Abstract
The inclusion complexes of poorly water-soluble cephalosporin, cefuroxime axetil (CFA), were prepared with β-cyclodextrin (βCD) with or without addition of l-arginine (ARG) to improve its physicochemical properties. We also investigated the effect of ARG on complexation efficiency (CE) of βCD towards CFA in an aqueous medium through phase solubility behaviour according to Higuchi and Connors. Although phase solubility studies showed AL (linear) type of solubility curve in presence and absence of ARG, the CE and association constant (Ks) of βCD towards CFA were significantly promoted in presence of ARG, justifying its use as a ternary component. The solid systems of CFA with βCD were obtained by spray drying technique with or without incorporation of ARG and characterized by differential scanning calorimetry (DSC), X-ray powder diffractometry (XRPD), scanning electron microscopy (SEM), and saturation solubility and dissolution studies. The molecular modeling studies provided a better insight into geometry and inclusion mode of CFA inside βCD cavity. The solubility and dissolution rate of CFA were significantly improved upon complexation with βCD as compared to CFA alone. However, ternary system incorporated with ARG performed better than binary system in physicochemical evaluation. In conclusion, ARG could be exploited as a ternary component to improve the physicochemical properties of CFA via βCD complexation.
Keywords: Cefuroxime axetil, β-cyclodextrin, Inclusion complex, Molecular modeling, Physicochemical characterization
1. Introduction
Cefuroxime axetil (CFA) (Fig. 1) is a β-lactamase-stable 1-acetoxyethyl ester prodrug of cefuroxime cephalosporin with high effectiveness against gram-positive and gram-negative microorganisms. It is used orally to treat respiratory tract infections, pharyngitis, tonsillitis, acute bacterial otitis, urinary tract infections and uncomplicated skin infections. The important structural feature of CFA is the presence of lipophilic 1-acetoxyethyl ester which facilitates its intestinal absorption after oral administration. However, CFA exists in crystalline and amorphous state and has poor aqueous solubility and dissolution rate in gastrointestinal tract. Consequently, these physicochemical properties of CFA are responsible for its limited and variable oral bioavailability (30%–60%) [1], [2], [3], [4], [5], resulting in poor therapeutic outcome.
Fig. 1.
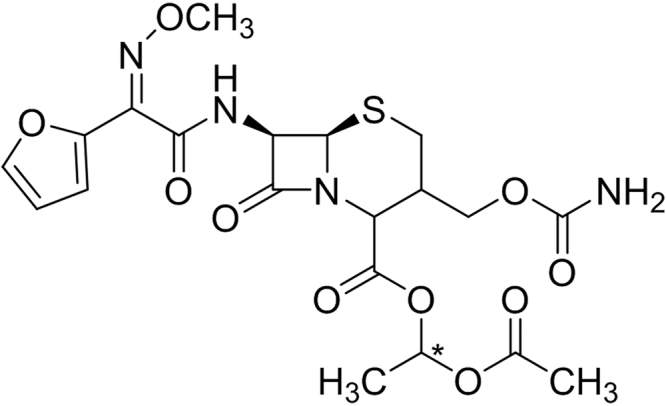
Chemical structure of cefuroxime axetil.
Cyclodextrins (CDs) are cyclic oligosaccharides with hydrophobic central cavity and hydrophilic exterior, rendering them as powerful complexing and solubilizing agents [6], [7], [8]. They are classified into α-, β- and γ-CDs consisting of (α−1, 4)-linked six, seven and eight α-d-glucopyranose units, respectively [9]. The inclusion complexation of drug (guest) molecules with CDs (host) is an industrially feasible technique used to improve the physicochemical properties of the drug, such as solubility, dissolution rate, and bioavailability [10], [11], [12]. Unfortunately, due to lower complexation efficiency (CE) of CDs, solubility enhancement via cyclodextrin (CD) complexation is limited to certain extent [13]. Several papers have reported enhancement in CE of CDs with the addition of small amounts of hydrophilic polymers [14], [15], hydroxyl acids [16], [17] and/or amino acids [9], [18], [19], [20], [21] as ternary components to the complexation media resulting in the formation of ternary complexes. The basic amino acid, l-arginine (ARG), was proved to be a better choice as an auxiliary substance to increase the solubilizing capacity of CDs through electrostatic interaction and salt formation during the multi-component complex formation of weekly acidic drugs [21], [22]. Thus, considering the advantage of incorporation of amino acid as a ternary component during complexation of drug with CD, the proposed work was carried out to promote the physicochemical properties of poorly water-soluble CFA via β-cyclodextrin (βCD) complexation using ARG as an auxiliary substance, which to our knowledge has not been reported yet.
The present study primarily focused on the investigation of the physicochemical properties of the inclusion complexes of CFA with βCD in presence of ARG as a ternary component. Initially, the possibility of complex formation was studied by molecular modeling approach and subsequently by phase solubility measurements to determine the stoichiometry of the complex formation. The binary and ternary inclusion complexes were prepared by spray drying method and characterized by differential scanning calorimetry (DSC), X-ray powder diffractometry (XRPD) and scanning electron microscopy (SEM). The saturation solubility and dissolution experiments were further conducted in distilled water and 0.07M HCl respectively to examine the physicochemical performance of the prepared complexes.
