Abstract
There is an increasing interest in the icy moons of the Solar System due to their potential habitability and as targets for future exploratory missions, which include astrobiological goals. Several studies have reported new results describing the details of these moons’ geological settings; however, there is still a lack of information regarding the deep subsurface environment of the moons. The purpose of this article is to evaluate the microbial habitability of Europa constrained by terrestrial analogue environments and sustained by radioactive energy provided by natural unstable isotopes. The geological scenarios are based on known deep environments on Earth, and the bacterial ecosystem is based on a sulfate-reducing bacterial ecosystem found 2.8 km below the surface in a basin in South Africa. The results show the possibility of maintaining the modeled ecosystem based on the proposed scenarios and provides directions for future models and exploration missions for a more complete evaluation of the habitability of Europa and of icy moons in general.
Subject terms: Microbial ecology, Astrobiology
Introduction
Except for Earth, the moons of the giant planets, especially the icy moons such as Enceladus, Europa, Ganymede and Callisto, are the only other places in the Solar System with considerable evidence of the existence of liquid water in abundance. The oceans under the icy crusts on these moons may be able to host living organisms1–8 and thus be able to host habitable environments. Two critical factors that can make these moons compelling environments for living systems, besides the presence of water, are the availability of energy and a chemical disequilibrium based on reductant-oxidant pairs f or biological processes3. The surface of the Jovian moon Europa is young, with a resurfacing age determined to be approximately 30–70 Myr9, and it represents one of the most important targets for astrobiology research in the Solar System10. Studies also indicate active geological processes on a global scale on this icy moon such as the movement of lithospheric blocks and the upwelling of material that fills the rocky core3,11,12. These aspects could help to maintain the chemical disequilibrium in the oceans under its icy layer, which is powered by tidal and radiolysis phenomena (the latter is emphasized in this paper).
Radiolysis has already been proposed for the Europan framework. However, it was mainly focused on radiolysis caused by the bombardment of the superficial ice with energetic charged particles, such as electrons or ions, that are accelerated by the magnetic field of a nearby planet (in this case, Jupiter)3,13. Another possibility for the radiolysis on Europa comes from the bottom of the ocean, where there is a water-rock interface. Radioactive decay also occurs from fissionable materials that exist in every rocky celestial body in the Solar System. These materials, primordial radioactive elements, emit ionizing radiation that can interact with the ocean water that breaks its molecules, causes excitation and ionization, and consequently and locally forms very reactive ionized or radical species4,14–17. This defines the water radiolysis, the focus of this work. Research on the radiation effects on water and mainly radical formation induced by radiolysis increased largely in the mid-20th century14; however, the association of radiation from nuclear decay as a possible source of energy for a living system was proposed near the end of the century18. Recent studies of the so-called fossil natural reactors on Earth18 have provided a basis for the debate on the importance of ionizing energy from radioactive decay as a localized source of energy for biological processes. Notwithstanding, the recent discovery of peculiar ecosystems in deep subsurface environments, which are maintained by nutrients produced via radionuclide radiolysis19–21, has garnered attention for its feasibility.
On Earth, water radiolysis is significant in the deep environments where water and fissionable materials exist17,19,22,23 and consequently form several chemical species that contribute to microbial activity20,21. Chivian et al.20 and Lin et al.21 reported an important occurrence in nature of metabolism dependent on this type of radioactivity interaction. In the depths of the Mponeng gold mine in South Africa20,21 and located at the region of the Witwatersrand basin, it was found that a single-species ecosystem based on the bacterium Candidatus Desulforudis audaxviator, which uses this source of energy, was independent of sunlight. This discovery opened new venues to the study of other non-illuminated environments of the Solar System and the Universe, including Europa and other icy moons.
