Abstract
Antibiotics are the chemotherapeutic agents that kill or inhibit the pathogenic microorganisms. Resistance of microorganism to antibiotics is a growing problem around the world due to indiscriminate and irrational use of antibiotics. In order to overcome the resistance problem and to safely use antibiotics, the correct measurement of potency and bioactivity of antibiotics is essential. Microbiological assay and high performance liquid chromatography (HPLC) method are used to quantify the potency of antibiotics. HPLC method is commonly used for the quantification of potency of antibiotics, but unable to determine the bioactivity; whereas microbiological assay estimates both potency and bioactivity of antibiotics. Additionally, bioassay is used to estimate the effective dose against antibiotic resistant microbes.
Simultaneously, microbiological assay addresses the several parameters such as minimal inhibitory concentration (MIC), minimum bactericidal concentration (MBC), mutation prevention concentration (MPC) and critical concentration (Ccr) which are used to describe the potency in a more informative way. Microbiological assay is a simple, sensitive, precise and cost effective method which gives reproducible results similar to HPLC. However, the HPLC cannot be a complete substitute for microbiological assay and both methods have their own significance to obtain more realistic and precise results.
Keywords: Potency, Antibiotics, Antibiotic resistance, Microbiological assay, Bioactivity, HPLC
1. Introduction
Antibiotics are the chemotherapeutic agents that kill or inhibit the growth of microorganisms. These chemical agents are used to treat disease by destroying pathogenic microorganisms or inhibiting their growth at concentration low enough to avoid undesirable damage to the host. Antibiotics are drugs preparations which contain some chemical substances that are produced by microorganisms and by chemical synthesis. These substances at very low concentrations are known to totally destroy or partially inhibit microorganisms [1], [2]. Antibiotics have widespread application in the treatment of bacterial disease [3].
Antimicrobial chemotherapy plays a critical role in fighting against infectious disease caused by microorganisms but antibiotic resistant microorganisms are an increasing problem of public health. The misuse of antibiotics fosters the increase and spread of antibiotic resistance, and may lead to super-infections [4], [5]. Due to increasing resistant problems, the quantification of the actual concentration of active ingredient in antibiotic preparation is critical.
The effectiveness of antibiotic agents depends on many factors such as route of administration, location of infection, presence of interfering substances, concentration of the drug in the body, nature of the pathogen, presence of drug allergies and resistance of microorganism to the drug [5]. The effectiveness of antibiotics is described in terms of potency and accurate measurement of potency is critical in pharmacology to safe and proper use of antibiotics [6].
Antibiotics are considered most commonly faked and adulterated pharmaceutical products [7]. A mild difference in the concentration of active ingredient in antibiotic preparations may have impact in actual efficacy. Therefore, quantification of active pharmaceutical ingredient (API) in antibiotic preparation is very necessary [4], [8]. The quantification of active ingredient is critical for drug regulatory agencies around the world [9]. The quantification of active ingredient in antibiotic preparations is important to obtain the same pharmaceutical equivalence of generics as compared to innovate products.
The potency of antibiotics can be determined by chemical and biological methods [4]. These methods include microbiological assays, automated chemical assays (e.g. high performance liquid chromatography (HPLC)), immunological assays (e.g. fluorescence polarization immune assay, fluorescence immunoassay) and radioimmunoassay [10]. However, the assay methodology has changed to chromatographic assays for many antibiotics as they provide quantitative measurements of the purity and the amounts and types of impurities in antibiotics. Nevertheless, a number of commercially important antibiotics continue to require potency determination by microbiological assays [11]. The measurement of antibiotic components is generally done by chemical methods such as HPLC and UV spectrophotometry, but these methods cannot provide a true indication of biological activity which is the main limitation of these methods. However, microbiological assay can precisely determine both potency and bioactivity of an antibiotic. Besides, microbiological assay does not require specialized equipment or toxic solvents [12], [13]. Impurities and related substances do not affect the results of microbiological assay [14]. It also precisely quantifies the actual concentration of active ingredient in antibiotic preparation when microbial resistant problem arises. Microbiological method is the most convenient way to determine the potency of antibiotics [15].
A reduction in antimicrobial activity will also reveal subtle changes not demonstrable by chemical methods [16], [17]. The microbiological assay is the only standard method for the estimation of loss in the activity of an antibiotic [17]. The inhibition of microbial growth in standardized conditions may be utilized in demonstrating the therapeutic efficacy of antibiotics. However, the effective and fully characterized microbial strain is required for microbiological assay. The identification and characterization of microbial strain are performed by cultural and non-cultural techniques [18], [19].
Determination of antimicrobial potency is extremely important for the quality control and quality assurance of antibiotic preparations. Hence, it is necessary to select practical and economical method for quality control of antibiotics [20], [21]. Recently an application of microbiological assay has been developed for intravenously administered antibiotics. This method is highly acceptable by regulating authorities to control antibiotic potency [9]. Being a biological assay, microbiological assay is subject to some biological errors, but after validating all variable parameters it is possible to obtain meaningful results and to achieve a precision similar to that of many chemical methods [14]. Microbiological assay in comparison to chemical methods measures the true response of antibiotics on biological system and is used to obtain more realistic and precise measurement of potency to overcome the antibiotic resistance problem [4].
