Abstract
For quality control purpose, an approach of combining chromatographic fingerprint of Huaijiao pill (HP) and simultaneous determination of its major bioactive components was developed using high performance liquid chromatography coupled with diode array detector (HPLC--DAD). For fingerprint analysis, 16 peaks were selected as the characteristic peaks to evaluate the similarities of different samples collected from different batches of three manufacturers. The similarities of 17 Huaijiao pill samples were beyond 0.966, indicating that samples from different batches and manufacturers were, to some extent, consistent. Additionally, simultaneous quantification of seven bioactive markers, namely sophoricoside, baicalin, naringin, genistein, rutin, quercetin and 5-O-methylvisammioside, in HP was performed to interpret the quality consistency. The validation of the proposed approach was acceptable, with the accuracy of 90.2%–106.9% in recovery test. The intra-day and inter-day precisions of the method were evaluated and the RSD values were less than 2.81%. The results from the quantitative data showed that the contents of six marker compounds (except for 5-O-methylvisammioside) were quite consistent between batches produced by one manufacturer and significantly distinctive among different manufacturers. The proposed approach was expected to be developed as a powerful tool for the quality control of HP.
Keywords: Chromatographic fingerprint, HPLC--DAD, Huaijiao pill
1. Introduction
Huaijiao pill (HP), an ancient traditional Chinese medicine (TCM) widely used across China, is prepared from six Chinese medicinal herbs including Sophorae Fructus (Sophora japonica L.), Sanguisorbae Radix (Sanguisorba officinalis L.), Scutellariae Radix (Scutellaria baicalensis Georgi), Aurantii Fructus (Citrus aurantium L.), Angelicae Sinensis Radix (Angelica sinensis (Oliv.) Diels) and Saposhnikoviae Radix (Saposhnikovia divaricata (Turcz.) Schischk). It is commonly applied in clinical practice for the treatment of hematochezia, edema and carbuncle caused by haememorrhoids through clearing heat in bowels and dispelling wind, cooling blood for hemostasis, eliminating swelling and easing pain [1]. Moreover, HP is reported to treat hypertension, chronic pharyngitis and acne, and satisfactory effects were obtained [2], [3], [4]. Modern pharmacological studies have demonstrated its efficacy in inhibiting anti-Lea agglutinin, stopping bleeding, anti-tumor, anti-inflammation, anti-aging and antiatheroscloresis [5], [6], [7], [8]. According to Chinese medicine therapy, Sophorae Fructus is the principal component of HP [9]. According to modern pharmacological researches, the efficacy of Sophorae Fructus is associated with its flavonoides including sophoricoside, rutin, genistein and quercetin. Sophoricoside, the main bioactive constituent of Sophorae Fructus, shows inhibitory effects on chemical mediators involving in inflammatory response. It was identified as a selective inhibitor of cyclooxygenase (COX)-2 activity [10]. Meanwhile, sophoricoside and genistein (well known as a phytoestrogen of soy products, and were reported to prevent cancer and osteoporosis) exhibit inhibitory effects on proinflammatory cytokines, IL-5, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-6 bioactivities [11]. All these supplementary herb components provide a therapeutic function via synergistic effects with the principal drug.
Unlike the synthetic drugs, TCMs exert the curative effects based on the synergic effects of their multi-components. More than 100 manufacturers produce HP across China. The composition of these herbs can vary depending on geographical source, cultivation conditions, harvest time, storage, and pretreatment. However, only three bioactive components of it, i.e., sophoricoside, naringin and baicalin, have been determined through officially conducted quality control of HP presently [1]. Anaphylactic reaction reported is induced by taking HPs [12], [13]. Therefore, an integral quality control approach based on the multiple constituents of HP is urgently needed to ensure the efficacy and safety of the drug. Fingerprint has been internationally accepted as an efficient technique over the last two decades for the authentication and quality control of TCMs [14], [15]. Chromatographic fingerprint technique can be used to characterize both the marker compounds and the unknown components in a complex system, a strategy recommended to assess the quality and consistency of botanical products by the State Food and Drug Administration of China (SFDA), the European Medicines Evaluation Agency (EMEA), and the US Food and Drug Administration (USFDA) [16], [17], [18].
However, fingerprint is insufficient to control the overall quality of TCMs since it cannot quantitate bioactive constituents which are directly related to the quality of the TCMs [19], [20]. Recently, some methods combining chromatographic fingerprint and quantitative determination have been developed and validated for quality control of herbal preparations [21], [22]. So far, several approaches have been developed for the determination of the bioactive constituents of HP, such as high performance liquid chromatography (HPLC) [23], [24]. However, to the best of our knowledge, quality control approaches, which have been published, are mainly focused on constituents in one or two of the six Chinese medicinal herbs in HP [25], [26], [27]. In HP, sophoricoside, baicalin, naringin, genistein, rutin, quercetin and 5-O-methylvisammioside are generally considered as the bioactive components, and their determinations are respectively well-documented. We report, for the first time, a combinative method using HPLC for fingerprint and simultaneous quantitation of seven major bioactive constituents in HP. Meanwhile, the combinative method can be readily utilized as a comprehensive quality control approach for the TCM formula as well.
2. Experimental
2.1. Materials and chemicals
Seventeen samples of Huaijiao pill from three manufacturers were collected: S1–S7 (sample 1, S1; the abbr. of other samples were similar) were from Chongqing TongJunGe Pharmaceutical Co., Ltd, Taiji Group, Chongqing, China; S8–S15 were from Chongqing Second TCM Co., Ltd, Taiji Group, Chongqing, China; S16 and S17 were from Wuhan TaiFu Pharmaceutical Co., Ltd, Wuhan, China. All the samples were water-honeyed pills. Chemical standards of sophoricoside, baicalin, genistein, rutin, quercetin and 5-O-methylvisammioside were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), and naringin was obtained from Fluka BioChemika (Switzerland). Acetonitrile and methanol of HPLC grade were purchased from Dikma Technology Inc.. Ultrapure water was obtained from Southwest Institute of Technical Physics, Chengdu, China. All other chemicals and reagents were of analytical reagent grade and used without further purification.