2. Experimental
2.1. Materials and reagents
CFA was obtained as a gift sample from Okasa Pharma Ltd. (Satara, India). βCD (Average molecular weight 1135 g/mol, purity 98%) was purchased from Himedia Laboratories Pvt., Ltd. (Mumbai, India). ARG (purity 99%) was procured from Loba Chemie Pvt., Ltd. (Mumbai, India). Analytical grade chemicals and double distilled water were used for all experimental procedures. All substances were used directly without further purification.
2.2. Molecular modeling studies
Molecular modeling simulations were performed using VLifeMDS 4.3 software Suit (VLife Sciences and Technologies, Pune, India) on Intel i3 CORE processor operated with Windows XP. The molecular structure of βCD was downloaded from Pubchem Structural Database (CID444041). The chemical structure of CFA was built on workspace of VLife Engine module. The individual structures (2D) of host and guest were converted to 3D and optimized to achieve minimum energy using Merck Molecular Force Field (MMFF) program with RMS gradient of 0.01 kcal/mol and dielectric constant of 1. Subsequently, CFA was allowed for conformational analysis using Monte Carlo simulations. One of the CFA conformers with least energy was positioned into the βCD cavity at different orientations and the structures were again minimized. The stoichiometry of the complex formation showing least energy was considered optimum from the total energies obtained after optimization of 1:1, 1:2 and 2:1 stoichiometries of CFA:βCD complexes. The stability of the complex was predicted from relative thermodynamic relationship in molecular mechanic (MM) calculations by calculating complexation energies (ΔE) [23], [24].
2.3. Phase solubility studies
The solubility behaviour of CFA was examined in distilled water at room temperature (25±2 °C) according to the method described by Higuchi and Connors [25]. Excess amount of CFA was added to 20 mL of aqueous solutions containing various concentrations of βCD (0–0.01 M) with or without addition of ARG (0.25%, m/v). The suspensions were mechanically shaken subsequently on a rotary shaker for 72 h at 125 rpm until equilibrium was achieved. The samples were filtered through Whatman filter paper 41, diluted if necessary and analyzed spectrophotometrically (Shimadzu UV–vis spectrophotometer 1800, Japan) at 281 nm. The association constant (Ks) of complex and CE of βCD were calculated according to the Eqs. (1) and (2), respectively [26].
| (1) |
is the solubility of CFA in absence of βCD and slope is obtained from the phase solubility diagram constructed by plotting concentration of drug on y-axis and concentration of βCD on x-axis. It gives idea about the stoichiometry of the complex formation. Linear dependence of drug concentration to βCD concentration, with slope ratio below one, usually assumes 1:1 ratio of the complex and refers to AL (linear) type of the phase solubility curve.
| (2) |
The thermodynamic parameter, Gibbs free energy of transfer (∆Gtr°), was also determined with the following equation:
| (3) |
Where Sc/S0 is the ratio of molar solubility of CFA in aqueous solutions of βCD with or without ARG to that in distilled water in absence of βCD. ∆Gtr° values demonstrate the process of transfer of CFA from pure water to aqueous solutions of βCD [5].
2.4. Preparation of binary and ternary inclusion complexes
Equimolar quantities of CFA and βCD with or without addition of ARG (0.25%, m/v) were dissolved in 100 mL of methanol and sonicated for 10 min. The mixture was stirred for 72 h at room temperature (25±2 °C) on a magnetic stirrer (2MLH, Remi Laboratory Instruments, Mumbai, India) at 125 rpm. The resultant suspension was spray dried using Lab spray dryer (SPD-D-111 Techno Search Instruments, Thane, India) under the following set of conditions: Inlet temperature 65 °C, outlet temperature 45 °C, cool temperature 20 °C, aspirator speed 40 mBar and feed rate 5 mL/min. The spray dried product was collected and stored in desiccators to prevent it from moisture absorption.
2.5. DSC analysis
Thermograms of CFA, βCD, ARG and complexes were recorded on a DSC analyzer (Mettler DSC 823E Mettler Toledo Pvt. Ltd, Switzerland). A sample (5 mg) was placed in an aluminum pan and heated under a nitrogen atmosphere (flow rate 100 mL/min) at a heating rate of 10 °C/min over the temperature range of 30–300 °C. The thermal behaviour of samples was recorded and studied.
2.6. XRPD analysis
The XRPD analysis of all samples including pure CFA was performed using X-ray diffractometer (PW 1729, Philips, the Netherland) with copper (Cu) anode, generator tension 40 kV, generator current 30 mA, and scanning speed 2°/min over the interval of 10–90°/2θ.
2.7. SEM analysis
The surface morphological features of all samples were investigated using a scanning electron microscope (SEM-JOEL Instruments, JSM-6360, Japan) operated at an acceleration voltage of 20 kV and the obtained microphotographs were examined at ×500 and ×2000 magnifications.
2.8. Saturation solubility studies
Saturation solubility studies of CFA and inclusion complexes were conducted in triplicate as follows: an excess amount of CFA and/or complexes were added to 20 mL of distilled water in vials sealed with stoppers and shaken in a rotary flask shaker at room temperature (25±0.5 °C) for 24 h. A portion of solution was withdrawn, filtered through Whatman filter paper 41 and analyzed spectrophotometrically (Shimadzu UV–vis spectrophotometer 1800, Japan) at 280 nm. The data of saturation solubility were analyzed statistically using ANOVA (Instat®, GraphPad software Inc. Version 3.05).