2. Microbiological assay methods
Microbiological assays are prescribed for the drug substances and preparations where the potency cannot be adequately determined by chemical means. Additionally, the resistance and sensitivity of pathogenic microorganisms is also determined by microbiological assays. These are performed daily on bacterial isolates in clinical and quality control laboratories. All microbiological assay techniques involve either diffusion of antimicrobial agent in the agar or dilution of antibiotic in agar or broth. Different designs of microbiological assay are available for the estimation of potency, bioactivity and resistance of antibiotics.
Kirby-Bauer disk diffusion method is used to determine the sensitivity or resistance of pathogenic aerobic and facultative anaerobic bacteria to various antimicrobial compounds. The pathogenic microorganism is grown on Mueller-Hinton agar in the presence of various antimicrobial impregnated filter paper disks. The absence of microbial growth around the disks indicates the ability of that antimicrobial compound to inhibit the growth of microorganism [5]. The broth dilution method involves subjecting the isolate to a series of concentrations of antimicrobial agents in a broth environment. Microdilution testing uses about 0.05–0.1 mL total broth volume and can be conveniently performed in a Microtiter plate. Macrodilution testing uses broth volumes at about 1.0 mL in standard test tubes. For both of these broth dilution methods, the lowest concentration at which the isolate is completely inhibited is recorded as minimal inhibitory concentration (MIC). In agar dilution method an antimicrobial agent is incorporated into a solid agar medium in a Petri dish. A standard concentration of organisms is inoculated onto the surface of this medium. No growth of the test organism after incubation indicates that it is susceptible at the antimicrobial concentration incorporated into the medium [5]. The agar dilution method provides a specific MIC of an antimicrobial agent.
E-test (Epsilometer test) is a quantitative method for antimicrobial susceptibility testing. In E-test, a thin inert carrier strip having antimicrobial agent is applied onto an inoculated agar plate. After incubation, the asymmetrical inhibition ellipse is produced. The intersection of the inhibitory zone edge and the calibrated carrier strip indicates the MIC value over a wide concentration range (>10 dilutions) with inherent precision and accuracy [22].
The agar diffusion method (Cylindrical-plate or Cup-plate) is a most widely employed method for the estimation of potency and bioactivity of antibiotics. The agar diffusion method depends on diffusion of the antibiotic from a vertical cylinder through a solidified agar layer in a Petri dish. The growth of the specific microorganisms inoculated into the agar is prevented in a circular area or zone around the cylinder containing the antibiotics [23], [24]. The principle of such assay is to compare how much of a sample under examination produces the similar biological effect as in a given quantity of a standard preparation [17], [23], [24]. The agar diffusion method relates the size of the inhibition zone and the dose of the antibiotic assayed. The relation of the diameter of inhibitory zones and the concentration of antibiotic in a solution applied in cups has been considered theoretically [1].
3. Different assay designs for agar diffusion assay
Different pharmacopoeias recommended different designs of agar diffusion assays. The 2×2 and 3×3 assay designs are adopted by Indian Pharmacopoeia [23], British Pharmacopoeia [24] and Brazilian Pharmacopoeia [25]. The 5×1 assay design is adopted by Indian Pharmacopoeia [23] and United States Pharmacopoeia [17] whereas 3×1 assay design is adopted by the European Pharmacopoeia. The use of an adequate experimental design in relation to the criteria of linearity, precision and accuracy of the analytical results are fundamental requirement. It is highly advisable to adopt an assay design which, without further effort, gives better results. The number and nature of the samples are the most important factors to be taken into account, in the selection of a design [21].
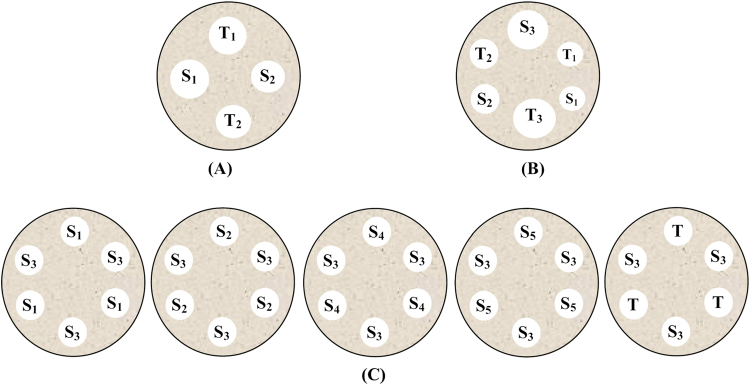
The 2×2 assay design also known as symmetrical and balanced assay is simple and effective which employs two doses of standard and two doses of sample with the same concentration. Each Petri dish includes four doses of both preparations in an alternative manner and in such a way that the number of replicates equals that of dishes (Fig. 1A) [21]. In 3×3 assay design three dose levels of each standard and sample are used as shown in Fig. 1B. Six dishes were employed for each sample assayed through 3×3 experimental design [26]. The 5×1 assay includes five preparations of standard and one preparation of sample which is equal to the median standard concentration. In 5×1 assay design, five Petri dishes were used for each assay in order to test the reference concentration concomitantly with each standard or sample concentration (Fig. 1C) [1], [27].
Fig. 1.
Assay designs for agar diffusion bioassay: (A) 2×2 bioassay; (B) 3×3 bioassay; and (C) 5×1 bioassay. S: standard solutions; T: test solutions.