2.2. Apparatus and chromatographic conditions
Instrument for analysis was a Shimadzu LC-20A module consisting of an LC-20AB binary pump, a DGU-20A3 degasser, a CTO-20A thermostatted column compartment and an SPD-M20A UV–vis diode array detector (Shimadzu, Japan). HPLC fingerprints and quantitative determinations were performed with LC solution software (Shimadzu, Japan). The chromatographic separation was carried out on a Dikma Diamonsil C18 column (250 mm×4.6 mm, 5 µm) with a Dikma Diamonsil ODS (10 mm×4.0 mm, 5 µm) pre-column from Dikma Technologies maintained at 35.2 °C. The mobile phases were composed of mobile phase A (acetonitrile-0.05% phosphoric acid (95:5, v/v) and mobile phase B (acetonitrile-0.05% phosphoric acid (20:80, v/v) with a gradient program as follows: 0–5 min, linear gradient 0%–10% B; 5–15 min, linear gradient 10%–20% B; 15–25 min, linear gradient 20%–25% B; 25–30 min, linear gradient 25%–30% B; 30–36 min, linear gradient 30%–45% B; 36–47 min, linear gradient 45%–100% B; 47–52 min, isocratic 100% B; and 52–57 min, linear gradient 100%–0% B at a flow rate of 1.0 mL/min. The UV absorbance was monitored at 254 nm using DAD. All injection volumes of sample and standard solutions were 10 μL.
2.3. Preparation of sample and standard solution
1.0 g of HP powder was extracted by ultrasonication for 45 min with 20 mL of solvent (70% ethanol-glacial acetic acid (80:1, v/v)) in a 50 mL conical flask with cover. The extract was cooled to room temperature, adjusted the weight back to the pre-extractioin weight with solvent, and then centrifuged at 10,000 rpm for 10 min. 2 mL of supernatant was transferred to a 10 mL volumetric flask, and then methanol was added to the scale and shaken evenly. The solutions were filtered with a 0.45 µm membrane filter prior to HPLC analysis.
Each standard was accurately weighed and dissolved in methanol to produce standard stock solution. A mixed standard stock solution was then prepared in methanol from the individual standard stock solutions. And various standard solutions were obtained through diluting the stock solution to a series of concentrations in order to construct the calibration curves. All the standard solutions were stored in a refrigerator at 4 °C and brought to room temperature before analysis.
2.4. Preparation of negative control solution and unilateral sample solution
According to the proportion and the preparation technology of the prescription, the negative sample was prepared without Sophorae Fructus, Scutellariae Radix and Aurantii Fructus. Then, the negative control solution was prepared in accordance with the preparation method of the test solution in Section 2.3. Similarly, six solutions of the unilateral sample were prepared respectively.
2.5. Method validation
2.5.1. Specificity
The specificity test was performed by analyzing the negative control solution, and then compared its chromatogram with those of the mixed standard solution and the sample solution.
2.5.2. Linearity, limit of detection (LOD) and limit of quantification (LOQ)
The linearity study was achieved by diluting stock solution into a series of concentrations. The calibration curves were constructed with six concentrations in triplicate. Calibration curves for all the compounds were generated by plotting the integrated chromatography peak area (Y) versus the concentrations (X, μg/mL) of the mixed standard solutions. Results were expressed as the values of the correlation coefficient (r2). LOD and LOQ were calculated by diluting the standard solution when signal-to-noise ratios (S/N) of analytes were almost 3 and 10, respectively.
2.5.3. Precision, repeatability and accuracy
Intra- and inter-day precision tests were performed by analyzing sample solutions (S1) during a single day (n=6) and on six different days (n=3), respectively. For repeatability test, six independent sample solutions (S1) were prepared by the same procedure noted in Section 2.3. An accurately known amount of the corresponding marker compounds at three concentration levels (0.5, 1.0 and 1.5 times of the concentration) was spiked into a previously analyzed HP sample solution (S1), and three concentration levels of solutions were prepared and then analyzed. Recovery tests were performed by comparatively analyzing spiked and unspiked samples. Relative standard deviation (RSD) was used to describe precision, repeatability and recovery.
2.6. HPLC fingerprints
2.6.1. Similarity calculation of the HPLC fingerprints
All determinations were carried out in triplicate, and the data were analyzed by the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine software (Version 2004 A) which was developed by the Chinese Pharmacopoeia Committee. The median method was chosen to fit the fingerprint chromatograms and the correlation coefficient was employed to evaluate the similarity.
2.6.2. Assignment of the 16 characteristic peaks of the HPLC fingerprint
Chromatograms of six unilateral sample solutions were compared with the sample fingerprint chromatogram, respectively. And the 16 characteristic peaks of the HPLC fingerprint were individually assigned to the corresponding herbs.
3. Results and discussion
3.1. Optimization of HPLC chromatographic conditions
HPLC conditions including mobile phase and detection wavelength were investigated to optimize chromatographic separation. The effect of mobile phase composition on chromatographic separation was investigated, using methanol-0.05% phosphoric acid, acetonitrile-0.2% acetic acid, and acetonitrile-0.05% phosphoric acid, respectively. Finally, the proposed mobile phase of acetonitrile-0.05% phosphoric acid (v/v) had the most effective HPLC result.