2.9. Dissolution studies
The dissolution studies of CFA and complexes were carried out in eight station dissolution test apparatus (Disso 2000 Tablet dissolution test apparatus, Lab India, Mumbai, India) according to USP type II. A total of 125 mg of pure CFA or complexes were added into a dissolution vessel containing 900 mL of 0.07 M HCl, maintained at 37±0.5 °C at 55 rpm. A total of 6 mL of samples were withdrawn at appropriate time intervals. The volume of dissolution medium was adjusted to 900 mL by replacing each 6 mL of aliquot withdrawn with 6 mL of fresh 0.07 M HCl. The solution was immediately filtered through Whatman filter paper 41, suitably diluted and analyzed spectrophotometrically (Shimadzu UV–vis spectrophotometer 1800, Japan) at 281 nm. The data of dissolution studies were analyzed statistically using ANOVA (Instat®, GraphPad software Inc. Version 3.05).
3. Results and discussion
3.1. Molecular modeling studies
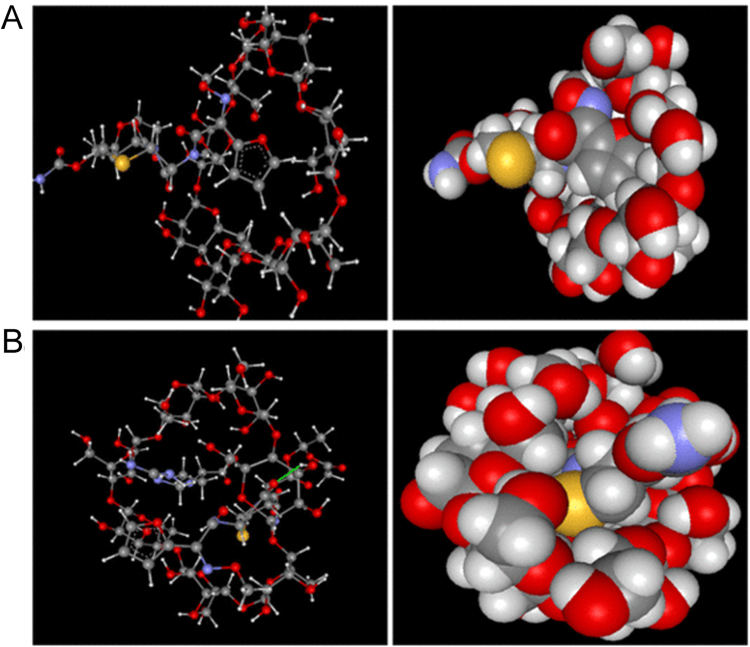
The possibility of complex formation was assessed with the molecular mechanic calculations. The total energies of complex formation for 1:1, 1:2, 2:1 stoichiometries were found to be 422.5, 9072, and 4235 kcal/mol, respectively. As the lowest total energy was shown by 1:1 stoichiometry of CFA:βCD inclusion complex, it was considered to be the most stable inclusion complex among all the stoichiometries examined (Fig. 2).
Fig. 2.
Optimized geometric models of (A) CFA:βCD binary system (B) CFA:ARG:βCD ternary system.
As shown in Fig. 2, insertion of furan ring from narrow rim of βCD (Fig. 2A) and insertion of dihydrothiazine ring from wider rim of βCD (Fig. 2B) were the most appropriately predicted inclusion geometries for binary and ternary complexes, respectively, corresponding to the greatest absolute value of heat of formation and the lowest complexation energies (ΔE) (Table 1) [23].
Table 1.
Enthalpies (ΔH) and complexation energies (ΔE) of optimized CFA:βCD binary and CFA:ARG:βCD ternary complexes (kcal/mol).
| Possible geometries of CFA inside βCD cavity | CFA:βCD |
CFA:ARG:βCD |
||
|---|---|---|---|---|
| ΔH | ΔE | ΔH | ΔE | |
| Furan ring in narrow rim | −63,552 | −157.5 | −708,902 | 305.63 |
| Furan ring in wider rim | −13,207 | −30.5 | −746,427 | 663.34 |
| Dihydrothiazine ring in narrow rim | −98,571 | −30.5 | −352,425 | −21.5 |
| Dihydrothiazine ring in wider rim | −2,099,959 | 655.41 | −132073.03 | −27.5 |
Enthalpy (ΔH): Enthalpy of formation of complex – sum of enthalpies of formation of guest and host; Complexation energy (ΔE): Energy of the complex – sum of the energies of guest and host in their respective equilibrium geometry; CFA: cefuroxime axetil; βCD: β-cyclodextrin; ARG: l-arginine.
Thus, it could be concluded that βCD could accommodate furan ring from narrow rim inside the βCD cavity in absence of ARG while insertion of dihydrothaizine moiety in βCD cavity was favored through wider rim in presence of ARG, indicating ARG involvement in the hydrogen bonding interactions with βCD and CFA outside the βCD cavity.