Recently, the debate on radiolysis under the surface of Europa has gained new perspectives. Atri24 discusses the importance of galactic cosmic rays (GCR), which are primary charged particles, mostly protons, that originated beyond the Solar System. If a celestial body has a reasonably thick atmosphere, primary GCR particles strike the atmospheric molecules, producing secondary particles such as kaons, pions and muons that can propagate deep underground and are highly unstable, quickly decaying to produce particles such as β and γ particles and possibly triggering radiolysis. The radiolysis discussed in that work24 is galactic cosmic ray-induced and may be important when considering small rocky bodies such as planets not tied to any planetary system or comet, but it depends on the presence of an atmosphere. However, radiolysis from radioactive isotope decay has shown potential importance in powering life on the deep subsurface of icy moons where solar energy cannot reach and galactic cosmic rays cannot provide enough energy. Considering charged particles of reasonable primary energy, only muons could reach 3 km below the surface level of an icy moon, and the energy deposition rate would still become nearly zero below this depth25,26.
However, there is still a modest number of references in the literature related to the effect of water radiolysis as a consequence of radioactive minerals in the deep subsurface icy moons and its implications for habitability. Recently, the radiolytic production of H2 in the subsurface of several of the Solar System’s icy moons was proposed13,27, although there is a necessity for complementary models to associate radiolytic energy production with biological metabolism to assess the actual habitability of extensive extraterrestrial water bodies.
Models related to the survival of bacterial cells based on radiolysis-produced chemical species, such as H2, have been proposed28. This model focuses on terrestrial context and on the primary radiolysis product. In contrast, in this study, we present the model based on the production of a secondary chemical species, sulfate, and apply it to the extraterrestrial context. For this model, we compared the radiolysis-produced sulfate rate to in situ sulfate demand for a deep subsurface environment where Ca. D. audaxviator was found.
Thus, this paper approaches the possibility of maintaining an ecosystem based on the chemical energy provided as a consequence of the direct radiolysis of water by primordial long-living radionuclides such as 238U, 232Th and 40K on a modeled setting for Europa. This hypothesis was based on the importance of such species for the natural radioactivity on Earth and assuming a similar elemental distribution on rocky planets and on Europa. The potential habitability of Europa is the main reason for the choice of this moon for the model in addition to the reasonable and growing body of information on its subsurface ocean and crust11 as well as for its importance for future space missions such as the ESA mission JUpiter ICy moons Explorer (JUICE)10. It was possible to show the availability, under certain conditions, of enough chemical energy to sustain an ecosystem of chemoautotrophic extremophilic candidate species such as Ca. D. audaxviator, which has a metabolism based on sulfur reduction. Such an energy source could be used to sustain basic metabolism and/or repair damage caused by radiation exposure20, thus creating a habitable environment, from the energetic point of view. Ca. D. audaxviator was found in fractured water in the gold mine of Mponeng, located in the Witwatersrand basin region20, South Africa, surviving at depths up to 2.8 km below the surface with temperatures between 40 °C and 60 °C, a pH of 9.3, pressures comparable to those of the abyssal regions of Earth’s oceans, low availability of nutrients and some concentration of radioactive minerals such as uraninite (UO2)19,20. This work proposes the use of this microorganism as a biological model and deep terrestrial environments as analogues to better understand the limits of habitability on deep, non-illuminated environments of the Solar System, although this scenario is still poorly explored for this purpose.
Biological Energy Transduction
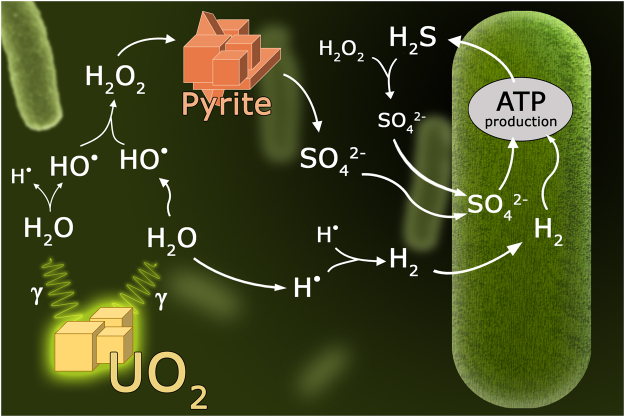
Since the first examples of a single-species microbial ecosystem were reported from deep subsurface environments20,29, attention has been drawn to bacteria similar to Ca. D. audaxviator on other extreme regions. Figure 1 illustrates a simplified model of the metabolic pathway of Ca. D. audaxviator, which obtains energy from the radiolysis of water. The bacterium extracts energy from the sulfate reduction reaction, as shown in reaction (1)21.
| 1 |
Figure 1.
