Abstract
A rapid and sensitive ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) method is described for determination of letrozole in human plasma. Following solid phase extraction (SPE) of letrozole and letrozole-d4 on Orochem DVB-LP cartridges, chromatography was performed on Acquity UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) column using methanol-0.1% formic acid in water (85:15, v/v) as the mobile phase. Detection was carried out on a triple quadrupole mass spectrometer with an electrospray source, operated under positive ionization mode. Quantitation of letrozole and letrozole-d4 was done using multiple reaction monitoring (MRM) following the transitions at m/z 286.2→217.0 and m/z 290.2→221.0, respectively. The calibration plots were linear through the concentration range of 0.10–100 ng/mL (r2≥0.9990) using 100 µL human plasma. The extraction recovery of letrozole ranged from 94.3% to 96.2% and the intra-batch and inter-batch precision was ≤5.2%. The method was successfully applied to a bioequivalence study of letrozole after oral administration of 2.5 mg tablet formulation to 16 healthy postmenopausal Indian women. The assay reproducibility was also established through incurred sample reanalysis (ISR) of 74 subject samples.
Keywords: Letrozole, Letrozole-D4, UPLC–MS/MS, Solid phase extraction, Bioequivalence study
1. Introduction
Letrozole (LTZ) is a third-generation, potent and selective non-steroidal aromatase inhibitor. It is approved for anti-hormonal therapy in postmenopausal women having estrogen receptor positive breast cancer. Aromatases (cytochrome P-450 [CYP] 19) are enzymes that convert adrenal androgens into estrogens in the peripheral tissues, which are responsible for the promotion and progression of breast cancer in postmenopausal women. LTZ effectively suppresses the production of estrogen by preventing the aromatase enzyme from producing estrogens through competitive binding to the heme moiety of cytochrome P-450 subunit [1], [2].
LTZ is rapidly and completely absorbed after oral administration with mean absolute bioavailability of 99.9%. It is weakly bound to proteins (60%), primarily to albumin and has 1.9 L/kg volume of distribution at steady state. It is metabolized by cytochrome P450 isoenzymes (CYP 3A4 and CYP 2A6) into a pharmacologically inactive carbinol metabolite, which further undergoes glucuronide conjugation [1], [2]. Approximately 90% of LTZ is recovered in urine, with a major part of about 75% corresponding to the glucuronide conjugate of the carbinol metabolite. The terminal elimination half-life of LTZ is about two days [2]. However, due to its low therapeutic dose (2.5 mg) and wide distribution in tissues, the plasma concentration of LTZ is rather low. Thus, it is essential to establish a reliable, selective and sensitive analytical method for the quantitation of LTZ, especially for pharmacokinetic applications.
Several methods are reported for the determination of LTZ as a single analyte [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], together with its inactive metabolites [13], [14] and with other drugs like tamoxifen and anastrozole [15] in different biological matrices. Mainly liquid chromatography with UV [4], [5], [12], fluorescence [3], [14] or mass spectrometry [6], [7], [8], [9], [10], [11], [13], [15] detection has been used for the quantification of LTZ in various matrices like human urine, rat plasma and human plasma. All previous methods based on liquid chromatography with mass spectrometric detection have sensitivity less than 0.2 ng/mL and chromatographic analysis time ranging from 3.0 to 16.0 min. The salient features of reported liquid chromatography–tandem mass spectrometry (LC–MS/MS) methods developed for LTZ in human plasma are summarized in Table 1.
Table 1.
Comparative summary of liquid chromatography–tandem mass spectrometry methods developed for letrozole in human plasma.