In 3×1 assay design, three preparations of standard and one preparation of sample equal to the median standard concentration are used. Six dishes were employed for each sample assayed through 3×1 experimental designs [12].
Different designs are recommended for different purposes considering cost, errors, and simplicity of the assay. The 2×2 and 3×3 assays are recommendable for research and development, as they give information about the regression significance and parallelism between the standard curve and the sample that permits the evaluation of the test validity. The 5×1 assay is proved to be appropriate for routine analysis in quality control laboratories where a high number of samples can be evaluated simultaneously. The 3×1 assay is adequate for routine analysis in quality control laboratories, as it simplifies the test execution and the potency calculation, besides offering advantages in terms of low cost and the material involved. As per different pharmacopoeias, different assay designs and their recommended dose ratios are mentioned in Table 1.
Table 1.
Different bioassay designs for agar diffusion assay.
| Assay design | Dose ratio | Pharmacopoeia | Informative statistical parameters | References |
|---|---|---|---|---|
| 2×2 | 2:1, 4:1 | Indian Pharmacopoeia, British Pharmacopoeia, Brazilian Pharmacopoeia | Regression significance, Parallelism | [21] |
| 3×3 | 2:1 | Indian Pharmacopoeia, British Pharmacopoeia, Brazilian Pharmacopoeia | Regression significance, Parallelism | [28] |
| 3×1 | 2:1 | European Pharmacopoeia | Regression significance, Linearity | [21] |
| 5×1 | 4:5 | Indian Pharmacopoeia, United States Pharmacopoeia | Regression significance, Linearity | [21], [27] |
2×2, 3×1 and 5×1 assay designs were compared for gentamycin. The statistical analysis demonstrated that there was no significant difference between the results obtained through 2×2, 3×1 and 5×1 assays and they were equivalent and interchangeable [21].
4. Parameters used to define potency of antibiotics
Potency measurements by microbiological assays are more informative in comparison to HPLC and provide true information about the bioactivity of a given antibiotic. Several in vitro parameters can be used to determine the potency of antibiotics, including MIC, minimum bactericidal concentration (MBC), mutation prevention concentration (MPC) and critical concentration (Ccr).
MIC is considered as the standard parameter to determine the susceptibility of microorganism to antimicrobial agents. MIC can be defined as the lowest concentration of an antimicrobial agent that can inhibit the visible growth of microorganisms after overnight incubation [29]. MIC is used as a research tool to determine the in vitro activity of new antimicrobial agents and from such studies important data can be obtained to determine MIC breakpoints [30]. MIC is also used in diagnostic laboratories to confirm unusual resistance [31], [32], to compare the in vitro activity of different antimicrobial agents [33], [34], to assess the in vitro activity of antibiotic combinations against resistant organism [35] and to give dose recommendations of antibiotics [36]. MIC values indicate antibiotic potency and can only be compared within the respective classes of antibiotics. When comparing antibiotics that have the same molecular class and mode of eradication, the antibiotic with the lowest MIC is more potent because less antibiotic is required to eradicate the bacteria. Several methods such as broth dilution method [35], agar dilution method [37] and agar diffusion method [38] have been used to calculate MIC.
MBC is recognized as the standard quantitative index of the bactericidal potency of antimicrobial agents. MBC is defined as the lowest concentration of antimicrobial agent that prevents the growth of a microorganism after subculturing to an antibiotic free medium [39]. MBC values reflect the antibiotic concentration at which 99.9% eradication of bacterial isolates occurs. MBC can be determined by broth dilution methods combined with agar plate methods. The bactericidal activity of antibiotic is an important parameter for pathogen eradication; and the more potent is an antimicrobial agent, the less likely is resistant selection [40]. MIC and MBC values are similar for bactericidal agents that kill bacteria. MBC values provide less information when applied to bacteriostatic agents that inhibit bacterial growth such as macrolides.
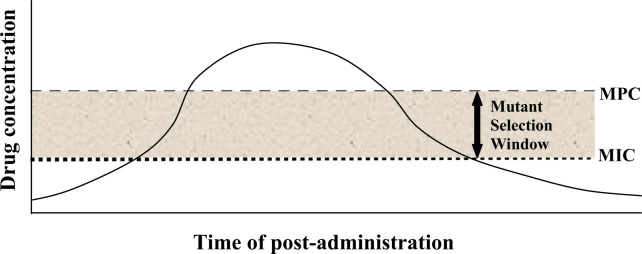
MPC is the antibiotic concentration that allows no mutant to grow [41], or a concentration above which bacterial cells require the presence of two or more resistant mutants for growth [42]. MPC is a new concept applied to face the increased prevalence of antibiotic resistance problems by using antibiotic concentration that can prevent the selection of resistant mutants [43]. MPC values represent the antibiotic concentration at which 100% eradication of isolates occurs. MPC can be applied to calculate potency of antibiotics, to compare the efficacy of different antibiotics against single step resistant mutants and to compare the incidence of resistant mutant occurrence [44]. Due to the increasing resistance problem of antibiotics for many infectious diseases, it is necessary to prevent further selection of resistant mutants so that a concentration of antibiotic can be attained at which no mutant is recovered even when 1010 to 1011 cells are plated [45]. For technical convenience and comparative purpose MPC is defined as the concentration at which no colony recovery was observed when ≥1010 cells are applied. MPC can be determined by the same agar dilution method used for determination of MIC and more (1010) cells are required for MPC determination [43]. MPC has been used to examine the fluoroquinolone potency against clinical isolates of Mycobacterium tuberculosis [42] and Streptococcus pneumonia [46]. MPC testing gives a theoretical profile of antimicrobial characteristics, but has not been proven reliable in animal studies or clinical trials. An antibiotic concentration window exists in which resistant mutants are selectively amplified. The upper boundary of the window is an antibiotic concentration that blocks the growth of least susceptible, first-step resistant mutants. The lower boundary is the lowest antibiotic concentration that blocks the growth of the majority of antibiotic susceptible cells. Because the upper and lower limits of the window are measurable, antibiotics can be compared to find compounds that have narrower windows and that are best suited for administration above the upper boundary (Fig. 2).
