration for fresh weight determination. The harvested cells were frozen in liquid nitrogen and then stored at − 80 °C until analysis.
For elicitor treatment, 6-day-old suspension-cultured cells were treated with different concentrations of MeJA (50, 100, and 250 µM), COR (0.5, 1, and 2.5 µM), or solvent alone as the mock control and further incubated for 4 days. The cells were harvested by vacuum filtration and then frozen in liquid nitrogen.
Determination of total saponin
The content of total saponin was determined using an anisaldehyde reagent as described by Vador et al. (2012). 50 mg (fresh weight) of the sample was suspended in hexane to remove fat. The defatted samples were air dried and mixed with 2 ml of methanol (6 h × 3 times) for saponin extraction. All the supernatants of the methanol extracts were pooled, and methanol was evaporated using a rotary evaporator (IKA RV8, IKA, Staufen, Germany). The crude saponin was dissolved in 1 ml of ethyl acetate. About 500 μl of the sample was mixed with 250 μl A reagent [0.5% (v/v) p-anisaldehyde in ethyl acetate] and 250 μl B reagent [50% (v/v) H2SO4 in ethyl acetate]. The reaction mixtures were immediately incubated at 60 °C for 10 min for color development. Each tube was cooled, and the absorbance was taken at 430 nm. The amount of crude saponin was calculated as diosgenin equivalent from the equation obtained from the standard diosgenin graph.
Determination of oleanolic acid content
The freeze-dried cells (1 g) were soaked in methanol [1:15 dry weight material to MeOH (ml)] for 1 h and sonicated for 10 min using an ultrasonic bath (DAIHAN-Sci, Wonju-si, South Korea). This process was repeated three times, and the extracts were concentrated by evaporation.
To determine the concentration of oleanolic acid in the extracts, the HPLC analysis was conducted with an Agilent 1200 chromatograph equipped with a diode array detector (Agilent Technologies, Waldbronn, Germany). The separation was performed using a Shiseido CAPCELL PAK-C18 column (4.6 mm × 150 mm, particle size 5.0 μm) using the mobile phase of MeOH/H2O (95:5, v/v) at a flow rate of 0.4 ml/min, and the reading was taken at 215 nm as described by Xu et al. (2012). A standard calibration curve of oleanolic acid was constructed in the concentration range of 100 ng to 10 μg (y = 159.09x − 5.8357, R2 = 0.9999).
Identification and expression analysis of genes involved in saponin biosynthesis
To identify the genes involved in the biosynthesis of saponin, we downloaded SRA (Sequence Read Archive) dataset for the transcriptome sequencing of K. septemlobus non-embryogenic callus (SRX375488). Moreover, the CLC Genomics Workbench and Main Workbench (Insilicogen, Yongin-si, South Korea) were used for the assembly and generation of a local BLAST database. The protein and mRNA sequences of Panax ginseng squalene epoxidase (FJ393274), cycloartenol synthase (AB009029), and beta-amyrin synthase (AB014057) were used as queries in the search against the K. septemlobus transcriptome dataset.
Total RNA was extracted using the FavorPrep™ plant total RNA purification mini kit (FAVORGEN Biotech Corporation, Ping-Tung, Taiwan) according to the manufacturer’s instructions. cDNA was synthesized using the ReverTra Ace® qPCR RT Master Mix with qDNA Remover (TOYOBO, Co., Ltd, Osaka, Japan) in accordance with the manufacturer’s recommendations. qRT-PCR was performed using the SYBR® Green Real-time PCR Master Mix (TOYOBO, Co., Ltd, Osaka, Japan) in the CFX96™ Real-time system (BIO-RAD) with default parameters. The expression level of each gene was normalized to actin, and three technical replicates were performed in each biological replicate. The specific primer pairs used in qRT-PCR are listed in Table 1.
Table 1.