| Sr. No. | Linear range (ng/mL) | Plasma volume (µL) | Extraction procedure; internal standard | Mean recovery (%) | Chromatographic run time (min); retention time (min) | Application; Incurred sample reanalysis | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 0.20–100 | 250 | LLE with methyl tert-butyl ether; LTZ-d4 | 65.7 | 3.0; 1.25 | Pharmacokinetic study with 2.5 mg LTZ in 72 healthy volunteers under fasting & fed condition; – | [7] |
| 2 | 0.40–50 | 100 | PP with methanol-water and acetonitrile; LTZ-d4 | 90.5 | 4.0; 2.7 | Clinical pharmacokinetic study with 2.5 mg LTZ in 20 healthy, post-menopausal Chinese women; % change within 20% | [8] |
| 3 | 0.25–100 | 500 | SPE on SOLA cartridges; olanzapine | 87.1 | 3.0; 2.043 | Pharmacokinetic study with 2.5 mg LTZ in 32 healthy, post-menopausal women; – | [9] |
| 4 | 1.0–60 | 50 | PP with methanol; anastrozole | – | 5.5; 3.81 | Analysis of samples from patients treated with 2.5 mg/day LTZ; – | [10] |
| 5a | 5.7–428 | 150 | SPE on BondElut C18 96-well plates; LTZ-d4 | 98.5 | 4.75; 3.86 | Quantification of LTZ and its metabolites in the plasma samples of 20 post-menopausal patients with early breast cancer receiving a daily dose of 2.5 mg LTZ; – | [14] |
| 6b | 10–300 | 1000 | SPE on Strata X-C; bunitrolol | 49.0 | 16; 8.0 | Analysis of plasma samples of 310 breast cancer patients undergoing anti-estrogen therapy; – | [15] |
| 7c | 0.10–100 | 100 | SPE on Orochem DVB-LP cartridge; LTZ-d4 | 95.5 | 2.0; 1.21 | Bioequivalence study with 2.5 mg LTZ in 16 healthy post-menopausal Indian women; % change within ±16% | PM |
cLTZ: letrozole; PP: protein precipitation; LLE: liquid-liquid extraction; SPE: solid phase extraction; PM: present method.
Together with its inactive metabolites, carbinol and carbinol glucuronide;
Along with tamoxifen and anastrozole;
UPLC--MS/MS method;
Thus so far, there is only one report on the use of ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) for the determination of letrozole in rat plasma. The linear concentration range was established from 2.0 to 2000 ng/mL using acetonitrile as protein precipitant for sample preparation with recovery of 77.9%–79.5%. However, there are no UPLC based methods for the determination of LTZ in human plasma. Herein we describe development and validation of a sensitive, selective and fast analytical method for quantification of LTZ in human plasma by UPLC–MS/MS using a deuterated internal standard (LTZ-d4). The method presents an efficient extraction procedure based on solid phase extraction (SPE) with minimal matrix interference. The proposed method was successfully applied to support a bioequivalence study of 2.5 mg letrozole tablet formulation in 16 healthy postmenopausal Indian women under fasting condition.
2. Experimental
2.1. Chemicals and materials
The details of reference standards, their purity and other materials used in the study are given in Appendix A – Supplementary data.
2.2. Liquid chromatographic and mass spectrometric conditions
A Waters Acquity UPLC system (MA, USA) consisting of binary solvent manager, sample manager and column manager was used to set the reversed-phase liquid chromatographic conditions. The analysis of LTZ was performed on Waters Acquity UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) column and maintained at 35 °C in a column oven. The mobile phase consisted of methanol-0.1% formic acid in water (85:15, v/v). The flow rate of the mobile phase was kept at 0.300 mL/min. Ionization and detection of LTZ and internal standard (IS) was carried out on Waters Quattro Premier XE triple quadrupole mass spectrometer (MS; Milford, MA, USA), equipped with electro spray ionization (ESI) source and operated in the positive ionization mode. The source-dependent and compound-dependent parameters optimized for LTZ and LTZ-d4 are presented in Supplementary Table 1. Quadrupole 1 and 3 were maintained at unit mass resolution and MassLynx software version 4.1 was used to control all parameters of UPLC and MS.
2.3. Standard stock, calibration standards and quality control samples
Calibration standards (CSs) were made at 0.10, 0.20, 0.50, 1.00, 2.00, 5.00, 10.0, 20.0, 50.0, and 100 ng/mL concentrations. Quality control (QC) samples were prepared at 80.0 ng/mL (HQC, high quality control), 40.0/15.0 ng/mL (MQC-1/2, medium quality control), 0.30 ng/mL (LQC, low quality control) and 0.10 ng/mL (LLOQ QC, lower limit of quantification quality control). The details of solution preparation are provided in Appendix A – Supplementary data.