3.2. Phase solubility studies
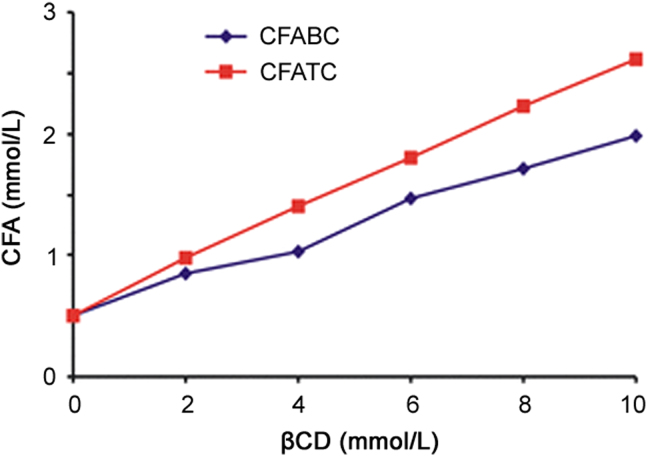
The phase solubility diagrams of CFA in aqueous βCD solution in presence and absence of 0.25% ARG exhibited AL type of solubility curve with linear increase in solubility of CFA upon increasing the concentration of βCD (Fig. 3).
Fig. 3.
Phase solubility diagram of CFA:βCD inclusion complexes in water at 25±2 °C. CFABC: binary system; CFATC: ternary system with ARG.
The slopes of phase solubility curves were less than 1, indicating the formation of water soluble complexes with 1:1 stoichiometry [13], [25]. As shown in Table 2, the values of S0, slopes, Ks, and CE of complex increased with the addition of ARG to the complexation media, indicating greater effectiveness of ternary systems over binary one.
Table 2.
Phase solubility data of binary and ternary inclusion complexes of CFA with βCD.
| Complexes | S0 | Slope | r2 | Ks (M−1)a | KTS/KBS | CE |
|---|---|---|---|---|---|---|
| CFA:βCD | 0.00051 | 0.1485 | 0.9922 | 339.74±1.5 | – | 0.17 |
| CFA:ARG:βCD | 0.00054 | 0.2096 | 0.9989 | 490.98±2.7b | 1.44 | 0.27 |
CFA: cefuroxime axetil; βCD: β-cyclodextrin; ARG: l-arginine; : solubility of CFA in absence of βCD; r2: regression coefficient of phase solubility plot; Ks (M−1): association constant of complexes; KTS/KBS: the ratio of Ks for ternary and binary systems; CE: complexation efficiency.
indicates mean±SD (n=3); SD: Standard deviation.
Significant difference compared to Ks of βCD binary system (p<0.001).
This enhancement in phase solubility parameters of CFA upon complexation with βCD in presence of ARG might be attributed to its electrostatic/hydrogen bonding interaction and salt formation with βCD and CFA [11], [19]. In addition to that, molecular interactions based on solublization of the drug such as hydrophobic bonding, Van der Waals dispersion forces and/or promoting the release of high-energy water molecules present in the cavity might have also contributed to beneficial effects of ARG as a ternary component [20], [21], [22], [26].
Table 3 shows Gibbs free energy change (ΔGtr°) values to predict the thermodynamics of process of transfer of CFA from pure water to aqueous solution of βCD. ΔGtr° values were found to be negative at various concentrations of βCD in all cases, indicating the spontaneous nature of CFA solubilization. The values were decreased upon increasing concentration of βCD, indicative of more favorable solubilization reaction as the concentration of βCD increased [5], [11], [27].
Table 3.
Gibbs free energy of transfer (∆Gtr°) of CFA from pure water to aqueous solutions of βCD in presence and absence of auxiliary substance ARG (0.25%, m/v).
| Concentration of βCD (mmol/L) | ΔGtr° (J/mol) |
|
|---|---|---|
| CFA:βCD | CFA:ARG:βCD | |
| 0.002 | −108.82 | −139.67 |
| 0.004 | −146.97 | −212.80 |
| 0.006 | −221.53 | −265.05 |
| 0.008 | −254.39 | −309.59 |
| 0.010 | −284.66 | −342.30 |
CFA: cefuroxime axetil; βCD: β-cyclodextrin; ARG: l-arginine.
These values indicated that the system was releasing energy upon complexation undergoing Vander Waals and electrostatic interactions and became more favorable in presence of ARG, suggesting its effectiveness as ternary component to prepare ternary systems.
3.3. DSC analysis
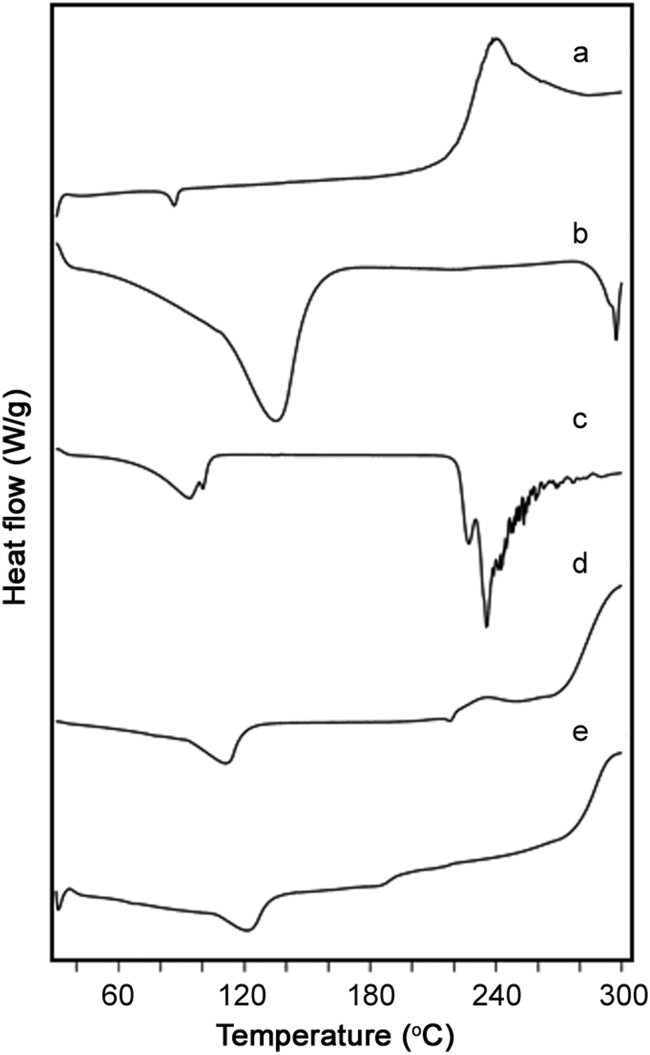
DSC technique has attracted attention to examining the interaction between host and guest molecules during the complex formation. When guest molecules are embedded in the βCD cavity, their melting points usually shift to a different temperature or disappear [22], [28]. The thermograms of CFA, βCD, ARG and their complexes are shown in Fig. 4.