List of primers used in the quantitative real-time PCR analysis
| Primer name | Nucleotides (5′ to 3′) |
|---|---|
| KsSQE1-F | CAAAGGTGGATGTGCCTTCT |
| KsSQE1-R | GGGTGATGGGTCTGCTAAGA |
| KsSQE2-F | GCGATCTCCATGACTCATCA |
| KsSQE2-R | TAAAGTGCACCTGCCAATGT |
| KsSQE3-F | GTGTGAATGAAATCGATGCG |
| KsSQE3-R | AGCTTCTGCCTGCTACATCC |
| KsbAS-F | TAAGCAGCCGATACCTTTGC |
| KsbAS-R | TAATCAGGAATTCGGGCAAG |
| Act-F | ATCATGAAGTGCGATGTGGA |
| Act-R | TGCTCATACGATCCGCAATA |
Statistical analysis
Statistical analyses were conducted by the ANOVA test using a statistical package program (SPSS 10.0). Duncan’s test was used to determine the significance of the differences between the groups. Differences at p < 0.05 were considered significant.
Results
Production of saponins in a K. septemlobus suspension culture
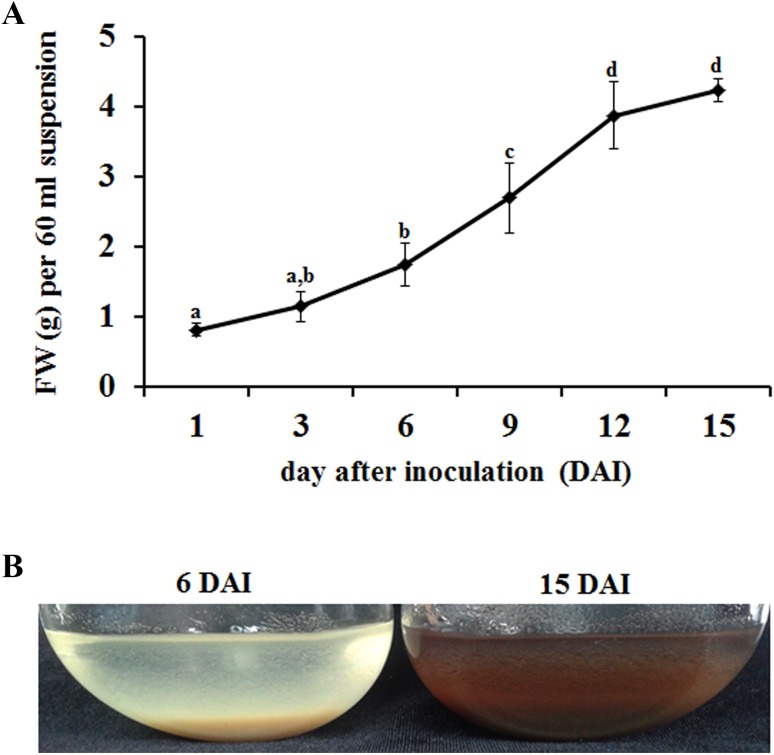
Cell suspension cultures were initiated from friable calli grown on a callus induction medium. The suspended cells were yellowish and presented dispersed cells, thus resulting in a fine milky suspension (Fig. 1). Cell growth was determined in a liquid MS medium containing 3% sucrose by recording the fresh weight of cells every three days. As shown in Fig. 1, biomass was increased from 0.7 to 4 g in 15 days. The typical growth curve was obtained with a lag phase until 3 days, and the log phase growth began on the third day until the 12th day. Although biomass was increased on the 15th day of cell culture, the cells became dark brown because of the senescence of the cells or cell death (Fig. 1).
Fig. 1.