2.4. Plasma sample extraction
Prior to analysis, all frozen subject samples, CSs and QC samples were thawed and allowed to equilibrate at room temperature for 30 min. To an aliquot of 100 µL of spiked plasma sample, 50 µL of IS solution and 300 µL of 0.1 M HCl in water were added and vortex-mixed for 20 s. The samples were then centrifuged at 3200g for 5 min at 10 °C and loaded on Orochem DVB-LP (30 mg, 1 cc) extraction cartridges, which were preconditioned with 1.0 mL of methanol, followed by 1.0 mL of water. Washing of samples was done with 2×1 mL of water. Elution of LTZ and LTZ-d4 was completed using 2×0.5 mL of methanol into pre-labeled vials. The eluates were evaporated to dryness under gentle stream of nitrogen (20 psi) at 40 °C. The dried samples were reconstituted with 100 µL of the mobile phase, vortex-mixed for 30 s and 10 µL was used for injection in the chromatographic system.
2.5. Assay validation
The bioanalytical method validation was performed as per the United States Food and Drug Administration (USFDA) guidance [16] and was similar to the one described in our previous report [17]. Details of validation procedure and acceptance criteria are given in Appendix A – Supplementary data.
2.6. Application of the method and incurred sample reanalysis (ISR)
A bioequivalence study was conducted with a single oral dose of a test (2.5 mg letrozole tablets from a Generic Company, India) and a reference (FEMARA®, 2.5 mg letrozole tablets from Novartis Pharmaceutical Corporation, East Hanover, NJ 07936-1080, USA) formulation in 16 healthy postmenopausal Indian women under fasting. The study was conducted as per International Conference on Harmonization, E6 Good Clinical Practice guidelines [18]. ISR was performed as reported previously [19]. The details of both the experiments are provided in Appendix A – Supplementary data.
3. Results and discussion
3.1. UPLC–MS/MS method development
To attain optimal analytical performance for the assay, MS settings, sample clean-up and UPLC conditions were suitably investigated to achieve the desired sensitivity and short analysis time. A standard solution (1000 ng/mL) of LTZ and LTZ-d4 was directly infused into the MS using ESI as the ionization source. The mass spectrometer was tuned in both positive and negative ionization modes to obtain consistent and abundant product ions in the mass range of 100–340 amu, based on previous studies [6], [7], [9], [10], [11]. Due to the presence of tertiary nitrogen groups in LTZ the response in the positive ionization mode was greater than that in the negative mode. The characteristic protonated precursor ions for LTZ and LTZ-d4 were observed at m/z 286.2 and m/z 290.2 respectively in the Q1 spectra. The most abundant and consistent product ion was found at m/z 217.0 and 221.0 for LTZ and LTZ-d4, respectively, which can be attributed to the loss of triazole moiety from their precursor ions (Supplementary Figs. 1 and 2). For quantification of LTZ and LTZ-d4, a dwell time of 150 ms was adequate to generate sufficient data points.
To establish a linear concentration range from 0.1 to 100 ng/mL, it was essential to optimize an efficient extraction protocol to obtain quantitative and precise recovery of LTZ and LTZ-d4 with minimal matrix effect. As all three conventional extraction methodologies, namely protein precipitation (PP) [8], [10], liquid-liquid extraction (LLE) [7], [12] and SPE [6], [9], [11], have been used previously, a systematic study was undertaken to optimize the best extraction conditions with all three approaches. As the PP has the advantage of simplicity and speed of analysis, the initial attempts were done with methanol and acetonitrile as protein precipitants. However, this technique did not provide cleaner extracts which were apparent from strong matrix interferences and poor recovery especially at LLOQ and LQC concentration levels. A similar observation was also reported in a previous study [12]. Further, LLE was tested using methyl tert-butyl ether, dichloromethane, n-hexane and ethyl acetate as single solvents and also as binary mixtures in different proportions. In majority of the extraction trials the recovery was in the range of 64 %–73 %, but there was some inconsistency at lower concentration levels. As a result, SPE was explored to obtain cleaner extract for better signal intensity and recovery of LTZ. In this experiment two different extraction cartridges, Orochem DVB-LP and Lichrosep DVB HL, were tested. With both the cartridges extraction was performed under acidic as well as basic conditions. However, the response obtained under alkaline conditions was too low compared to that under acidic conditions. Additionally, the mean recovery found was somewhat less with Lichrosep DVB HL (85.7%) compared to Orochem DVB-LP. Nevertheless, superior results were obtained under acidic conditions (0.1 M HCl) on Orochem DVB-LP cartridges and hence they were chosen in the present work. The mean extraction recovery of LTZ and LTZ-d4 was 95.5% and 96.2%, respectively, which was highly consistent at all QC levels (Table 2).