Fig. 4.
DSC thermograms of CFA (a), βCD (b), ARG (c), CFA:βCD binary system (d) and CFA:ARG:βCD ternary system (e).
The DSC curve of CFA (Fig. 4a) exhibited glass transition temperature (Tg) at 86.52 °C, indicating its amorphous nature. The appearance of a broad endotherm at 134.02 °C in DSC curve of βCD was attributed to loss of water from βCD cavity (Fig. 4b). ARG exhibited broad peaks (Fig. 4c) at 93.45 and 99.55 °C due to loss of water of crystallization from small portions of l-arginine 2H2O and two melting endotherms at 225.83 and 232.96 °C with different intensities, indicating melting with decomposition of anhydrous ARG and total decomposition of the melt, respectively [20], [22].
The binary and ternary complexes showed appearance of broad peaks at 110.76 °C and 120.84 °C, respectively (Fig. 4d and e), assignable to water loss and disappearance of Tg of CFA ensuring entrapment of CFA inside βCD cavity with replacement of water molecules. The peaks of ARG also disappeared in ternary systems, indicating ARG involvement in the complexation process. These results strongly suggested an existence of strongly physical interaction between CFA and βCD and formation of stable inclusion complex in solid state.
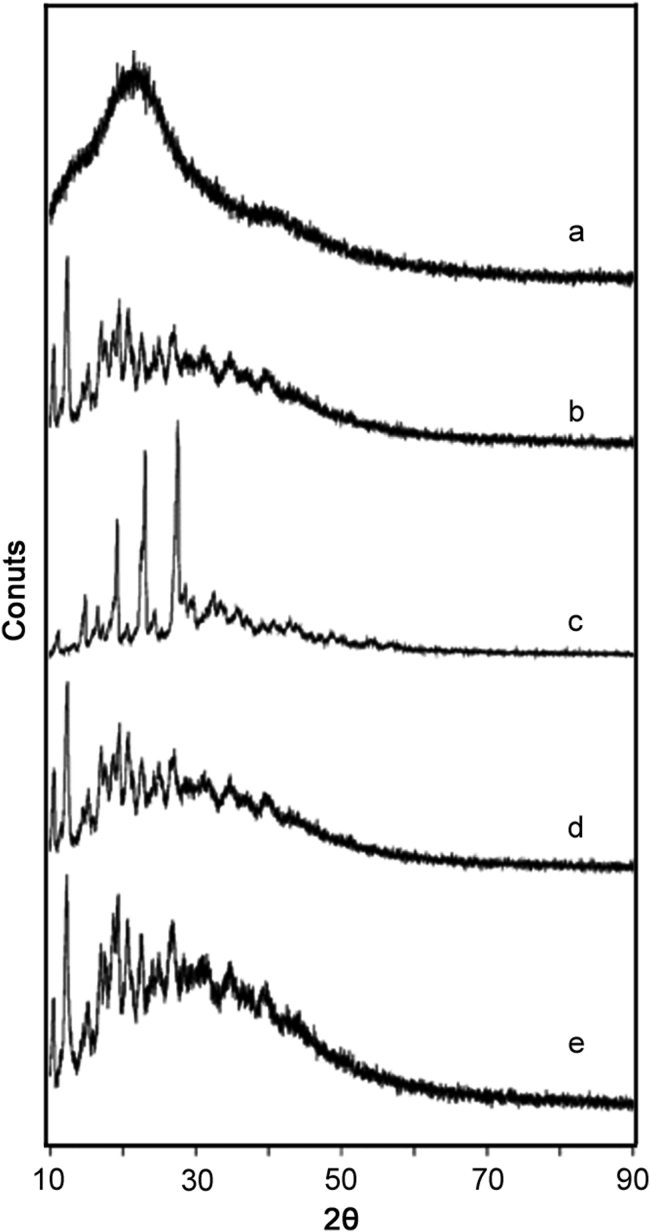
3.4. XRPD analysis
The physical state of pure drug and its complexes could be assessed by examining their XRPD patterns as shown in Fig. 5. The diffractogram of CFA (Fig. 5a) exhibited diffused peaks, indicating its amorphousness. However, there were certain peaks still detectable at 2θ° values of 11.17 (515), 13.95 (642), 16.42 (715), 19.28 (979), 20.23 (949), 21.49 (1026), 22.18 (982), 22.93 (977), 23.56 (956) 26.84 (727), and 29.62 (599), revealing the presence of some crystalline traces in the diffractogram of CFA. The crystalline nature of βCD was clearly shown by the appearance of sharp peaks at 10.60 (807), 10.71 (539), 11.94 (639), 12.57 (786), 12.49 (1111), 12.37 (1404), 12.41 (1349), 15.29 (700), 17.07 (982), 19.50 (1128), 20.74 (1070), and 22.56 (902) in its diffractogram (Fig. 5b). ARG displayed major peaks at 11.19 (519), 14.88 (1004), 16.63 (850), 19.26 (2111), 23.09 (3126), and 27.55 (3560) in crystalline state (Fig. 5c).