Cell growth pattern in the K. septemlobus suspension culture. a The growth of cell suspension cultures was determined by measuring the fresh weight. Data represent the mean of three independent experiments ± SE. b K. septemlobus suspension culture obtained 6 or 15 days after inoculation
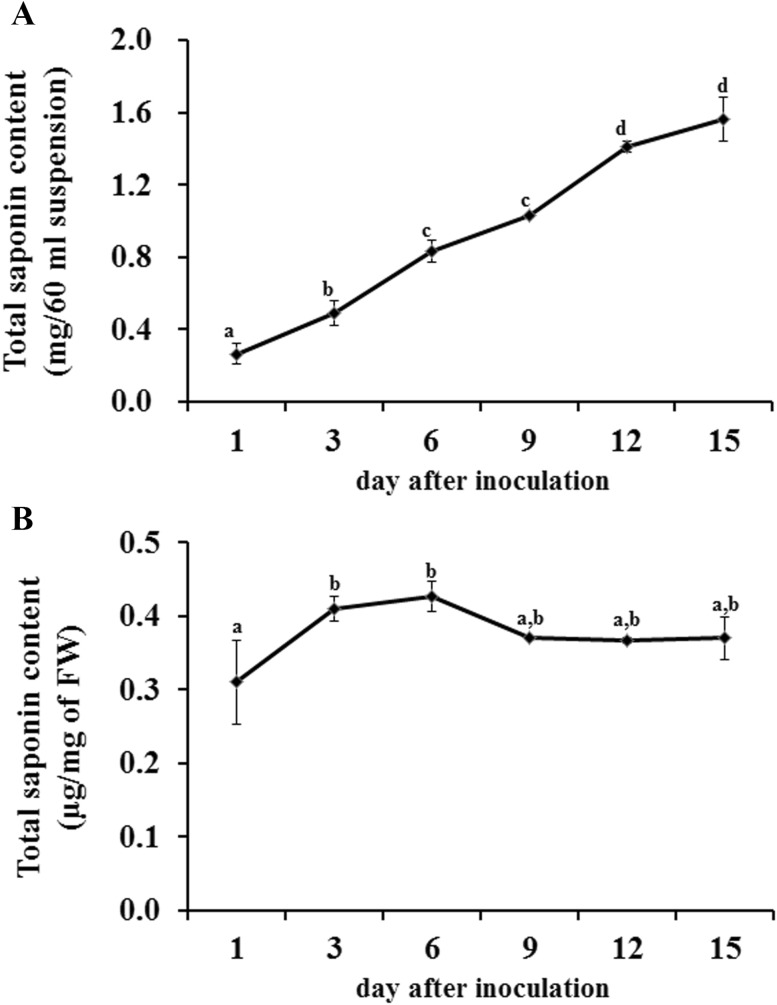
To investigate the capacity of the K. septemlobus suspension culture to synthesize saponins, the variation of total saponin content during the culture period was analyzed. The total saponin production increased significantly according to the culture period, and the maximum yield of total saponin produced by the K. septemlobus suspension culture was reached at 15 days (1.56 mg/60 ml suspension) (Fig. 2a). At the end of the culture, the production of saponin obtained was almost six times higher than that at the beginning. The highest saponin production capacity of cells was observed after day 6 (0.42 μg/mg of fresh weight), and it decreased after day 9 (0.37 μg/mg of fresh weight) (Fig. 2b). These findings indicate that increased biomass is correlated with saponin accumulation according to time courses, but the capacity of saponin production may not be linked to cell growth. The physiological state of the cells for elicitation is critical in the manipulation of secondary metabolite accumulation. In most cases, the maximum response of the cells was observed when they were stimulated at the end of the log phase or at the beginning of the stationary phase (Ramirez-Estrada et al. 2016). In the case of the K. septemlobus suspension culture, the beginning of the cell color change from yellow to dark brown was observed after day 12 (beginning of the stationary phase). Therefore, cells in the mid-log phase (day 6) showed the highest capacity of saponin production (Fig. 2b), and they were used for further analysis.
Fig. 2.