The wavelength of the target compounds in HP was selected by a DAD full wavelength scan (190–400 nm). Most chemical constituents in HP had strong UV absorbance at 254 nm. Therefore, 254 nm was chosen for obtaining the HP fingerprints. Meanwhile, the maximum absorption wavelengths, 260 nm (for flavonoides including sophoricoside, genistein, rutin and quercetin), 277 nm (for baicalin), 283 nm (for naringin) and 293 nm (for 5-O-methylvisammioside), were selected for the detection, respectively. Optimal HPLC condition used in this study is shown as follows. The mobile phases were composed of mobile phase A (acetonitrile-0.05% phosphoric acid (95:5, v/v)) and mobile phase B (acetonitrile-0.05% phosphoric acid (20:80, v/v)) with a gradient program. The UV absorbance was monitored at 254 nm using DAD. All injection volumes of sample and standard solutions were 10 μL.
3.2. Optimization of extraction conditions
Optimization of extraction conditions was investigated through single factor experiment. Sample pretreatment conditions were optimized by investigating the effect of extraction solvents and methods on the extraction efficiency of chemical markers used for HPLC fingerprinting and quantification. 70% ethanol-glacial acetic acid (80∶1, v/v) was found to be the most effective solvent for extracting more compounds based on the HPLC results.
Extraction methods including ultrasonication and heat-reflux were then investigated for extraction efficiency using 70% ethanol-glacial acetic acid (80∶1, v/v) as the extraction solvent. The results showed that the extraction efficiencies of the two techniques were comparable, and ultrasonication was chosen because of its simple and rapid performance.
Then, the ratio of sample-extraction solvent with 1 g∶10 mL, 1 g∶20 mL, 1 g∶40 mL was studied respectively, and a ratio of 1 g∶20 mL was selected according to the HPLC results, which was economic and environmental friendly. Extraction time under ultrasonication was also tested, and the results showed that all the marker compounds were extracted within 45 min, and that longer period of ultrasonication did not increase the contents significantly. The optimal extraction conditions for HP used in this study are detailed as follows. 1.0 g of HP powder was extracted by ultrasonication for 45 min with 20 mL of solvent (70% ethanol-glacial acetic acid (80∶1, v/v)) in a 50 mL conical flask with cover. The extract was cooled to room temperature, adjusted the weight back to the pre-extractioin weight with solvent, and then centrifuged at 10,000 rpm for 10 min. 2 mL of supernatant was transferred to a 10 mL volumetric flask, and then methanol was added to the scale and shaken evenly. The solutions were filtered with a 0.45 µm membrane filter prior to HPLC analysis.
3.3. Method validation
3.3.1. Specificity
No interfering peaks were observed in any chromatograms of the negative control solution compared with the sample solution and the mixed standard solution at the same retention time.
3.3.2. Linearity, LOD and LOQ
As shown in Table 1, acceptable results of the regression analysis, the correlation coefficients (r2), LOD and LOQ were obtained for all the analytes. The LOD and the LOQ were in the range of 0.005–0.303 μg/mL and 0.017–0.954 μg/mL for all the analytes, respectively. DAD detections have acceptable linearity beyond 0.9995.
Table 1.
Statistical results of linear regression equation analysis in the determination of seven bioactive components in HP.
| Analyte | Regression equation (Y= aX+ b) | r2 (n = 6) | Linear range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Sophoricoside | Y=53656X+1856 | 0.9998 | 5.94–118.9 | 0.021 | 0.059 |
| Baicalin | Y=32129X-6974.2 | 0.9996 | 5.61–112.2 | 0.109 | 0.353 |
| Naringin | Y=15874X+1492.8 | 1.0000 | 8.91–178.2 | 0.303 | 0.954 |
| Genistein | Y=70623X-6707.8 | 0.9995 | 0.59–11.8 | 0.053 | 0.145 |
| Rutin | Y=16522X+3036 | 0.9999 | 2.32–46.4 | 0.075 | 0.216 |
| Quercetin | Y=37178X+1813.1 | 0.9995 | 0.54–10.8 | 0.020 | 0.065 |
| 5-O-methylvisammioside | Y=16522X+3036 | 1.0000 | 1.16–23.2 | 0.005 | 0.017 |
For the regression equation, Y = aX +b, Y is the peak area while X is the concentration (μg/mL).
3.3.3. Precision, repeatability and accuracy
For precision test, statistical data showed that the RSD values of six compounds (except that 5-O-methylvisammioside was not detected in sample solutions under the optimized method. One possible explanation was that its content was far too low.) were in the range of 0.34%–1.36% for intra-day precision and 0.79%–2.81% for inter-day precision. The RSD values of repeatability were less than 2.41% (Table 2). For recovery test, mean recoveries of the seven standard substances were between 96.1% and 101.0%, with RSD values less than 1.35% (n=9) (Table 3). These validation results described above indicated that the developed method is acceptable.
Table 2.
Results of intra- and inter-day precisions and repeatability (n=6).
| Analyte | Precision |
Repeatability |
||||
|---|---|---|---|---|---|---|
| Intra-day |
Inter-day |
Mean (μg/mL) | RSD (%) | |||
| Mean (μg/mL) | RSD (%) | Mean (μg/mL) | RSD(%) | |||
| Sophoricoside | 81.92 | 1.36 | 83.04 | 2.81 | 70.24 | 2.41 |
| Baicalin | 102.54 | 0.34 | 106.67 | 0.79 | 91.38 | 0.95 |
| Naringin | 42.63 | 0.89 | 43.88 | 1.98 | 31.39 | 2.18 |
| Genistein | 5.55 | 1.11 | 5.83 | 1.04 | 1.58 | 0.99 |
| Rutin | 24.81 | 1.04 | 26.46 | 0.88 | 30.83 | 0.79 |
| Quercetin | 0.99 | 1.15 | 1.01 | 1.93 | 0.97 | 1.32 |
5-O-methylvisammioside was not detected in sample solutions under the separation condition described in Section 2.2.