Fig. 5.
XRPD patterns of CFA (a), βCD (b), ARG (c), CFA:βCD binary system (d), and CFA:ARG:βCD ternary system (e).
The examination of XRPD patterns of binary (Fig. 5d) and ternary (Fig. 5e) complexes showed maximum appearance of peaks of βCD and an absence of crystalline traces of CFA, confirming spatial entrapment of CFA inside βCD cavity. In ternary systems, the overlapping of βCD and ARG crystalline peaks was noticed. However, the peaks of ARG were diffused to certain extent due to solid state interaction during complex formation [9].
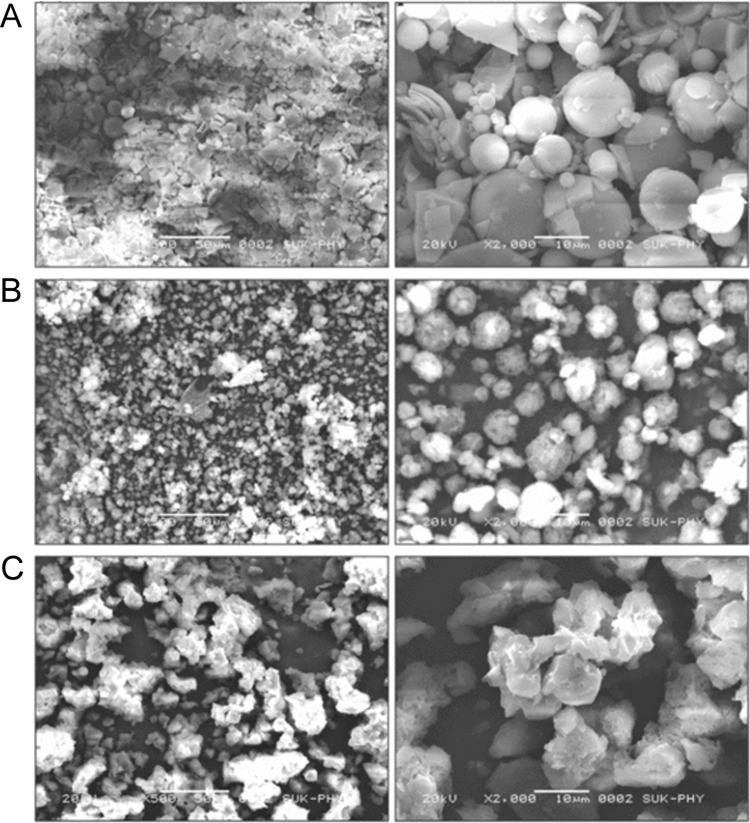
3.5. SEM analysis
The surface morphological features of pure CFA and complexes are shown in Fig. 6. Pure CFA appeared as amorphous broken spherical particles as separate entities (Fig. 6A). There were distinct changes observed in morphology of spray dried complexes. The particles of binary complexes exhibited altered shape and showed bulky spherical agglomerate type morphology (Fig. 6B) due to crystalline nature of βCD. The crystalline nature of βCD and ARG also further contributed to change in morphology of ternary complexes (Fig. 6C) showing characteristic bulky agglomerated crystalline structure images. The alteration in the morphology of particles in spray dried complexes confirmed the presence of a new solid phase in the complex achieving maximum complexation [29].
Fig. 6.
SEM of (A) CFA, (B) CFA:βCD binary system and (C) CFA:ARG:βCD ternary system.
3.6. Saturation solubility studies
The saturation solubility studies of binary and ternary systems showed remarkable enhancement in the solubility as compared to pure CFA (p<0.001). Pure CFA exhibited a solubility of 0.355±0.04 mg/mL in distilled water. The binary and ternary complexes showed solubility of 8.66±0.30 and 16.87±0.72 mg/mL, respectively. There was 24.39 fold increment in the solubility of binary complex observed, whereas 47.50 fold increment in the solubility of ternary complex was noted, which was almost double than that of binary complex. The enhancement in solubility of complex was attributed to the formation of stable inclusion complex of CFA with βCD. In ternary systems, ARG played a significant role as a ternary component resulting in better performance than binary system.
The improvement in water solubility of CFA from the complexes could be explained in terms of wetting property and hydrophilicity of βCD, altered surface morphological features of the complexes due to spray drying technique and inclusion into the hydrophobic βCD cavity [30].