Variation in the total saponin content during the culture period of K. septemlobus suspension culture. Total saponin content was expressed as mg of diosgenin equivalents per total fresh weight obtained from a 60 ml culture (a) or μg of diosgenin equivalents per mg of fresh weight (b). Data are expressed as mean values, with the standard error obtained from three independent experiments
Effect of MeJA and COR on saponin production
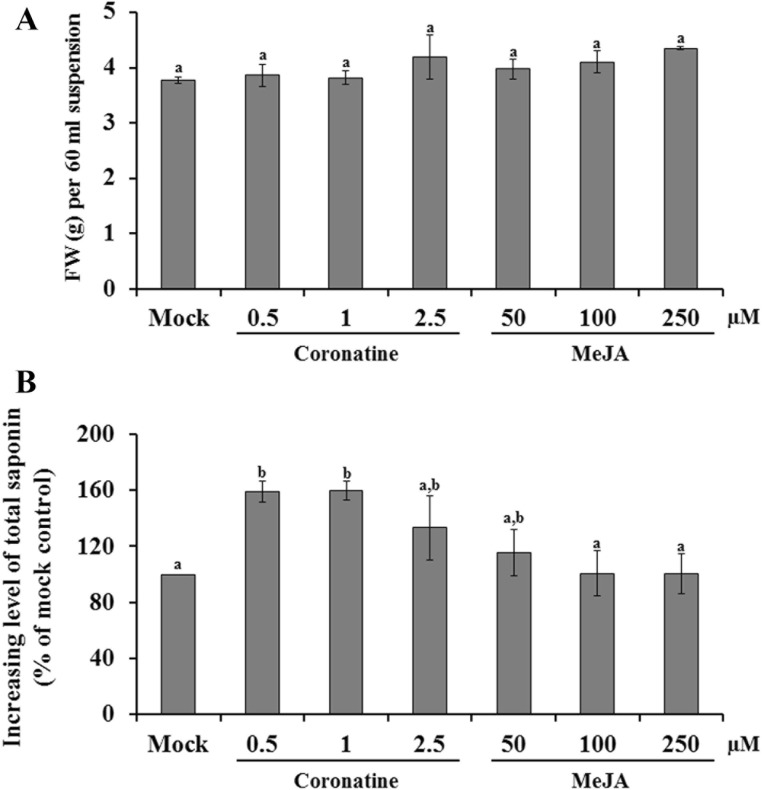
MeJA is an important signal molecule that regulates a wide range of plant physiology, including plant growth and development, pollen production, and plant resistance against abiotic and biotic stresses (Wu et al. 2008), whereas COR is a non-host-specific phytotoxin produced by various strains of Pseudomonas syringae and a structural mimic of the plant hormone jasmonic acid isoleucine (Geng et al. 2014). Although MeJA and COR play a role during incompatible plant-pathogen interactions, they have been successfully used as an effective elicitor to enhance the production of secondary metabolites from the cell suspension culture of various plants (Giri and Zaheer 2016; Ramirez-Estrada et al. 2016). Therefore, to investigate the effect of MeJA and COR on the product yield of saponins in the K. septemlobus suspension culture, the cell suspension of K. septemlobus was treated with different levels of MeJA or COR after 6 days of cultivation. Four days after the treatment of each elicitor, the fresh weight and total saponin content were analyzed. As shown in Fig. 3a, all the tested concentrations of MeJA or COR did not exhibit an inhibitory effect on cell growth. When COR was applied to cell cultures, the increasing level of saponins was observed (Fig. 3b). The total saponin content was increased about 159 and 160% with 0.5 and 1 μM of COR, respectively, compared with that in the mock-treated cells. However, further dosage increase (2.5 μM) decreased the total saponin production compared with the 1 μM COR treatment. In the case of MeJA, total saponin production was less affected by the addition of MeJA than that of COR. The maximum level of saponin production increased to 115% when the cell cultures were treated with MeJA (Fig. 3b). These findings indicate that COR is a more powerful elicitor to improve the saponin production yield in K. septemlobus suspension culture.
Fig. 3.
Effect of methyl jasmonate (MeJA) and coronatine (COR) on the growth (a) and saponin accumulation (b) of a K. septemlobus suspension culture. Four days after treatment with different concentrations of MeJA or COR, the cells were harvested by vacuum filtration. Data are expressed as the mean ± SE of three independent experiments
Effect of COR on the expression of the genes involved in saponin biosynthesis
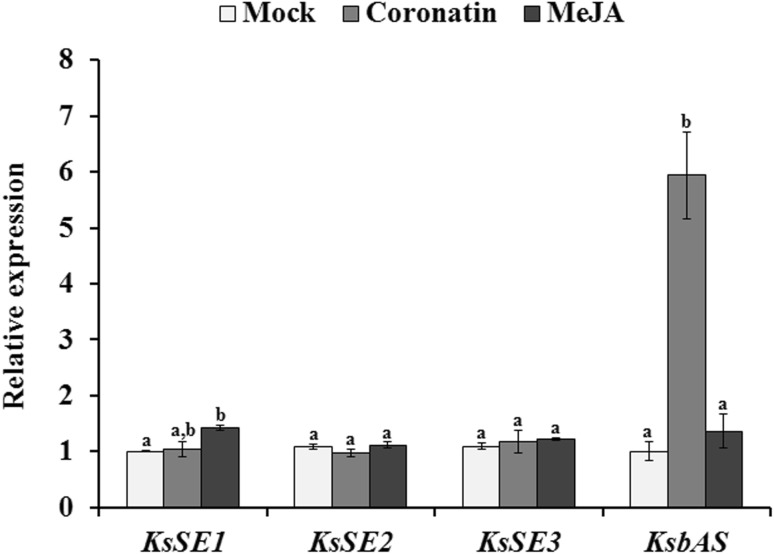
Structurally, saponins are classified into steroid and triterpenoid saponins that are synthesized from isopentenyl diphosphate produced by the methylerythritol phosphate pathway and mevalonate pathway. Both triterpenoid and steroidal aglycone backbones are derived from the linear 30-carbon precursor squalene, which is oxidized to 2,3-oxidosqualene by squalene epoxidase (SQE) (Moses et al. 2014a). Cycloartenol synthase (CAS) catalyzes 2,3-oxidosqualene to cycloartenol (the precursor for steroidal saponins), whereas oxidoreductases, including beta-amyrin synthase (bAS) and lupeol synthase, catalyze 2,3-oxidosqualene to beta-amyrin (basic precursor for oleanane-type saponins) or alpha-amyrin (basic precursor for ursane-type saponins) (Moses et al. 