Table 3.
Statistical results of recovery of extraction of analytes in HP (n=9).
| Analyte | Spiked amount (mg) | Recorded amount (mg) | RSD (%) | Calculated recovery (%) | Mean recovery (%) |
|---|---|---|---|---|---|
| Sophoricoside | 0.144 | 0.151 | 0.89 | 104.9 | 100.3 |
| 0.288 | 0.279 | 0.45 | 96.8 | ||
| 0.432 | 0.428 | 0.21 | 99.1 | ||
| Baicalin | 0.109 | 0.106 | 0.32 | 97.6 | 100.0 |
| 0.218 | 0.224 | 0.55 | 102.5 | ||
| 0.327 | 0.327 | 0.04 | 99.9 | ||
| Naringin | 0.101 | 0.108 | 1.35 | 106.9 | 100.6 |
| 0.202 | 0.182 | 0.76 | 90.2 | ||
| 0.303 | 0.317 | 1.04 | 104.6 | ||
| Genistein | 0.006 | 0.005 | 0.67 | 92.7 | 96.1 |
| 0.011 | 0.010 | 0.34 | 94.4 | ||
| 0.017 | 0.017 | 0.58 | 101.1 | ||
| Rutin | 0.107 | 0.105 | 0.25 | 98.1 | 101.0 |
| 0.214 | 0.216 | 0.49 | 101.0 | ||
| 0.321 | 0.333 | 0.81 | 103.8 | ||
| Quercetin | 2.45×10-3 | 2.41×10-3 | 0.34 | 98.4 | 96.3 |
| 4.90×10-3 | 4.80×10-3 | 0.28 | 98.0 | ||
| 7.35×10-3 | 6.81×10-3 | 0.54 | 92.6 | ||
| 5-O-methylvisammioside | 5.2×10-3 | 5.3×10-3 | 0.65 | 101.9 | 99.1 |
| 10.4×10-3 | 10.2×10-3 | 0.23 | 98.1 | ||
| 15.6×10-3 | 15.2×10-3 | 0.44 | 97.4 |
3.4. Quantitative determination of the seven marker compounds in HP
In this study, the proposed HPLC--DAD method was successfully applied to the simultaneous determination of the seven markers in HP samples. The identity of the marker compound peaks in the chromatogram was confirmed by their retention time and their DAD profiles. The contents of the marker compounds in 17 samples from three manufacturers are summarized in Table 4. Significant variances among the contents of the same markers were observed from different samples. For example, the highest content of baicalin was 10.38 mg/g in sample S7 and the lowest (3.44 mg/g) in sample S8. The concentrations of the analytes were in the order of baicalin> sophoricoside> rutin> genistein>quercetin, determined in samples S1, S2, S3, S4, S5, S6, S7 (S1–S7 were obtained from one manufacturer), S16 and S17 (these two samples were from the same manufacturer). However, the order (sophoricoside>baicalin>naringin>rutin>genistein>quercetin) was different in samples S8, S9, S11, S12, S13 and S14 which were produced by the same manufacturer. Moreover, naringin was only detected in samples S1, S8, S9, S10, S11, S12, S13 and S14. These data suggested that the batch-to-batch consistency of HP was good under the same manufacturing conditions, which were consistent with results from the chromatographic fingerprints. However, the content of each marker determined between manufacturers, was significantly different with RSD values of 12.83%–64.87%, indicating the large variations in their quality. A possible explanation of the result is that the herbal materials and manufacturing processes applied by manufacturers are quite different. It is noteworthy that chromatographic fingerprints may not fully monitor the quality consistency. Therefore, more attention should be paid to quality consistency of HP to ensure its clinical efficacy. Our results accorded with the previous studies. Compared to chromatographic fingerprint alone, chromatographic fingerprint combined with quantitative techniques for determining marker compounds is a better tool for quality consistency evaluation of herbal preparations [22], [28], [29].
Table 4.
Quantitative determination of seven markers in 17 HP samples.