3.7. Dissolution studies
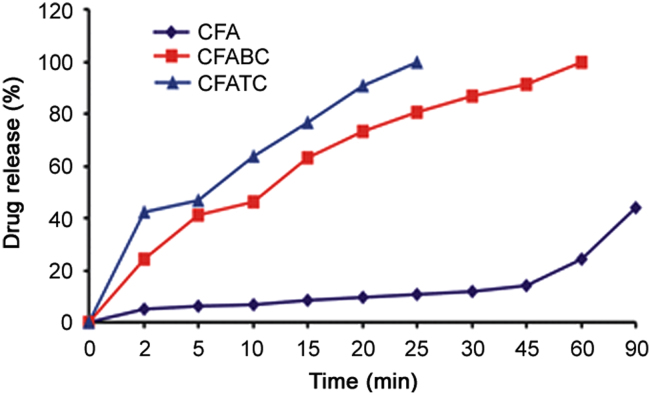
The dissolution curves of CFA and spray dried complexes are shown in Fig. 7. According to the results, an increment in dissolution profile was noted for solid complexes as compared to pure CFA (p<0.001). The binary systems showed almost complete drug release in 60 min. Indeed, ternary complexes demonstrated faster dissolution profile than binary complexes with 99.87% drug release within 25 min in dissolution media. However, the release of pure drug was incomplete even in 90 min.
Fig. 7.
The dissolution profile of CFA, binary and ternary inclusion complexes at 37±0.5 °C. CFA: cefuroxime axetil; CFABC: binary system; CFATC: ternary system with ARG.
The significant improvement in dissolution rate of CFA from inclusion complexes could be ascribed to greater hydrophilicity, wetting property, increased contact between the drug and βCD due to spray drying technology and ability to form stable inclusion complex with βCD [30]. The greater effectiveness of ternary complex for higher release rate was due to positive effect of addition of basic amino acid ARG which drastically promoted phase solubility parameters such as Ks and CE, interacting simultaneously both with βCD (via hydrogen bonding) and CFA (via electrostatic interactions and salt formation) [11], [22], [31]. Thus, it can be concluded that ternary systems of CFA with βCD and ARG could be a reliable approach for improved dissolution properties.
4. Conclusions
The present study demonstrated a successful application of basic amino acid ARG as a ternary component to improve the physicochemical properties of CFA via ternary complexation with βCD. The significant enhancement in association constant and complexation efficiency of βCD towards CFA in presence of ARG could be possibly helpful in reducing the workable amount of βCD during formulation of complexes. In conclusion, CFA can form stable inclusion complexes with βCD in presence of ARG as an auxiliary substance to offer ternary systems with better performances.
Acknowledgments
The authors are thankful to Okasa Pharma Ltd., Satara, India, for providing gift sample of drug for the research. The authors are also thankful to Shivaji University, Kolhapur, India and India Shri Shivaji Memorial College of Pharmacy, Pune, Maharashtra, India, for providing analytical facilities to perform characterization studies. The laboratory facilities provided by the Principal, Government College of Pharmacy, Karad, Maharashtra, India, are also gratefully acknowledged.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Sruti J., Patra C.N., Swain S. Improvement in the dissolution rate and tableting properties of cefuroxime axetil by melt-granulated dispersion and surface adsorption. Acta Pharm. Sin. B. 2013;3:113–122. [Google Scholar]
- 2.Parfitt K. Thirty-sixth ed. Pharmaceutical Press; London: 2009. Martindale, The Extra Pharmacopoeia. [Google Scholar]
- 3.Dhumal R.S., Biradar S.V., Yamamura S. Preparation of amorphous cefuroxime axetil nanoparticles by sonoprecipitation for enhancement of bioavailability. Eur. J. Pharm. Biopharm. 2008;70:109–115. doi: 10.1016/j.ejpb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Varshosaz J., Hassanzadeh F., Mahmoudzadeh M. Preparation of cefuroxime axetil nanoparticles by rapid expansion of supercritical fluid technology. Powder Technol. 2009;189:97–102. [Google Scholar]
- 5.Shah M., Pore Y., Dhawale S. Physicochemical characterization of spray dried ternary micro-complexes of cefuroxime axetil with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2013;76:391–401. [Google Scholar]
- 6.Loftsson T., Hreinsdottir D., Masson M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005;302:18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Rajewski R.A., Stella V.J. Pharmaceutical applications of cyclodextrins: in vivo drug delivery. J. Pharm. Sci. 1996;85:1142–1168. doi: 10.1021/js960075u. [DOI] [PubMed] [Google Scholar]
- 8.Loftsson T., Brewster M. Pharmaceutical applications of cyclodextrins: drug solubilization and stabilization. J. Pharm. Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 9.Udrescu L., Sbarcea L., Fulias A. Physicochemical characterization of zofenopril inclusion complex with hydroxypropyl-β-cyclodextrin. J. Serb. Chem. Soc. 2015;80:485–497. [Google Scholar]
- 10.Doiphode D., Gaikwad S., Pore Y. Effect of β-cyclodextrin complexation on physicochemical properties of zaleplon. J. Incl. Phenom. Macrocycl. Chem. 2008;62:43–50. [Google Scholar]
- 11.Jadhav P., Petkar B., Pore Y. Physicochemical and molecular modeling studies of cefixime-l-arginine-cyclodextrin ternary inclusion compounds. Carbohydr. Polym. 2013;98:1317–1325. doi: 10.1016/j.carbpol.2013.07.070. [DOI] [PubMed] [Google Scholar]