Table 2.
Extraction recovery for letrozole and letrozole-d4.
| QC level | Letrozole |
Letrozole-d4 |
||||
|---|---|---|---|---|---|---|
| Area response (n=6) |
Extraction recovery (%) | Area response (n=6) |
Extraction recovery (%) | |||
| Pre-extraction spiking | Post-extraction spiking | Pre-extraction spiking | Post-extraction spiking | |||
| LQC | 1246 | 1302 | 95.7 | 207,349 | 216,892 | 95.6 |
| MQC-2 | 63,247 | 65,746 | 96.2 | 210,341 | 217,294 | 96.8 |
| MQC-1 | 168,144 | 178,307 | 94.3 | 214,493 | 222,272 | 96.5 |
| HQC | 336,457 | 351,943 | 95.6 | 211,539 | 220,583 | 95.9 |
Extraction recovery: pre-extraction spiking/post-extraction spiking.
Chromatographic conditions were optimized to obtain high sensitivity and sample throughput. Different mobile phase compositions and mobile phase additives were explored to achieve optimal peak shape, adequate retention and response on UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm). The mobile phase systems consisting of acetonitrile–water and methanol–water with ammonium acetate/formate buffers and formic acid/acetic acid in different volume ratios were tested. A summary of various trials conducted with different mobile phases on this column is presented in Table 3. Methanol was selected as the organic modifier since it provided a better chromatographic peak shape and response for LTZ compared to acetonitrile. Further, formic acid rather than acetic acid or the acidic buffers assisted better to accomplish good peak shape and response in the positive mode. To set the optimum mobile phase conditions, the ratio of the organic diluent varied from 60% to 90% and the concentration of formic acid was tested in the range of 0.05%–0.2%. The best chromatographic conditions as a function of analyte peak intensity, peak shape, adequate retention and analysis were obtained using methanol-0.1% formic acid in water (85:15, v/v) as the mobile phase under isocratic elution. The retention time for LTZ and LTZ-d4 was 1.21 and 1.20 min, respectively within 2.0 min at a flow rate of 0.3 mL/min, ensuring high throughput of the method. The reproducibility of retention time for LTZ, expressed as coefficient of variation (CV) was ≤0.6% for a minimum of 100 injections on the same column. Deuterated internal standard, LTZ-d4 used in the present work was useful in maintaining overall accuracy and precision of the data. The specificity of the method was evident from the representative multiple reaction monitoring (MRM) ion chromatograms of blank plasma, LLOQ sample and a subject sample in Fig. 1. No significant interference in the drug free blank plasma samples was observed at the retention time of the analyte. The post column infusion experiment showed no interfering signals at the retention time of the analyte and IS from endogenous plasma components (Supplementary Fig. 3). Likewise, none of the medications commonly used by healthy subjects interfered at the retention time of LTZ. Matrix effect, expressed as IS-normalized matrix factors (MFs) was found in the range of 0.981–1.032, indicating minimal ion suppression/enhancement (Table 4). The relative matrix effect calculated in terms of % CV value with ten different plasma sources was 2.60, which is within the acceptance criteria of ≤3.0% using labeled IS (Supplementary Table 2).
Table 3.
Results of chromatographic trials on Waters Acquity UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) analytical column.