Fig. 2.
Pharmacodynamic depiction of the mutant selection window.
Ccr is the minimal concentration of antibiotic that inhibits microorganism growth and prevent bacterial concentration to reach critical point [38]. Antibiotic activity can be expressed in terms of Ccr which is a parameter of the microorganism's sensitivity under particular assay condition [39]. Ccr can be two to four times greater than the MIC, which is determined under different conditions [47].
5. Factors influencing variability and error in microbiological assays
Microbiological assay provides a valid measure of antibiotic activity with some problem of interference from biologically active compounds or degraded products [28]. Several factors are investigated by scientists that normally cause variation in zone diameters in conventional agar diffusion bioassay. Among these factors the most considerable factor is the unequal exposure of the individual plates at the top or bottom of the stacks. Another major variable is the variable in the time interval between pouring seeded agar in the plates and the time of applying the solution of the antibiotic to the plates [48].
Other factors that lead to variability and error in microbiological assay include agar thickness, inoculums concentration, incubation temperature, exposure-time duration and sample preparation. Factors affecting microbial growth rates include pH and chemical composition of media and pH of buffer solution used. The effects of different pHs of buffer on the growth of different microbial strains were observed [1], [4]. The thickness of the agar layer greatly affects the resulting zone diameter. The higher the thickness of agar layers, the smaller the zone diameter. Similarly, an influence of inoculums concentration on resulting zone size is widely recognized [1], [4], [14]. Experiments were performed to determine how critical the concentration of inoculums might be, when other factors are constant [1], [4]. High inoculum concentration of test microorganism shows hazy growth appearance on the medium plate and no appearance of antimicrobial activity of antibiotic, whereas low concentration of inoculum shows light and immeasurable zone.
Variation in incubation temperature of plates in different positions within stacks (i.e., top and bottom) is unavoidable but can be minimized by enclosure of stacks in close-fitting steel cylinders and avoidance of large temperature shifts, particularly between room and incubator temperature. Chemical composition of the media can be fixed and optimized that favors rapid growth of microorganism. pH of media can be adjusted initially and maintained by the addition of hydrochloric acid or sodium hydroxide in sufficient quantities. The maintenance of pH of media is an essential step because the growth of indicator microorganism can lead to change in pH of media. It was observed that germination of Bacillus subtilis spores led to an upward shift in pH [49].
Experimental variability in microbiological assay can be minimized by improved handling, using multiple disks or cylinders on the same Petri dish to eliminate the differential effects from growth, time and temperature by reducing the wedge effect. Variability of microbiological assays can also be minimized by incorporation of external reference plates at spiced locations and by statistical validation of method [9]. A validated agar diffusion bioassay has a capability to give high level of reproducibility and precision.
6. Quantification of antibiotics by HPLC method
HPLC is a chromatographic technique used for the identification, quantification and purification of individual components of a mixture in analytical chemistry [50]. HPLC is used extensively throughout the pharmaceutical industries for the quantification of antibiotics in pharmaceutical preparations. It is used to provide information on the composition of drug related samples. The information obtained may be qualitative, indicating what compounds are present in the sample, or quantitative, providing the actual amounts of compounds in the sample [50]. HPLC is used at all the different stages in the creation of a new drug, and is also used routinely during drug manufacturing. It is more attractive than the classical bioassay in terms of speed, accuracy and precision. Hence, it has largely replaced the microbiological assays to determine the antibiotic concentrations in body fluids and pharmaceutical preparations [9].
7. Comparison of microbiological assay and HPLC method
When comparing the microbiological assay and chemical assay, each exhibits several advantages and deficiencies [51]. Both microbiological and HPLC methods are used to evaluate the potency of antibiotics [26], [27] and to determine drug concentration in body fluids [52] and in therapeutic drug monitoring [53].
Microbiological assay methods are one of the most widely accepted methods for determination of antibiotic potency. However, in spite of some advantages of HPLC method, microbiological assay because of its simplicity, accuracy and inexpensiveness is still recommended as a method of choice in routine potency measurement when immediate results are not required and therapeutic regimes are known [51], [54], [55]. Validated chemical methods are also considered specific and accurate, but these tests are likely to be very narrow in their application and much more difficult to establish than microbiological test. Although automated chemical methods are more attractive than the classical bioassay in terms of speed, accuracy and precision [56] and have largely replaced the microbiological assays to determine the antibiotic concentrations in body fluids (serum, plasma or urine) [9], [53], [57], these methods have limitations when a drug resistance against a particular antibiotic arises.