2014b). Most of the triterpenoid saponins from K. septemlobus were oleanane-type saponins (Quang et al. 2011), thus indicating that CAS, SQE, and bAS are the key enzymes involved in saponin biosynthesis of K. septemlobus. Therefore, to investigate the mode of action of COR in saponin biosynthesis, we identified four unigenes that encode three putative SQEs and bAS from the SRA dataset for the transcriptome sequencing of a K. septemlobus non-embryogenic callus. However, the sequence information of CAS could not be found from the assembled unigenes probably because of the low expression level of CAS in the non-embryogenic callus. The expression pattern of KsSQEs and KsbAS in elicitor-treated cell cultures was analyzed by qRT-PCR. When the cell cultures were treated with MeJA or COR, no or low induction of KsSQEs was observed (Fig. 4). However, the KsbAS transcript level in COR-treated cultures was 5.9 times higher than that in the mock control, and no significant induction in the KsbAS gene expression was observed in the MeJA-treated cultures. This expression analysis suggests that the enhanced expression of KsbAS is required for the accumulation of saponins in K. septemlobus. The elicitation of COR results in the accumulation of oleanane-type saponins can be hypothesized. To investigate our hypothesis, we analyzed the effect of elicitation on the accumulation of oleanolic acid, a precursor for oleanane-type triterpene saponins. The elicitation of COR achieved an oleanolic acid production of 2.3 μg/mg extract. Conversely, the oleanolic acid content was not detectable or was low in the mock control and MeJA-treated cell cultures (Table 2), thus indicating a clear relationship between the expression pattern of KsbAS and the oleanolic acid production. Overall, these findings suggest that COR is a powerful elicitor for inducing saponin biosynthesis, especially oleanane-type triterpene saponins, in K. septemlobus cell cultures.
Fig. 4.
Expression pattern of squalene epoxidases (KsSQEs) and beta-amyrin synthase (KsbAS) in K. septemlobus suspension culture exposed to methyl jasmonate (MeJA) and coronatine (COR). The expression level for each gene was calculated relative to its expression in the mock-treated control. The means and standard errors were calculated from three independent experiments
Table 2.
Oleanolic acid contents of the elicitor-treated K. septemlobus suspension culture
| Elicitor | Oleanolic acid (μg/mg of extract) |
|---|---|
| Coronatine | 2.369 ± 0.98 |
| Methyl jasmonate | –a |
| Mock control | –a |
aNot detectable
Discussion
Given that K. septemlobus is used in oriental medicine to treat rheumatic arthritis, furuncle, dysentery, lumbago, and neuralgia (Hyun and Kim 2009), the presence of valuable secondary metabolites, including hederagenin glycosides, in this plant has stimulated the interest of the cosmetic and pharmaceutical industries. However, the direct isolation of secondary metabolites from this plant is not economically feasible. Therefore, we established in this study that the cell suspension culture of K. septemlobus is an attractive alternative in producing valuable secondary metabolites. In the log phase, the accumulation of secondary metabolites was observed from the suspension-cultured cells of various plants (Ouyang et al. 2005; Liu et al. 2007; Sivanandhan et al. 2014). Similarly, we observed the highest saponin production capacity of K. septemlobus cells in the log phase (Fig. 2b), thus indicating that the productivity of secondary metabolites is dependent on the growth phase of the cells.