| Sr. No. | Mobile phase, flow rate (0.300 mL/min) | Retention time for LTZ (min) | Observation |
|---|---|---|---|
| 1 | MeOH:10.0 mM AF buffer (80:20, v/v) | 2.37 | Peak shape was slightly broad and response was also low, run time 3.5 min |
| 2 | ACN:10.0 mM AF buffer (80:20, v/v) | 2.45 | Peak shape was slightly broad and response was also low, run time 3.5 min |
| 3 | 0.1% FA in water: 0.1% FA in MeOH (20:80, v/v) | 2.22 | Peak shape and response was better compared to AF buffer (Sr. No. 1 and 2), run time 3.5 min |
| 4 | 0.1% FA in water: MeOH-ACN (50:50, v/v) (20:80, v/v) | 2.34 | Peak shape was good but the response was somewhat unacceptable, run time 3.5 min |
| 5 | MeOH:0.1% FA in water (70:30, v/v) | 2.06 | Adequate retention, response was acceptable but the peak was slightly broad |
| 6 | ACN:0.1% FA in water (70:30, v/v) | 2.04 | Adequate retention, response was acceptable but the peak was broad |
| 7 | MeOH:0.1% FA in water (80:20, v/v) | 1.27 | Slight peak tailing, adequate response and retention, run time 2.0 min |
| 8 | ACN:0.1% FA in water (80:20, v/v) | 1.28 | Adequate retention, better response but the peak shape was slightly broad, run time 2.0 min |
| 9 | MeOH:0.1% FA in water (85:15, v/v) | 1.21 | Good peak shape, adequate response, adequate retention and short analysis time (2.0 min) |
ACN: acetonitrile; MeOH: methanol; AF: ammonium formate; FA: formic acid; LTZ: letrozole.
Fig. 1.
MRM ion chromatograms of (A) double blank plasma (without IS), (B) blank plasma with letrozole-d4 (IS), (C) letrozole at LLOQ and IS, and (D) subject sample at Cmax after administration of 2.5 mg dose of letrozole and IS.
Table 4.
Matrix factors for letrozole and letrozole-d4 (IS).
| QC level | Letrozole |
Letrozole-d4 |
|||||
|---|---|---|---|---|---|---|---|
| Area response (n=6) |
Matrix factor | Area response (n=6) |
Matrix factor | IS-normalized matrix factor | |||
| Post-extraction spiking | Neat samples in mobile phase | Post-extraction spiking | Neat samples in mobile phase | ||||
| LQC | 1302 | 1305 | 0.998 | 216,892 | 224,281 | 0.967 | 1.032 |
| MQC-2 | 65,746 | 64,457 | 1.020 | 217,294 | 208,986 | 1.040 | 0.981 |
| MQC-1 | 178,307 | 178,844 | 0.997 | 222,272 | 225,839 | 0.984 | 1.013 |
| HQC | 351,943 | 361,709 | 0.973 | 220,583 | 225,344 | 0.979 | 0.994 |
Matrix factor: post-extraction spiking/neat samples in mobile phase.
3.2. Assay validation
The precision (% CV) values for system suitability test varied from 0.12% to 0.32% for the retention time and 0.45% to 0.78% for the area response of LTZ and LTZ-d4. The signal-to-noise (S/N) ratio for system performance was ≥24. The auto-sampler carryover experiment showed minimal carryover of analyte (≤0.03% of LLOQ area) in the extracted blank sample injected immediately after ULOQ sample.
The calibration curves showed good linearity (r2≥0.9990) in the studied concentration range of 0.10–100 ng/mL for LTZ. The mean linear equation for calibration curve concentrations was y=(1.0148x+0.0214)x−(0.1223±0.2435). The accuracy (%) and precision (% CV) values for CSs ranged from 98.2% to 102.0% and 1.76% to 5.46%, respectively. The LLOQ (0.10 ng/mL) was measured at an S/N ≥24.
The intra-batch precision (% CV) varied from 1.5% to 4.5% while the accuracy was within 96.7%–102.5%. For the inter-batch experiments, the precision (% CV) ranged from 2.4% to 5.2% and the accuracy was between 96.3% and 102.0% (Supplementary Table 3).
The stability of LTZ was thoroughly evaluated in stock solutions prepared in methanol and in plasma samples under different storage conditions. Stock solutions of LTZ and LTZ-d4 kept for short-term stability remained stable at room temperature up to 7 h, and for long-term stability (a minimum of 7 days) at refrigerated temperature of 5 °C with % change ≤1.8 for both the experiments. The detailed results of LTZ stability in plasma are shown in Supplementary Table 4. Further, the % change for whole blood sample stability was less than 4.2% when compared with freshly spiked blood samples at the LQC and HQC levels.
The precision (% CV) and accuracy values on different columns and from analysts for method ruggedness ranged from 0.72% to 1.15% and 96.8% to 101.5%, respectively, across five QC levels. The precision values for dilution integrity of 1/5th and 1/10th dilution were 1.5% and 2.1%, respectively, while the corresponding accuracy was 97.4% and 102.6%, respectively.