Potency estimation of antimicrobial agent by using a microbiological assay is based on its inhibitory effect on microorganism under suitable conditions. Moreover, in contrast to microbiological assays, chemical assays require expensive instrumentation and are time-consuming [58]. A validated bioassay that can be used accurately and in a reproducible manner is suitable for both research and pharmaceutical industry. Microbiological assay plays an essential role in manufacturing and quality control of antibiotic medicines [14], [59].
However, automated assays and bioassays are frequently used and accepted by generic manufacturers as interchangeable and complementary of the other methods [17], [60]. Microbiological and chemical assays were compared to measure drug concentration in body fluid and both the assays performed equally well with a minor difference in the results [52]. Microbiological assays were found to determine higher contents of API as compared to HPLC [52], [56]. Different antibiotic concentrations in body fluids and in vitro potencies of different antibiotics by means of both assays were compared and a strong correlation was found, which reflected the accuracy of microbiological assay in comparison to HPLC (Table 2).
Table 2.
Correlation between HPLC and agar diffusion bioassay.
| Antibiotic | Correlation coefficient (R) | Medium | References |
|---|---|---|---|
| Clarithromysin | 0.871 | Serum | [51] |
| Cefoparazone | ≥0.95 | Serum | [61] |
| Cefoxitin | ≥0.95 | Serum | [61] |
| Ofloxacin | 0.845 | Serum | [52] |
| Fluconazole | 0.988 | In vitro | [27] |
| Cefuroxime | 0.991 | Plasma | [62] |
| Vancomysin | 0.975 | Plasma | [10] |
| Amoxicillin capsule | 0.996 | Plasma | [63] |
| Amoxicillin injection | 0.997 | Plasma | [63] |
| Amoxicillin granule | 0.998 | Plasma | [63] |
Antimicrobial activity of degraded antibiotic such as fluoroquinolone can be determined by microbiological assay and their presence can be confirmed in parallel by HPLC [64]. HPLC is used to determine the potency of antibiotics, but that is not an actual measurement of potency [59]. On the other hand, microbiological assay using a living system gives a more realistic measurement of antibiotic potency. A reduction in antimicrobial activity due to slight changes in antibiotic structure can be only detected by microbiological assay. Moreover, using a microbiological assay to measure antibiotic potency is more informative and also gives an idea about susceptibility profile of pathogenic organism to that antibiotic [65].
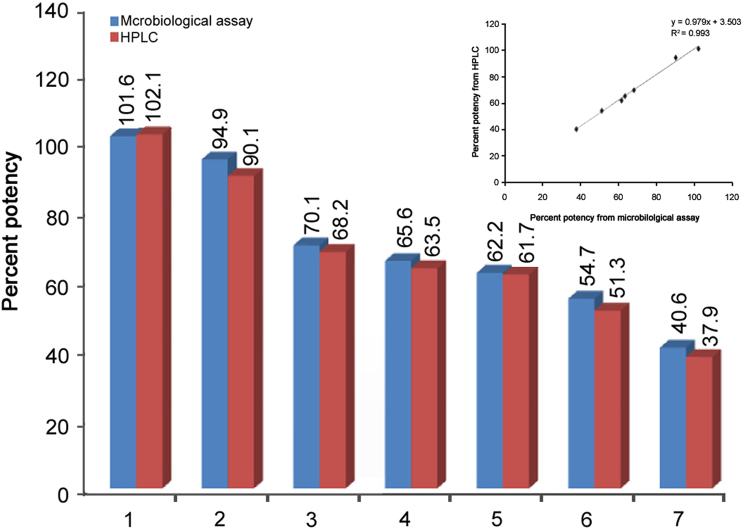
Articles show that microbiological assay can be used with almost the same accuracy to determine the potency of antibiotics with HPLC. M.C. Hsu and P.W. Hsu [63] compared the percent potency of amoxicillin in capsule, injection and granule formulations by microbiological assay and HPLC method. No significant difference in the assay values was obtained by these two methods. The correlation coefficients were found to be 0.996 for capsule, 0.997 for injection, and 0.998 for granule dosage forms. The potency of amoxicillin injection obtained by microbiological assay and HPLC method is shown in Fig. 3. Results showed a strong correlation between both the methods [63]. When a graph was plotted between the potency estimated by both the methods, a good linearity was obtained (Fig. 3 inset).
Fig. 3.
Comparison of the potency of amoxicillin injection determined by microbiological assay and HPLC method. Inset: The linearity of microbiological assay and HPLC method.
8. Conclusion
Antibiotics are a type of antimicrobial agents used in the treatment and prevention of bacterial infection. The resistance of microorganism to antibiotics is one of the most serious problems around the world. To overcome the resistance problem and to safely use antibiotics, the correct measurement of potency and bioactivity of antibiotics is essential. Generally microbiological assay and HPLC method are used to measure the potency of antibiotics, but still there is controversy regarding the selection of an appropriate method among the researchers and pharmaceutical industries. The literature finding shows that both microbiological assay and HPLC method exhibit several advantages and inadequacies. Although HPLC method is fast, accurate and precise for quantification of potency of antibiotics, it can not determine bioactivity. However, microbiological assay is simple, sensitive, accurate, precise and cost effective to estimate both potency and bioactivity. Besides this, microbiological assay become the most important method to quantify the concentration of active ingredient required for the inhibition of growth of antibiotic resistant microorganism.