An elicitor is defined as a substance that can stimulate any type of plant defense, and it is classified into chemical, biological, and physical elicitors (Giri and Zaheer 2016). The treatment of elicitors effectively promotes the production of secondary metabolites from various plants and cultured cells (Giri and Zaheer 2016). In the case of K. septemlobus cells, the enhanced production of total saponins was observed after treatment with COR, and the total saponin production was highly influenced by the treatment with COR than with MeJA (Fig. 3b), which are commonly used to enhance saponin production from plant cell/hairy root cultures (Lu et al. 2001; Scholz et al. 2009; Kim et al. 2015; Rahimi et al. 2015; Ramirez-Estrada et al. 2016). In addition, the production of total saponins from the K. septemlobus suspension culture is highly dependent on the COR concentration. These findings indicate that elicitation is an effective approach for improving the production yield of secondary metabolites. Nevertheless, elicitor type, dose, and treatment schedule are critical factors affecting the response of the suspension-cultured cells to elicitation (Wang and Wu 2013). COR, a phytotoxin produced by Pseudomonas syringae, has been successfully used as an effective elicitor to enhance the production of taxane from Taxus media and Corylus avellana cell cultures (Onrubia et al. 2013; Gallego et al. 2015), alkaloid from Eschscholzia californica cell culture (Haider et al. 2000), viniferins from Vitis vinifera cell culture (Taurino et al. 2015), and hydroxycinnamic acids from Lemna paucicostata cell culture (Kim et al. 2017). These indicate that COR has strong potential as an elicitor triggering functional secondary metabolite production in plant cell suspension cultures. COR is a molecular mimic of the isoleucine-combined form of jasmonic acid, indicating that COR consequently has a similar mechanism of action to the elicitor MeJA (Ramirez-Estrada et al. 2016). Interestingly, COR usually activates the production of secondary metabolites in cell cultures at concentrations lower than MeJA (Onrubia et al. 2013; Gallego et al. 2015; Ramirez-Estrada et al. 2016), which is similar to our observation (Fig. 3b). In addition, COR-treated cells exhibited a higher level of KsbAS expression than MeJA-treated cells (Fig. 4). A possible explanation for this phenomenon might be that COR is more stable than MeJA due to the rigid cis-orientation in its bi-cyclic skeleton (Heitz et al. 2012; Onrubia et al. 2013), suggesting that COR exerts biological effects on plant at low concentration.
In conclusion, the overall results of the present study suggest that COR is a powerful elicitor for inducing saponin biosynthesis in K. septemlobus suspension culture. The analysis of oleanolic acid content and gene expression pattern indicates that KsbAS should be a major target gene for COR. These results are an important step toward improving the production yield of saponins in plant suspension cultures, and they provide an opportunity for intensive research on the modification of the COR structure to improve the biological activity of COR.
Acknowledgements
This work was supported by the research Grant from the National Research Foundation of Korea (NRF-2015R1C1A1A01053448) funded by the Ministry of Science.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Jae Kook Lee and Seung Hee Eom contributed equally to this work.