| Sample no. | Contents (n=3, mg/g; mean±SD) |
|||||
|---|---|---|---|---|---|---|
| Sophoricoside | Baicalin | Naringin | Genistein | Rutin | Quercetin | |
| S1 | 7.42±0.37 | 9.28±0.25 | 4.91±0.22 | 0.53±0.12 | 2.39±0.14 | 0.10±0.03 |
| S2 | 6.95±0.21 | 9.85±0.15 | nd | 0.11±0.04 | 2.68±0.08 | trace |
| S3 | 7.35±0.17 | 7.18±0.16 | nd | 0.15±0.00 | 2.61±0.05 | 0.01±0.00 |
| S4 | 7.03±0.25 | 9.57±0.43 | nd | 0.11±0.00 | 2.86±0.03 | trace |
| S5 | 6.68±0.13 | 9.14±0.31 | nd | 0.15±0.00 | 2.70±0.01 | 0.03±0.00 |
| S6 | 7.36±0.15 | 9.83±0.23 | nd | 0.11±0.01 | 2.89±0.01 | trace |
| S7 | 7.69±0.19 | 10.38±0.34 | nd | 0.13±0.00 | 3.06±0.04 | 0.01±0.00 |
| S8 | 6.38±0.08 | 3.44±0.08 | 4.29±0.24 | 0.19±0.03 | 3.46±0.05 | 0.06±0.00 |
| S9 | 5.89±0.07 | 4.98±0.03 | 5.86±0.09 | 0.18±0.01 | 3.25±0.01 | 0.05±0.01 |
| S10 | 5.51±0.09 | 6.13±0.15 | 5.26±0.11 | 0.22±0.00 | 1.31±0.00 | 0.01±0.00 |
| S11 | 6.56±0.23 | 4.74±0.01 | 3.56±0.07 | 0.17±0.00 | 3.65±0.09 | 0.03±0.00 |
| S12 | 6.33±0.16 | 4.70±0.03 | 3.90±0.05 | 0.19±0.01 | 3.56±0.06 | 0.02±0.00 |
| S13 | 6.15±0.06 | 4.08±0.01 | 5.38±0.13 | 0.18±0.01 | 3.41±0.04 | 0.06±0.01 |
| S14 | 5.80±0.07 | 4.45±0.01 | 5.89±0.04 | 0.19±0.00 | 3.27±0.01 | 0.03±0.00 |
| S15 | 5.97±0.01 | 4.50±0.00 | nd | 0.15±0.00 | 2.10±0.00 | 0.05±0.01 |
| S16 | 5.83±0.03 | 6.90±0.07 | nd | 0.17±0.00 | 1.77±0.03 | 0.04±0.00 |
| S17 | 4.50±0.05 | 5.88±0.01 | nd | 0.15±0.01 | 1.65±0.07 | 0.03±0.00 |
| Average | 6.44±0.83 | 6.77±2.42 | 4.88±0.88 | 0.18±0.10 | 2.74±0.70 | 0.04±0.02 |
| RSD (%) | 12.83 | 35.72 | 18.05 | 52.46 | 25.61 | 64.87 |
nd: under detected limit; trace: under quantification limit.
5-O-methylvisammioside was not detected in sample solutions under the separation condition described in Section 2.2.
3.5. HPLC fingerprints
3.5.1. Evaluation of the HPLC fingerprints
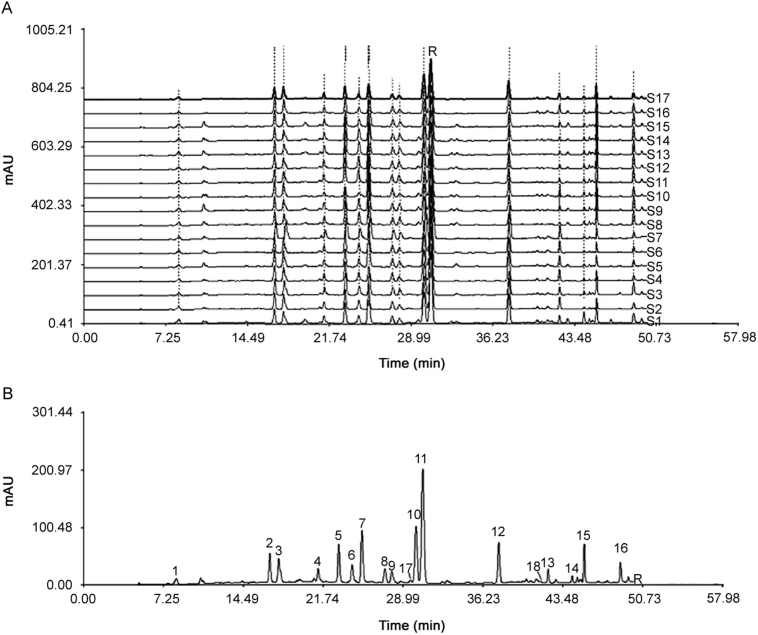
Chromatographic fingerprints were generated for 17 HP samples from three manufacturers, and 16 peaks were found in each individual sample (Fig. 1A). A simulative median fingerprint was generated by the professional software by analyzing all the 17 samples (Fig. 1B). The simulative median chromatogram of HP had 16 well-resolved “characteristic peaks”. Sophoricoside (peak 11) was an important bioactive component of HP with a consistently high content and a suitable retention time, so peak 11 was chosen as the reference peak to calculate the relative retention time (RRT) and relative peak area (RPA). RRT and RPA of the 16 characteristic peaks in 17 samples are shown in Table 5, Table 6.
Fig. 1.
HPLC chromatographic fingerprints of (A) 17 HP samples and (B) simulative median chromatogram obtained by Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine software (2004A). The chromatograms marked with S1–S17 and R represent 17 HP samples and the simulative median chromatogram, respectively. The peaks marked with 1–16 in the simulative median chromatogram represent 16 characteristic peaks, and peaks marked with 17 and 18 were naringin and quercetin, respectively. The separation condition was described in Section 2.2.
Table 5.
The relative retention time (RRT) of 16 characteristic peaks for 17 HP samples.