Microbiological assay has some biological errors, but after validating all variable parameters it is possible to achieve a precision similar to that of HPLC method. A validated microbiological assay can give significant results and is suitable for research and pharmaceutical industries. As HPLC is an automated fast and accurate method for the estimation of the potency of antibiotic, it is used for routine analysis in pharmaceutical industries where large numbers of samples are to be analyzed on a single day to fulfil the healthcare requirements. Both the methods should be used in parallel to obtain more realistic and precise measurement of potency of antibiotics.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Dafale N.A., Semwal U.P., Agarwal P.K. Quantification of ceftriaxone sodium in pharmaceutical preparations by new validated microbiological bioassay. Anal. Methods. 2012;4:2490–2498. [Google Scholar]
- 2.Denyer S.P., Hodges N.A., Gorman S.P. Hugo & Russell’s Pharmaceutical Microbiology. Seventh ed. Blackwell Publishing Company; UK: 2004. [Google Scholar]
- 3.Liu Y.Q., Zhang Y.Z., Gao P.J. Novel concentration-killing curve method for estimation of bactericidal potency of antibiotics in an in vitro dynamic model. Antimicrob. Agents Chemother. 2004;48:3884–3891. doi: 10.1128/AAC.48.10.3884-3891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dafale N.A., Semwal U.P., Agarwal P.K. Development and validation of microbial bioassay for quantification of levofloxacin in pharmaceutical preparations. J. Pharm. Anal. 2015;5:18–26. doi: 10.1016/j.jpha.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott L.M., Harley J.P., Klein D.A. Microbiology. Seventh ed. Mcgraw-Hill; New York: 2008. pp. 835–858. [Google Scholar]
- 6.Branson E. Clinical relevance of minimum inhibitory concentration. Aquaculture. 2001;11:289–296. [Google Scholar]
- 7.Ejikeme U.C., Ademola O.J. Microbiological assay of the active component of ampicillin in ampicillin and ampicillin/cloxacillin suspensions using Bacillus megatharium NCTC 10342A (76) as indicator organism. Afr. J. Microbiol. Res. 2010;4:51–54. [Google Scholar]
- 8.Hewitt W. Microbiological Assay: An Introduction to Qualitative Principles and Evaluation. First ed. Academic Press; New York: 1977. pp. 1–50. [Google Scholar]
- 9.A.F. Zuluaga, M. Agudelo, C.A. Rodriguez, etal., Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics, BMC Clin. Pharmacol. 2009, 10.1186/1472-6904-9-1 [DOI] [PMC free article] [PubMed]
- 10.Pfaller M.A., Krogstad D.J., Granich G.G. Laboratory evaluation of five assay methods for vancomycin: bioassay, high-pressure liquid chromatography, fluorescence polarization immunoassay, radioimmunoassay, and fluorescence immunoassay. J. Clin. Microbiol. 1984;20:311–316. doi: 10.1128/jcm.20.3.311-316.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco G.M. Microbiological Assay Systems for the Analysis of Antibiotics in Pharmaceutical Formulations (PhD. Dissertation) The State University Of New Jersey; USA: 1997. [Google Scholar]
- 12.T.J.A. Pinto, F.R. Lourenco, T.M. Kaneko, Microbiological assay of gentamycin employing an alternative experimental design, AOAC Annual Meeting and Exposition, Anais. Anahein-California, 2007, pp. 157.
- 13.Saviano A.M., Francisco F.L., Lourenco F.R. Rational development and validation of a new microbiological assay for linezolid and its measurement uncertainty. Talanta. 2014;127:225–229. doi: 10.1016/j.talanta.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Dafale N.A., Agarwal P.K., Semwal U.P. Development and validation of microbial bioassay for the quantification of potency of the antibiotic cefuroxime axetil. Anal. Methods. 2013;5:690–698. [Google Scholar]
- 15.Cazedey E.C.L., Salgado H.R.N. Development and validation of a microbiological agar assay for determination of orbifloxacin in pharmaceutical preparations. Pharmaceutics. 2011;3:572–581. doi: 10.3390/pharmaceutics3030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelaziz A.A., Elbanna T.E., Gamaleldeen N.M. Validated microbiological and HPLC methods for the determination of moxifloxacin in pharmaceutical preparations and human plasma. Braz. J. Microbiol. 2012:1291–1301. doi: 10.1590/S1517-83822012000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Pharmacopoeia, United States Pharmacopoeial Convention, Rockville, MD, U.S.A, 2009, pp. 86–93.