3.3. Application to a bioequivalence study and ISR results
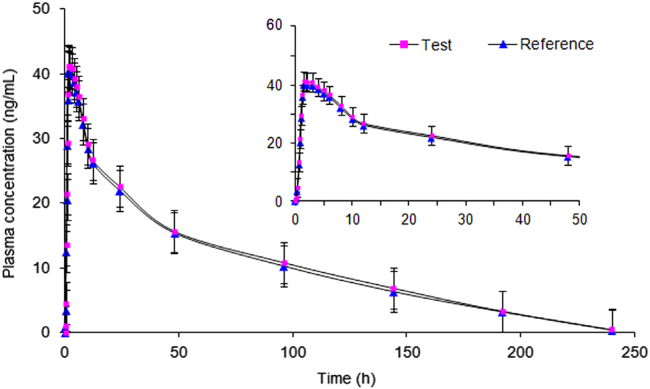
The proposed assay was successfully applied to a pharmacokinetic study in 16 healthy postmenopausal Indian women. The mean concentration (Cmax) in plasma (41.22±9.51 ng/mL) for LTZ was attained at 1.53±0.75 h (Tmax) with a half-life (t1/2) of 40.20±1.80 h. The area under the plasma concentration–time curve from time zero to the last measurable time point (AUC0–t) and area under the plasma concentration time curve from time zero to infinity (AUC0-inf) for LTZ were 2220.4±189.3 and 2181.2±210.7 h ng/mL, respectively. The mean plasma concentration and time profile for 2.5 mg test and reference formulation of LTZ is shown in Fig. 2. It has been reported that the efficacy of LTZ is not consistent and that there is some inter-individual variability. The mean Cmax, Tmax and t1/2 values obtained for LTZ in the present work were comparable with a similar study involving 20 healthy Portuguese postmenopausal female volunteers [6]. However, AUC0–240 and AUC0-inf values were much higher compared to those in Portuguese subjects for identical dose strength. On the contrary, these two parameters were in good agreement with the Chinese subjects but showed significant difference in the t1/2 values (69.0 h) [8]. This difference can be attributed to several factors including race of subjects, type of food and others. Nevertheless, the mean log-transformed ratios of the Cmax, AUC0–240 and AUC0-inf and their 90% confidence intervals were between 95% and 109%, which is within the defined bioequivalence range of 80%–125% (Supplementary Table 5). The study data was also authenticated through ISR study by reanalysis of 74 incurred samples. The results showed % change within ±16%, which is within the acceptance criterion of ±20% and thus demonstrated excellent reproducibility of the proposed method (Supplementary Fig. 4).
Fig. 2.
Mean plasma concentration--time profile of letrozole after oral administration of 2.5 mg (test and reference) tablet formulation to 16 healthy postmenopausal Indian women.
4. Conclusion
A selective, simple and rapid UPLC–MS/MS method was developed and fully validated for the quantification of LTZ in human plasma. The use of UPLC column with small particle size provided sharp peak shapes, enhanced peak capacity and speed of analysis without compromising sensitivity. LTZ and its deuterated IS were rapidly eluted in a run time of 2.0 min per sample and thus the method has the shortest analysis time for the determination of LTZ. In comparison with other reports, the proposed method was proved to be highly sensitive with LLOQ of 0.10 ng/mL, using small plasma volume for processing. Additionally, the method is specific and is unaffected by any endogenous matrix components. Further, the method was successfully applied to a pharmacokinetic study of 2.5 mg LTZ in healthy postmenopausal women and the reproducibility of the assay was demonstrated by incurred sample reanalysis.