| Sample no. | Peak no. |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| S1 | 0.274 | 0.549 | 0.575 | 0.692 | 0.752 | 0.792 | 0.820 | 0.888 | 0.909 | 0.980 | 1.000 | 1.225 | 1.371 | 1.442 | 1.477 | 1.584 |
| S2 | 0.273 | 0.549 | 0.575 | 0.691 | 0.752 | 0.792 | 0.821 | 0.888 | 0.908 | 0.980 | 1.000 | 1.224 | 1.370 | 1.441 | 1.477 | 1.584 |
| S3 | 0.273 | 0.550 | 0.576 | 0.692 | 0.753 | 0.792 | 0.821 | 0.889 | 0.909 | 0.980 | 1.000 | 1.224 | 1.369 | 1.440 | 1.475 | 1.582 |
| S4 | 0.273 | 0.549 | 0.576 | 0.692 | 0.753 | 0.792 | 0.821 | 0.888 | 0.909 | 0.980 | 1.000 | 1.225 | 1.371 | 1.442 | 1.478 | 1.585 |
| S5 | 0.273 | 0.549 | 0.575 | 0.692 | 0.752 | 0.792 | 0.820 | 0.889 | 0.909 | 0.980 | 1.000 | 1.224 | 1.370 | 1.441 | 1.477 | 1.584 |
| S6 | 0.273 | 0.548 | 0.575 | 0.691 | 0.752 | 0.792 | 0.820 | 0.888 | 0.908 | 0.980 | 1.000 | 1.224 | 1.370 | 1.441 | 1.477 | 1.583 |
| S7 | 0.273 | 0.551 | 0.579 | 0.693 | 0.753 | 0.793 | 0.822 | 0.889 | 0.909 | 0.980 | 1.000 | 1.222 | 1.366 | 1.436 | 1.472 | 1.578 |
| S8 | 0.273 | 0.549 | 0.576 | 0.692 | 0.753 | 0.792 | 0.821 | 0.889 | 0.909 | 0.980 | 1.000 | 1.224 | 1.370 | 1.441 | 1.476 | 1.583 |
| S9 | 0.273 | 0.549 | 0.575 | 0.691 | 0.752 | 0.791 | 0.820 | 0.888 | 0.909 | 0.963 | 1.000 | 1.224 | 1.370 | 1.441 | 1.477 | 1.583 |
| S10 | 0.273 | 0.549 | 0.575 | 0.691 | 0.752 | 0.792 | 0.820 | 0.888 | 0.909 | 0.980 | 1.000 | 1.224 | 1.370 | 1.441 | 1.476 | 1.583 |
| S11 | 0.273 | 0.549 | 0.575 | 0.691 | 0.752 | 0.791 | 0.820 | 0.888 | 0.908 | 0.980 | 1.000 | 1.225 | 1.371 | 1.442 | 1.478 | 1.585 |
| S12 | 0.273 | 0.549 | 0.575 | 0.691 | 0.752 | 0.792 | 0.820 | 0.888 | 0.908 | 0.980 | 1.000 | 1.225 | 1.371 | 1.442 | 1.478 | 1.584 |
| S13 | 0.273 | 0.549 | 0.576 | 0.692 | 0.752 | 0.792 | 0.820 | 0.888 | 0.909 | 0.980 | 1.000 | 1.224 | 1.369 | 1.440 | 1.475 | 1.582 |
| S14 | 0.273 | 0.548 | 0.575 | 0.692 | 0.752 | 0.791 | 0.820 | 0.888 | 0.909 | 0.980 | 1.000 | 1.224 | 1.369 | 1.440 | 1.475 | 1.582 |
| S15 | 0.273 | 0.548 | 0.575 | 0.691 | 0.752 | 0.792 | 0.820 | 0.888 | 0.909 | 0.980 | 1.000 | 1.224 | 1.369 | 1.439 | 1.475 | 1.581 |
| S16 | 0.268 | 0.549 | 0.576 | 0.692 | 0.753 | 0.792 | 0.821 | 0.889 | 0.909 | 0.980 | 1.000 | 1.224 | 1.370 | 1.441 | 1.476 | 1.583 |
| S17 | 0.273 | 0.549 | 0.575 | 0.692 | 0.753 | 0.792 | 0.821 | 0.888 | 0.908 | 0.980 | 1.000 | 1.225 | 1.370 | 1.441 | 1.477 | 1.584 |
| Average | 0.273 | 0.549 | 0.575 | 0.692 | 0.752 | 0.792 | 0.820 | 0.888 | 0.909 | 0.979 | 1.000 | 1.224 | 1.370 | 1.441 | 1.476 | 1.583 |
| S.D. | 1.3×10-3 | 0.7×10-3 | 1.0×10-3 | 0.6×10-3 | 0.5×10-3 | 0.5×10-3 | 0.6×10-3 | 0.5×10-3 | 0.5×10-3 | 4.1×10-3 | 0.000 | 0.7×10-3 | 1.2×10-3 | 1.5×10-3 | 1.5×10-3 | 1.7×10-3 |
Table 6.
The relative peak areas (RPA) of 16 characteristic peaks for 17 HP samples.