- 18.Dafale N., Agrawal L., Kapley A. Selection of indicator bacteria based on screening of 16S rDNA metagenomic library from a two-stage anoxic–oxic bioreactor system degrading azo dyes. Bioresour. Technol. 2010;101:476–484. doi: 10.1016/j.biortech.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Dafale N.A. Exploration of genetic information from dynamic microbial populations for enhancing the efficiency of azo-dye-degrading systems. Environ. Rev. 2011;19:310–332. [Google Scholar]
- 20.Yamamoto C.H., Pinto T.J.A. Rapid determination of neomycin by a microbiological agar diffusion assay using triphenyltetrazolium chloride. J. Assoc. Anal. Chem. 1996;79:434–440. [PubMed] [Google Scholar]
- 21.Lourenco F.R., Pinto T.J.A. Comparison of three experimental designs employed in gentamycin microbiological assay through agar diffusion. Braz. J. Pharm. Sci. 2009;45:559–566. [Google Scholar]
- 22.Black J.G. Microbiology: Principles and Explorations. Sixth ed. John Wiley & Sons Inc.; USA: 2005. pp. 352–384. [Google Scholar]
- 23.Indian Pharmacopoeia, Indian Pharmacopoeia Commission, Ghaziabad, India, 2014, pp. 50–59.
- 24.British Pharmacopoeia, The Stationary Office, London, 2015, pp. V396−V402.
- 25.Farmacopeia Brasileira. Fourth ed., Sao Paulo, Atheneu, 1988, pt. 1, pp. V.5.2.17–V.5.2.17-15.
- 26.Hurtado F.K., Souza M.J., Melo J.D. Microbiological assay and HPLC method for the determination of Fluconazole in pharmaceutical injectable formulations. Lat. Am. J. Pharm. 2008;27:224–228. [Google Scholar]
- 27.Queiroz K.M., Silva M.L.M., Prado N.D. Comparison of microbiological assay and HPLC–UV for determination of fluconazole in capsules. Braz. J. Pharm. Sci. 2009;45:693–700. [Google Scholar]
- 28.Simon H.J., Yin E.J. Microbial bioassay of antimicrobial agent. Antimicrob. Agents Chemother. 1970;19:167–171. [Google Scholar]
- 29.Andrews J.M. Determination of minimum inhibitory concentration. J. Antimicrob. Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 30.Sevillano D., Calvo A., Jose M. Bactericidal activity of amoxicillin against non-susceptible Streptococcus pneumoniae in an in vitro pharmacodynamic model simulating the concentrations obtained with the 2000/125 mg sustained-release co-amoxiclav formulation. J. Antimicrob. Chemother. 2004;54:1148–1151. doi: 10.1093/jac/dkh463. [DOI] [PubMed] [Google Scholar]
- 31.Struillou L., Cohen Y., Lounis N. Activities of roxithromycin against Mycobacterium avium infections in human macrophages and C57bl/6 MIC. Antimicrob. Agents Chemother. 1995;39:878–881. doi: 10.1128/aac.39.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh Y.C., Chang K.Y., Huang Y.C. Clonal spread of highly-lactam-resistant Streptococcus pneumoniae isolates in Taiwan. Antimicrob. Agents Chemother. 2008;52:2266–2269. doi: 10.1128/AAC.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagannathan R., Mahadevan P.R. Minimum inhibitory concentration of drugs against Mycobacterium leprae as determined by an in vitro assay. J. Biosci. 1986;10:137–144. [Google Scholar]
- 34.Guna R., Munoz C., Dominguez V. In-vitro activity of linezolid, clarithromysin and moxifloxacin against clinical isolates of Mycobacterium kansaii. J. Antimicrob. Chemother. 2005;55:950–953. doi: 10.1093/jac/dki111. [DOI] [PubMed] [Google Scholar]
- 35.Landman D., Bratu D., Alam M. Citywide emergence of Pseudomonas aeruginosa strains with reduced susceptibility to polymyxin B. J. Antimicrob. Chemother. 2005;55:954–957. doi: 10.1093/jac/dki153. [DOI] [PubMed] [Google Scholar]
- 36.Jureen P., Ngeby K.A., Sturegard E. Wild-type MIC distributions for amino glycoside and cyclic polypeptide antibiotics used for treatment of Mycobacterium tuberculosis infections. J. Clin. Microbiol. 2010;48:1853–1858. doi: 10.1128/JCM.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shryock T.R., White D.W., Taples J.M.S. Minimum inhibitory concentration breakpoints and disk diffusion inhibitory zone interpretive criteria for tilmicosin susceptibility testing against Pasteurella species associated with bovine respiratory disease. J. Vet. Diagn. Investig. 1996;8:37–44. doi: 10.1177/104063879600800310. [DOI] [PubMed] [Google Scholar]
- 38.Bonev B., Hooper J., Parisot J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008;61:1295–1301. doi: 10.1093/jac/dkn090. [DOI] [PubMed] [Google Scholar]
- 39.Silva E., Diaz J.A., Arias M.J. Comparative in-vitro study of the antimicrobial activities of different commercial antibiotic products for intravenous administration. BMC Clin. Pharmacol. 2010;10:3. doi: 10.1186/1472-6904-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doern G.V. Antimicrobial use and the emergence of antimicrobial resistance with Streptococcus pneumoniae in the United States. Clin. Infect. Dis. 2001;33:S187–S192. doi: 10.1086/321847. [DOI] [PubMed] [Google Scholar]