References
- Bang SY, Park GY, Park SY, Kim JH, Lee YK, Lee SJ, Kim Y. The stem bark of Kalopanax pictus exhibits anti-inflammatory effect through heme oxygenase-1 induction and NF-κB suppression. Immune Netw. 2010;10:212–218. doi: 10.4110/in.2010.10.6.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego A, Imseng N, Bonfill M, Cusido RM, Palazon J, Eibl R, Moyano E. Development of a hazel cell culture-based paclitaxel and baccatin III production process on a benchtop scale. J Biotechnol. 2015;195:93–102. doi: 10.1016/j.jbiotec.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Geng X, Jin L, Shimada M, Kim MG, Mackey D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta. 2014;240:1149–1165. doi: 10.1007/s00425-014-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri CC, Zaheer M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 2016;126:1–18. doi: 10.1007/s11240-016-0985-6. [DOI] [Google Scholar]
- Haider G, von Schrader T, Füsslein M, Blechert S, Kutchan TM. Structure–activity relationships of synthetic analogs of jasmonic acid and coronatine on induction of benzo[c]phenanthridine alkaloid accumulation in Eschscholzia californica cell cultures. Biol Chem. 2000;381:741–748. doi: 10.1515/BC.2000.094. [DOI] [PubMed] [Google Scholar]
- Heitz T, Widemann E, Lugan R, Miesch L, Ullmann P, Désaubry L, Holder E, Grausem B, Kandel S, Miesch M, Werck-Reichhart D, Pinot F. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone Jasmonoyl-isoleucine for catabolic turnover. J Biol Chem. 2012;287:6296–6306. doi: 10.1074/jbc.M111.316364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Huang C, Wang MH. Chemical composition, nutritional value, and antioxidant constituents of Kalopanax pictus leaves. Food Chem. 2012;131:449–455. doi: 10.1016/j.foodchem.2011.09.005. [DOI] [Google Scholar]
- Hyun TK, Kim J-S. The pharmacology and clinical properties of Kalopanax pictus. J Med Plants Res. 2009;3:613–620. [Google Scholar]
- Kim S-J, Moon H-K. Establishment of suspension culture condition for embryogenic callus proliferation and somatic embryo development of Kalopanax septemlobus. J Plant Biotechnol. 2009;36:7–12. doi: 10.5010/JPB.2009.36.1.007. [DOI] [Google Scholar]
- Kim YB, Reed DW, Covello PS. Production of triterpenoid sapogenins in hairy root cultures of Silene vulgaris. Nat Prod Commun. 2015;10:1919–1922. [PubMed] [Google Scholar]
- Kim J-Y, Kim H-Y, Jeon J-Y, Kim D-M, Zhou Y, Lee JS, Lee H, Choi HK. Effects of coronatine elicitation on growth and metabolic profiles of Lemna paucicostata culture. PLoS ONE. 2017;12:e0187622. doi: 10.1371/journal.pone.0187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Jin YW, Park JH, Yoo YM, Hong SM, Amir R, Yan Z, Kwon E, Elfick A, Tomlinson S, Halbritter F, Waibel T, Yun BW, Loake GJ. Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol. 2010;28:1213–1217. doi: 10.1038/nbt.1693. [DOI] [PubMed] [Google Scholar]
- Liu JY, Guo ZG, Zeng ZL. Improved accumulation of phenylethanoid glycosides by precursor feeding to suspension culture of Cistanche salsa. Biochem Eng J. 2007;33:88–93. doi: 10.1016/j.bej.2006.09.002. [DOI] [Google Scholar]
- Lu M, Wong H, Teng W. Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep. 2001;20:674–677. [Google Scholar]
- Moon H-K, Kim Y-W, Lee J-S, Choi Y-E. Micropropagation of Kalopanax pictus tree via somatic embryogenesis. In Vitro Cell Dev Biol Plant. 2005;41:303–306. doi: 10.1079/IVP2004608. [DOI] [Google Scholar]
- Moon HK, Lee HS, Paek KY, Park SY. Osmotic stress and strong 2,4-D shock stimulate somatic-to-embryogenic transition in Kalopanax septemlobus (Thunb.) Koidz. Acta Physiol Plant. 2015;37:1710. doi: 10.1007/s11738-014-1710-x. [DOI] [Google Scholar]
- Moses T, Papadopoulou KK, Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol. 2014;49:439–462. doi: 10.3109/10409238.2014.953628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses T, Pollier J, Almagro L, Buyst D, Van Montagu M, Pedreño MA, Martins JC, Thevelein JM, Goossens A. Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α hydroxylase from Bupleurum falcatum. Proc Natl Acad Sci USA. 2014;111:1634–1639. doi: 10.1073/pnas.1323369111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy HN, Lee E-J, Paek K-Y. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014;118:1–16. doi: 10.1007/s11240-014-0467-7. [DOI] [Google Scholar]