| Sample no. | Peak no. |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| S1 | 0.071 | 0.152 | 0.160 | 0.075 | 0.226 | 0.107 | 0.501 | 0.100 | 0.087 | 0.427 | 1.000 | 0.336 | 0.060 | 0.083 | 0.174 | 0.092 |
| S2 | 0.047 | 0.180 | 0.196 | 0.070 | 0.225 | 0.132 | 0.131 | 0.114 | 0.085 | 0.483 | 1.000 | 0.380 | 0.107 | 0.018 | 0.087 | 0.056 |
| S3 | 0.041 | 0.175 | 0.193 | 0.078 | 0.236 | 0.128 | 0.278 | 0.109 | 0.099 | 0.467 | 1.000 | 0.272 | 0.064 | 0.022 | 0.180 | 0.104 |
| S4 | 0.044 | 0.174 | 0.195 | 0.072 | 0.226 | 0.138 | 0.210 | 0.117 | 0.093 | 0.481 | 1.000 | 0.367 | 0.103 | 0.018 | 0.079 | 0.051 |
| S5 | 0.053 | 0.200 | 0.233 | 0.090 | 0.212 | 0.141 | 0.608 | 0.130 | 0.108 | 0.508 | 1.000 | 0.364 | 0.099 | 0.024 | 0.093 | 0.064 |
| S6 | 0.048 | 0.171 | 0.192 | 0.071 | 0.218 | 0.137 | 0.240 | 0.111 | 0.089 | 0.468 | 1.000 | 0.363 | 0.102 | 0.018 | 0.077 | 0.052 |
| S7 | 0.045 | 0.194 | 0.193 | 0.073 | 0.223 | 0.137 | 0.237 | 0.114 | 0.092 | 0.475 | 1.000 | 0.363 | 0.102 | 0.019 | 0.077 | 0.049 |
| S8 | 0.064 | 0.156 | 0.167 | 0.095 | 0.298 | 0.181 | 0.492 | 0.116 | 0.140 | 0.463 | 1.000 | 0.150 | 0.029 | 0.035 | 0.263 | 0.147 |
| S9 | 0.074 | 0.162 | 0.173 | 0.095 | 0.292 | 0.181 | 0.545 | 0.122 | 0.130 | 0.469 | 1.000 | 0.226 | 0.049 | 0.034 | 0.253 | 0.155 |
| S10 | 0.042 | 0.216 | 0.234 | 0.052 | 0.138 | 0.050 | 0.534 | 0.137 | 0.067 | 0.455 | 1.000 | 0.285 | 0.072 | 0.047 | 0.169 | 0.108 |
| S11 | 0.053 | 0.150 | 0.165 | 0.094 | 0.301 | 0.187 | 0.398 | 0.116 | 0.132 | 0.462 | 1.000 | 0.197 | 0.041 | 0.034 | 0.269 | 0.140 |
| S12 | 0.053 | 0.158 | 0.170 | 0.095 | 0.304 | 0.189 | 0.387 | 0.114 | 0.130 | 0.465 | 1.000 | 0.201 | 0.042 | 0.035 | 0.276 | 0.149 |
| S13 | 0.068 | 0.160 | 0.172 | 0.094 | 0.309 | 0.182 | 0.456 | 0.120 | 0.129 | 0.472 | 1.000 | 0.177 | 0.036 | 0.035 | 0.277 | 0.151 |
| S14 | 0.059 | 0.159 | 0.171 | 0.093 | 0.305 | 0.186 | 0.383 | 0.120 | 0.127 | 0.474 | 1.000 | 0.204 | 0.042 | 0.037 | 0.269 | 0.156 |
| S15 | 0.075 | 0.202 | 0.218 | 0.090 | 0.208 | 0.119 | 0.629 | 0.121 | 0.105 | 0.483 | 1.000 | 0.219 | 0.048 | 0.028 | 0.233 | 0.138 |
| S16 | 0.037 | 0.208 | 0.213 | 0.082 | 0.211 | 0.063 | 0.204 | 0.129 | 0.085 | 0.524 | 1.000 | 0.323 | 0.086 | 0.036 | 0.193 | 0.102 |
| S17 | 0.076 | 0.207 | 0.261 | 0.111 | 0.242 | 0.116 | 0.323 | 0.122 | 0.103 | 0.609 | 1.000 | 0.365 | 0.087 | 0.039 | 0.188 | 0.094 |
| Average | 0.056 | 0.178 | 0.195 | 0.084 | 0.245 | 0.140 | 0.386 | 0.118 | 0.106 | 0.481 | 1.000 | 0.282 | 0.069 | 0.033 | 0.186 | 0.106 |
| S.D. | 0.013 | 0.022 | 0.029 | 0.014 | 0.048 | 0.042 | 0.153 | 0.008 | 0.022 | 0.039 | 0.000 | 0.080 | 0.028 | 0.016 | 0.078 | 0.041 |
Chromatographic profiles were generally consistent although the absorption intensity of some peaks and the number of peaks were slightly different for some samples. The similarity of each chromatograph against simulative median chromatogram was calculated: the similarities of S1–S17 were 0.989, 0.975, 0.998, 0.985, 0.979, 0.987, 0.966, 0.987, 0.986, 0.981, 0.993, 0.993, 0.990, 0.993, 0.979, 0.988, and 0.990. All the similarity values were in the range of 0.966–0.998, indicating that similar chemical components were present in these samples regardless of manufacturer.
3.5.2. Assignment of the 16 characteristic peaks of the HPLC fingerprint
To determine which herb each compound originated from, a comparative study was conducted using the individual extracts of six herbs. In the light of comparisons, the results of this study are as follows: Peaks marked with 1 and 7 were from Sanguisorbae Radix; peaks marked with 2, 3, 4, 5, 6, 8, 9 10, 11 and 14 were from Sophorae Fructus; and peaks marked with 12, 13, 15 and 16 were from Scutellariae Radix. Moreover, naringin was from Aurantii Fructus and quercetin was from Sophorae Fructus.
4. Conclusions
The proposed HPLC fingerprint method combined with quantitative analysis is an efficient and comprehensive tool for quality consistency evaluations of HP. HPLC fingerprint of the chromatographic profiles could serve as the preferred tool for quality consistency of HP by similarity comparison. Furthermore, simultaneous quantification of seven marker compounds from each individual herbal present in HP can be carried out in a single HPLC chromatogram, and used as a supplemental tool for quality consistency evaluation. To the best of our knowledge, this is the first report for both the fingerprint of HP and simultaneous determination of its seven major bioactive components. Overall, this study sets a good example for quality consistency evaluation using a combination of HPLC fingerprint and quantitative analysis.