- 41.Dong Y., Zhao X., Domagala J. Effect of fluoroquinolones concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 1999;43:1756–1758. doi: 10.1128/aac.43.7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong Y., Zhao X., Kreiswirth B.N. Mutant prevention concentration as a measure of antibiotic Potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2000;44:2581–2584. doi: 10.1128/aac.44.9.2581-2584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquali F., Manfreda G. Mutant prevention concentration of ciprofloxacin and enrofloxacin against Escherichia coli, Salmonella typhimurium and Pseudomonas aeruginosa. Vet. Microbiol. 2007;119:304–310. doi: 10.1016/j.vetmic.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Roderfguez J.C., Cebrian L., Lopez M. Mutant prevention concentration: a new tool for choosing treatment in nontuberculous Mycobacterial infections. J. Antimicrob. Chemother. 2004;24:352–356. doi: 10.1016/j.ijantimicag.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X., Xu C., Domagala J. DNA topoisomerase targets of the fluoroquinolones: A strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blondeau J., Zhao X., Hansen G. Mutant prevention concentration (MPC) as a guide for treating Streptococcus pneumoniae with fluoroquinolones. Antimicrob. Agents Chemother. 2001;45:433–438. doi: 10.1128/AAC.45.2.433-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drugeon H.B., Juvin M.E., Caillon J. Assessment of formulas for calculating critical concentration by the agar diffusion method. Antimicrob. Agents Chemother. 1987;31:870–875. doi: 10.1128/aac.31.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis W.W., Stout T.R. Disc plate method of microbiological antibiotic assay factors influencing variability and error-1. Appl. Microbiol. 1971;22:659–665. doi: 10.1128/am.22.4.659-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis W.W., Stout T.R. Disc plate method of microbiological antibiotic assay factors influencing variability and error-2. Antimicrob. Agents Chemother. 1971;10:447–450. doi: 10.1128/am.22.4.659-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McPolin O. An Introduction to HPLC for Pharmaceutical Analysis. Mourne Training Services; Northern Ireland, UK: 2009. [Google Scholar]
- 51.Lotfipour F., Valizadeh H., Nezhadi S.H. Comparison of microbiological and high-performance liquid chromatographic methods for determination of clarithromycin levels in plasma. Iran. J. Pharm. Res. 2010;9:27–35. [PMC free article] [PubMed] [Google Scholar]
- 52.Auten G.M., Preheim L.C., Sookpranee M. High-pressure liquid chromatography and microbiological assay of serum ofloxacin levels in adults receiving intravenous and oral therapy for skin infections. Antimicrob. Agents Chemother. 1991;35:2558–2561. doi: 10.1128/aac.35.12.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Miles M.V., Hall W. An improved micromethod for vancomycin determination by high-performance liquid chromatography. Ther. Drug Monit. 1995;17:366–370. doi: 10.1097/00007691-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Pennick G.J., Perea S., Modak A. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 2000;44:1209–1213. doi: 10.1128/aac.44.5.1209-1213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bottcher S., Von B.H., Hoppe-Tichy T. An HPLC assay and a microbiological assay to determine levofloxacin in soft tissue, bone, bile and serum. J. Pharm. Biomed. Anal. 2001;25:197–203. doi: 10.1016/s0731-7085(00)00478-7. [DOI] [PubMed] [Google Scholar]
- 56.Brogard H.M., Jehl L.F., Monteil H. Comparison of high-pressure liquid chromatography and microbiological assay for the determination of biliary elimination of ciprofloxacin in body fluids. Antimicrob. Agents Chemother. 1985;28:311–314. doi: 10.1128/aac.28.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hewitt W. CRC Press; Llandysul, UK: 2003. Microbiological Assay for Pharmaceutical Analysis: A Rational Approach; pp. 1–260. [Google Scholar]
- 58.Rex J.H., Hanson H., Amantea M.A. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 1991;35:846–850. doi: 10.1128/aac.35.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salgado H.R.N., Lopes C.O., Lucchesi M.B.B. Microbiological assay for gatifloxacin in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2006;40:443–446. doi: 10.1016/j.jpba.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 60.Salgado H.R., Tozo G.C. Microbiological assay for cefoxitin sodium in dosage form. J. Assoc. Anal. Chem. 2007;90:452–455. [PubMed] [Google Scholar]
- 61.Bawdon R.E., David L., Hemsell D.L. Comparison of cefoperazone and cefoxitin concentrations in serum and pelvic tissue of abdominal hysterectomy patients. Antimicrob. Agents Chemother. 1982;22:999–1003. doi: 10.1128/aac.22.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hekster Y.A., Baars A.M., Vree T.B. Comparison of high performance liquid chromatography and microbiological assay in the determination of plasma cefuroxime concentrations in rabbits. J. Antimicrob. Chemother. 1980;6:65–71. doi: 10.1093/jac/6.1.65. [DOI] [PubMed] [Google Scholar]
- 63.Hsu M.C., Hsu P.W. High-performance liquid chromatographic method for potency determination of amoxicillin in commercial preparations and for stability studies. Antimicrob. Agents Chemother. 1992;36:1276–1279. doi: 10.1128/aac.36.6.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sunderland J., Tobin C.M., Hedges A.J. Antimicrobial activity of fluoroquinolone photodegradation products determined by parallel-line bioassay and high performance liquid chromatography. J. Antimicrob. Chemother. 2001;47:271–275. doi: 10.1093/jac/47.3.271. [DOI] [PubMed] [Google Scholar]
- 65.Patel N., Lubanski P., Ferro S. Correlation between vancomysin MIC values and those of other agents against gram positive bacteria among patients with blood stream infections caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009;53:5141–5144. doi: 10.1128/AAC.00307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]