Acknowledgments
The authors are indebted to Department of Chemistry, St. Xavier's College, Ahmedabad, India for supporting this work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2016.05.004.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Bhatnagar A.S. The discovery and mechanism of action of letrozole. Breast Cancer Res. Treat. 2007;105:7–17. doi: 10.1007/s10549-007-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating G.M. Letrozole: a review of its use in the treatment of postmenopausal women with hormone-responsive early breast cancer. Drugs. 2009;69:1681–1705. doi: 10.2165/10482340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Zarghi A., Foroutan S.M., Shafaati A. HPLC determination of letrozole in plasma using fluorescence detection: application to pharmacokinetic studies. Chromatographia. 2007;66:747–750. [Google Scholar]
- 4.Sekar V., Jayaseelan S., Subash N. Bioanalytical method development and validation of letrozole by RP-HPLC method. Int. J. Pharm. Res. Dev. 2009;1:1–7. [Google Scholar]
- 5.Rezaee M., Yamini Y., Hojjati M. Novel extraction method based on the dispersion of the extraction solvent for extraction of letrozole from biological fluids. Anal. Methods. 2010;2:1341–1345. [Google Scholar]
- 6.Filipe A., Almeida S., Spinola A.C.F. Bioequivalence study of two letrozole tablet formulations. Single dose, randomized, open-label, two-way crossover bioequivalence study of letrozole 2.5 mg tablets in healthy volunteers under fasting conditions. Arzneim. Forsch. 2008;58:419–422. doi: 10.1055/s-0031-1296530. [DOI] [PubMed] [Google Scholar]
- 7.Joshi C., Vishnubhatla S., Chakkirala S. Validation and application of a high performance liquid chromatography–tandem mass spectrometry assay for letrozole in human plasma. Asian J. Pharm. Clin. Res. 2011;4:107–112. [Google Scholar]
- 8.Song J., Zhan Y., Chen X. Quantification of letrozole in human plasma using LC- (–)ESI-MS/MS with d4-letrozole as internal standard: application in a pharmacokinetic study. J. Liq. Chromatogr. Relat. Technol. 2013;36:1762–1776. [Google Scholar]
- 9.Platova A.I., Miroshnichenko I.I., Ptitsina S.N. Rapid and sensitive LC-MS/MS assay for quantitation of Letrozole using solid-phase extraction from human blood plasma and its application to pharmacokinetic studies. Pharm. Chem. J. 2014;48:292–297. [Google Scholar]
- 10.Shao R., Yu L.Y., Lou H.G. Development and validation of a rapid LC-MS/MS method to quantify letrozole in human plasma and its application to therapeutic drug monitoring. Biomed. Chromatogr. 2016;30:632–637. doi: 10.1002/bmc.3607. [DOI] [PubMed] [Google Scholar]
- 11.Cao G., Zhang Q., Yang X. Determination of letrozole in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study. Lat. Am. J. Pharm. 2015;34:45–50. [Google Scholar]
- 12.Acharjya S.K., Bhattamisra S.K., Muddana B.R.E. Development of a high-performance liquid chromatographic method for determination of letrozole in wistar rat serum and its application in pharmacokinetic studies. Sci. Pharm. 2012;80:941–953. doi: 10.3797/scipharm.1206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Precht J.C., Ganchev B., Heinkele G. Simultaneous quantitative analysis of letrozole, its carbinol metabolite, and carbinol glucuronide in human plasma by LC-MS/MS. Anal. Bioanal. Chem. 2012;403:301–308. doi: 10.1007/s00216-012-5813-1. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez J., Castaneda G., Munoz L. Rapid determination of letrozole, citalopram and their metabolites by high performance liquid chromatography-fluorescence detection in urine: Method validation and application to real samples. J. Chromatogr. B. 2013;913–914:12–18. doi: 10.1016/j.jchromb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Beer B., Schubert B., Oberguggenberger A. Development and validation of a liquid chromatography–tandem mass spectrometry method for the simultaneous quantification of tamoxifen, anastrozole, and letrozole in human plasma and its application to a clinical study. Anal. Bioanal. Chem. 2010;398:1791–1800. doi: 10.1007/s00216-010-4075-z. [DOI] [PubMed] [Google Scholar]
- 16.Guidance for Industry, Bionanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), 2001.
- 17.Shah P.A., Sharma P., Shah J.V. An improved LC–MS/MS method for the simultaneous determination of pyrazinamide, pyrazinoic acid and 5-hydroxy pyrazinoic acid inhuman plasma for a pharmacokinetic study. J. Chromatogr. B. 2016;1017:52–61. doi: 10.1016/j.jchromb.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 18.Guidance for Industry: ICH E6 Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), 1996.
- 19.Yadav M., Shrivastav P.S. Incurred sample reanalysis (ISR): a decisive tool in bioanalytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material