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
References
- 1.The State Pharmacopoeia Commission of PR China, Chinese Pharmacopoeia Commission, 2015 ed., Beijing, 2015, 〈http://www.drugfuture.com/Pharmacopoeia/CP2015-1/1642-1643〉
- 2.Dong Y.X., Wang W. 63 cases of Huaijiao pill on hypertension. Shaanxi J. Tradit. Chin. Med. 2001;22:604. [Google Scholar]
- 3.Lu Z.L., Liu Y.H. Decoction of Huaijiao to treat 175 cases of chronic pharyngitis. China Naturop. 1997;6:28. [Google Scholar]
- 4.Xie Y.X., Dai P.L. 62 cases of Huaijiao pill on acne, Shaanxi. J. Tradit. Chin. Med. 1999;20:227. [Google Scholar]
- 5.Zhao W.Z., Wang Y., Ming L. Anti inflammatory, analgesic and antihemorrhagic effects of compound Huaijiao oral solution. Acta Univ. Med. Anhui. 1997;32:110–112. [Google Scholar]
- 6.Chen H., Huang S.F., Xu P.F. Pharmacodynamic test of Huaijiao granule. Anhui Med. Pharm. J. 2005;10:731–733. [Google Scholar]
- 7.Wang J.H., Tang Y.P., Lou F.C. Chemical components and pharmacological activities of Huaijiao. World Notes Plant Med. 2002;17:58–60. [Google Scholar]
- 8.Shi J.H., Miao M.S., Tian X.Z. Degradating lipid action of Huaijiao granule. Henan Tradit. Chin. Med. 1997;17:347–348. [Google Scholar]
- 9.Song T., Liu P., Jia F. Research Progress of Huaijiao Pills, China Pract. Med. 2014;5:263–264. [Google Scholar]
- 10.Kim B.H., Chung E.Y., Ryu J.C. Anti-inflammatory mode of isoflavone glycoside sophoricoside by inhibition of interleukin-6 and cyclooxygenase-2 in inflammatory response. Arch. Pharm. Res. 2003;26:306–311. doi: 10.1007/BF02976960. [DOI] [PubMed] [Google Scholar]
- 11.Yun J., Lee C.K., Chang Il-M. Differential inhibitory effects of sophoricoside analogs on bioactivity of several cytokines. Life Sci. 2000;67:2855–2863. doi: 10.1016/s0024-3205(00)00873-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z.H. A case of allergy reaction induced by Huaijiao pill. Hunan J. Tradit. Chin. Med. 1995;11:39. [Google Scholar]
- 13.Zhao R.Q. Two cases of allergy reaction induced by Huaijiao pill. Chin. J. Chin. Mater. Med. 1997;22:185. [Google Scholar]
- 14.Goodarzi M., Russell P.J., Heyden Y.V. Similarity analyses of chromatographic herbal fingerprints: a review. Anal. Chim. Acta. 2013;804:16–28. doi: 10.1016/j.aca.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Liu M., Li Y.G., Zhang F. Chromatographic fingerprinting analysis of Danshen root (Salvia miltiorrhiza Radix et Rhizoma) and its preparations using high performance liquid chromatography with diode array detection and electrospray mass spectrometry (HPLC-DAD-ESI/MS) J. Sep. Sci. 2007;30:2256–2267. doi: 10.1002/jssc.200700149. [DOI] [PubMed] [Google Scholar]
- 16.The European Agency for the Evaluation of Medicinal Products, Note for Guidance on Quality of Herbal Medicinal Products, London, 2001.
- 17.Drug Administration Bureau of China, Guidance for Experimental Research on HPLC Fingerprint of Traditional Chinese Injections (Draft), Beijing, 2002.
- 18.(US) Food and Drug Administration, Guidance for Industry Botanical Drug Product, Rockville, 2004.
- 19.Yang D.F., Liang Z.S., Duan Q.M. Quality assessment of cardiotonic pills by HPLC fingerprinting. Chromatographia. 2007;66:509–514. [Google Scholar]
- 20.Xie Y., Jiang Z.H., Zhou H. Combinative method using HPLC quantitative and qualitative analyses for quality consistency assessment of a herbal medicinal preparation. J. Pharm. Biomed. Anal. 2007;43:204–212. doi: 10.1016/j.jpba.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Leung K.S., Fu C. Improved chromatographic assessment of Chinese medicinal products by multi-chemical classes analysis. Planta Med. 2009;75:1171–1179. doi: 10.1055/s-0029-1185478. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Wu T., Zhu J.H. Combinative method using HPLC fingerprint and quantitative analyses for quality consistency evaluation of an herbal medicinal preparation produced by different manufacturers. J. Pharm. Biomed. Anal. 2010;52:597–602. doi: 10.1016/j.jpba.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Meng J., Wang L.J. Determination of sophoricoside, baicalin and naringin in Huaijiao pill by HPLC. J. Chin. Mater. Med. 2011;04:638–639. [Google Scholar]
- 24.Wei X.L., Wang D.M., Zhang Y. RP-HPLC analysis of simultaneous determination of 4 bioactive components in Huaijiao pills. Chin. J. Pharm. 2011;07:549–552. [Google Scholar]
- 25.Gong C.Q., Li X.H., Zhou L.Z. Determination of naringin and baicalin in Huaijiao pill by RP-HPLC. Heilongjiang Med. J. 2013;03:359–361. [Google Scholar]
- 26.Li X.H., Gong C.Q., Zhou L.Z. Determination of naringin in Huaijiao pills by RP-HPLC. Guide Chin. Med. 2013;16:115–116. [Google Scholar]
- 27.Bian Q.Q., Yang Z.P. Determination of genistein and quercetin in Huaijiao pills by RP-HPLC. Chin. Tradit. Patent Med. 2005;07:766–769. [Google Scholar]
- 28.Jiang Y., Li S.P., Wang Y.T. Differentiation of Herba Cistanches by fingerprint with high-performance liquid chromatography diode array detection-mass spectrometry. J. Chromatogr. A. 2009;1216:2156–2162. doi: 10.1016/j.chroma.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Che X.Y., Deng X.H., Zhou J. Characteristic chromatogram of Huaijiao pill and determination of naringin and neohesperidin. West China J. Pharm. Sci. 2013;06:645–647. [Google Scholar]