- Ochoa-Villarreal M, Howat S, Hong S, Jang MO, Jin YW, Lee EK, Loake GJ. Plant cell culture strategies for the production of natural products. BMB Rep. 2016;49:149–158. doi: 10.5483/BMBRep.2016.49.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onrubia M, Moyano E, Bonfill M, Cusidó RM, Goossens A, Palazón J. Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol. 2013;170:211–219. doi: 10.1016/j.jplph.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Wang XD, Zhao B, Wang YC. Enhanced production of phenylethanoid glycosides by precursor feeding to cell culture of Cistanche deserticola. Process Biochem. 2005;40:3480–3484. doi: 10.1016/j.procbio.2005.02.025. [DOI] [Google Scholar]
- Park C, Jeong JS, Jeong JW, Kim YJ, Jung YK, Go GB, Kim SO, Kim GY, Hong SH, Yoo YH, Choi YH. Ethanol extract of Kalopanax septemlobus leaf induces caspase-dependent apoptosis associated with activation of AMPK in human hepatocellular carcinoma cells. Int J Oncol. 2016;48:261–270. doi: 10.3892/ijo.2015.3233. [DOI] [PubMed] [Google Scholar]
- Quang TH, Ngan NT, Minh CV, Kiem PV, Nhiem NX, Tai BH, Thao NP, Tung NH, Song SB, Kim YH. Anti-inflammatory triterpenoid saponins from the stem bark of Kalopanax pictus. J Nat Prod. 2011;74:1908–1915. doi: 10.1021/np200382s. [DOI] [PubMed] [Google Scholar]
- Quang TH, Ngan NT, Van Minh C, Van Kiem P, Nhiem NX, Tai BH, Thao NP, Chae D, Mathema VB, Koh YS, Lee JH, Yang SY, Kim YH. Inhibitory effects of oleanane-type triterpenes and saponins from the stem bark of Kalopanax pictus on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch Pharm Res. 2013;36:327–334. doi: 10.1007/s12272-013-0031-8. [DOI] [PubMed] [Google Scholar]
- Rahimi S, Kim YJ, Yang DC. Production of ginseng saponins: elicitation strategy and signal transductions. Appl Microbiol Biotechnol. 2015;99:6987–6996. doi: 10.1007/s00253-015-6806-8. [DOI] [PubMed] [Google Scholar]
- Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182. doi: 10.3390/molecules21020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke BK, Shin J, Sawant SS, Alkotaini B, Lee S, Kim BS. Rapid biological synthesis of silver nanoparticles using Kalopanax pictus plant extract and their antimicrobial activity. Korean J Chem Eng. 2014;31:2035–2040. doi: 10.1007/s11814-014-0149-5. [DOI] [Google Scholar]
- Scholz M, Lipinski M, Leupold M, Luftmann H, Harig L, Ofir R, Fischer R, Prüfer D, Müller KJ. Methyl jasmonate induced accumulation of kalopanaxsaponin I in Nigella sativa. Phytochemistry. 2009;70:517–522. doi: 10.1016/j.phytochem.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M. Enhanced biosynthesis of withanolides by elicitation and precursor feeding in cell suspension culture of Withania somnifera (L.) Dunal in shake-flask culture and bioreactor. PLoS ONE. 2014;9:e104005. doi: 10.1371/journal.pone.0104005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurino M, Ingrosso I, Damico L, De Domenico S, Nicoletti I, Corradini D, Santino A, Giovinazzo G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv Negramaro cell cultures. Springerplus. 2015;4:49. doi: 10.1186/s40064-015-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vador N, Vador B, Hole R. Simple spectrophotometric methods for standardizing ayurvedic formulation. Indian J Pharm Sci. 2012;74:161–163. doi: 10.4103/0250-474X.103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wu JY. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. Adv Biochem Eng Biotechnol. 2013;134:55–89. doi: 10.1007/10_2013_183. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J. 2012;10:249–268. doi: 10.1111/j.1467-7652.2011.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang L, Baldwin IT. Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? Planta. 2008;227:1161–1168. doi: 10.1007/s00425-008-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-H, Su Q, Zang Z-H. Simultaneous determination of oleanolic acid and ursolic acid by RP-HPLC in the leaves of Eriobotrya japonica Lindl. J Pharm Anal. 2012;2:238–240. doi: 10.1016/j.jpha.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]