- 12.Aleem O., Kuchekar B., Pore Y. Effect of β-cyclodextrin and hydroxyl propyl-β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008;47:535–540. doi: 10.1016/j.jpba.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro L., Loftsson T., Ferreira D. Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether β-cyclodextrin binary and ternary complexes. Chem. Pharm. Bull. 2003;51:914–922. doi: 10.1248/cpb.51.914. [DOI] [PubMed] [Google Scholar]
- 14.Valero M., Pérezrevuelta B.I., Rodríguez L.J. Effect of PVP K-25 on the formation of the naproxen: β-cyclodextrin complex. Int. J. Pharm. 2003;253:97–110. doi: 10.1016/s0378-5173(02)00664-6. [DOI] [PubMed] [Google Scholar]
- 15.Gajare P., Patil C., Kalyane N. Effect of hydrophilic polymer on pioglitazone complexation with hydroxyl propyl β-cyclodextrin. Dig. J. Nanomater. Biostruct. 2009;4:891–897. [Google Scholar]
- 16.Pokharkar V., Khanna A., Venkatpurwar V. Ternary complexation of carvedilol-cyclodextrin and citric acid for mouth-dissolving tablet formulation. Acta Pharm. 2009;59:121–132. doi: 10.2478/v10007-009-0001-3. [DOI] [PubMed] [Google Scholar]
- 17.Redenti E., Szente L., Szejtli J. Drug/cyclodextrin/hydroxy acid multicomponent systems. Properties and pharmaceutical applications. J. Pharm. Sci. 2000;89:1–8. doi: 10.1002/(SICI)1520-6017(200001)89:1<1::AID-JPS1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Shah M., Karekar P., Sancheti P. Effect of PVP K30 and/or l-arginine on stability constant of etoricoxib-HP-β-CD inclusion complex: Preparation and characterization of etoricoxib-HP-β-CD binary system. Drug Dev. Ind. Pharm. 2009;35:118–129. doi: 10.1080/03639040802220292. [DOI] [PubMed] [Google Scholar]
- 19.Patil A., Pore Y., Kuchekar B. Effect of l-arginine on bicalutamide complexation with hydroxypropyl-β-cyclodextrin. Dig. J. Nanomater. Biostruct. 2008;3:89–98. [Google Scholar]
- 20.Mura P., Bettinetti G.P., Cirri M. Solid-state characterization and dissolution properties of naproxen–arginine–hydroxypropyl-β-cyclodextrin ternary system. Eur. J. Pharm. Biopharm. 2005;59:99–106. doi: 10.1016/j.ejpb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Bramhane D.M., Saindane N.S., Vavia P.R. Inclusion complexation of weakly acidic NSAID with β-cyclodextrin: selection of arginine, an amino acid, as a novel ternary component. J. Incl. Phenom. Macrocycl. Chem. 2011;69:453–460. [Google Scholar]
- 22.El-Maradny H.A., Mortada S.A., Kamel O.A. Characterization of ternary complexes of meloxicam-HPβCD and PVP or l-arginine prepared by the spray-drying technique. Acta Pharm. 2009;58:455–466. doi: 10.2478/v10007-008-0029-9. [DOI] [PubMed] [Google Scholar]
- 23.Jesus M.B.D., Pinto L.D.M.A., Fraceto L.F. Theoretical and experimental study of a praziquantel and β-cyclodextrin inclusion complex using molecular mechanic calculations and 1H-nuclear magnetic resonance. J. Pharm. Biomed. Anal. 2006;41:1428–1432. doi: 10.1016/j.jpba.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Pascal B., Maud G., Georges D. The effect of cyclodextrins on the aqueous solubility of a new MMP inhibitor: phase solubility, 1H NMR spectroscopy and molecular modeling studies, preparation and stability study of nebulizable solutions. J. Pharm. Pharm. Sci. 2005;8:164–175. [PubMed] [Google Scholar]
- 25.Higuchi T., Connors K.A. Phase-solubility techniques. Adv. Anal. Chem. Instr. 1965;4:117–122. [Google Scholar]
- 26.Mura P., Maestrelli F., Cirri M. Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and aminoacids. Int. J. Pharm. 2003;260:293–302. doi: 10.1016/s0378-5173(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 27.Patel R.P., Patel D.J., Bhimani D.B. Physicochemical characterization and dissolution study of solid dispersions of furosemide with polyethylene glycol 6000 and polyvinylpyrrolidone K30. Dissolut. Technol. 2008;15:17–25. [Google Scholar]
- 28.Liu X., Lin H.S., Thenmozhiyal J.C. Inclusion of acitretin into cyclodextrins: phase solubility, photostability and physicochemical characterization. J. Pharm. Sci. 2003;92:2449–2457. doi: 10.1002/jps.10495. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes C.M., Vieira M.T., Veiga F.J.B. Physicochemical characterization and in vitro dissolution behavior of nicardipine–cyclodextrins inclusion compounds. Eur. J. Pharm. Sci. 2002;15:79–88. doi: 10.1016/s0928-0987(01)00208-1. [DOI] [PubMed] [Google Scholar]
- 30.Shinde V.R., Shelake M.R., Shetty S.S. Enhanced solubility and dissolution rate of lamotrigine by inclusion complexation and solid dispersion technique. J. Pharm. Pharmacol. 2008;60:1121–1129. doi: 10.1211/jpp.60.9.0002. [DOI] [PubMed] [Google Scholar]
- 31.Moote D.C.A. Ibuprofen arginine in the management of pain. Clin. Drug Invest. 1996;11:1–7. [Google Scholar]