Model of the single-species ecosystem of the fractured Mponeng gold mine in South Africa. Ca. D. audaxviator’s pathway for obtaining energy from the decay of uranium of 238UO2 (uraninite) is shown, including the pathway of sulfate reduction. Adapted from Chivian et al.20.
The bacterial sulfate consumption model calculations depend on the presence of H2 in such a way that sulfate is the limiting reactant of reaction (1). Dissolved H2 may exist on icy moons such as Enceladus as a result of radiolysis or hydrothermal activity. The presence of this gas in the subsurface ocean has been reported for Enceladus13. On Europa, there are also speculations related to this component27,30.
Physicochemical Basis For Radiolysis
As a benchmark for this work, the experimental results of Lefticariu et al.17 and Lin et al.21 were used for the calculations. The first study provided a model for the rate of radiolytic sulfate production by the exposure of water and pyrite to a source of gamma radiation. The second work presents the in situ rate of microbial sulfate reduction between 0.22 and 1.45 nM per year for a cell density of 4 × 107 cells per liter or 5.5 × 10–18 to 3.6 × 10–17 mols per cell per year in the case of the deep subsurface of the Witwatersrand basin20.
The experimental work conducted by Lefticariu et al.17 showed that the sulfate production via radiolysis is Gs = 2.1 × 10−9 [mol/m2/(J/kg)]. An identified source of in this environment is the oxidation and dissolution of pyrite (FeS2). This mineral is known for its primordial origin on rocky planets31, with possible implications on prebiotic chemistry and early metabolism. It can react with a radiolysis product of water due to the radiation released by minerals such as UO2 or ThO232, as expressed in reaction (2).
| 2 |
The radical is one of the products of the primary reactions of the radiolysis of water32,33 (3).
| 3 |
The production rate of as a function of the dose Di of (gamma) radiation from different species is then given by Ys = Σi Di × Gs in units of mol/m2/year. A direct relationship between Gs and Ys and the exposed surface area of pyrite exists, which is a feature that is explored further in section 5 and in the supplementary material. The dose Di is given by Di = Ei × λi × ci × Na × Ai−1 [J/kg/year], where E [J/decay] is the energy per decay corrected by neutrino loss via beta decay, λ = 1/T1/2 [decay per year] is the decay constant (in conformity with Lefticariu17,), c [ppm] is the concentration of the radioactive element, Na is Avogadro’s number, and Ai [g/mol] is the atomic mass. The index i stands for the radioactive species involved in the process. Finally, the annual production of radiolytic sulfate is given by Ps = YS × Spy × w%, where Spy [m2/kgrock] is the surface area of pyrite per kilogram of sedimentary rock, which, for pyrite in the authors’ experiment, was measured to be 226.0 ± 6.5 cm2/g, and w% is the pyrite mass percentage for the rock.
Abundances Of Radioactive Materials And Pyrite Granulometry
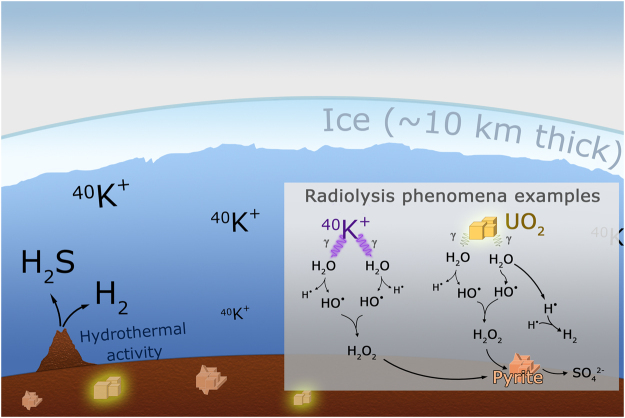
The results for radiolysis-produced sulfate, which was induced by natural radioactive decay in the Mponeng mine fracture water, suggests the possibility of an analogous process occurring in the subsurface environment of Europa, as schematized in Fig. 2. This work assumes that there are clumps of radioactive materials in the seabed, proximate to pyrite formations and far from hydrothermal vents that could be another source of sulfate and significantly increase the temperature of the medium. Models for the origin, composition and evolution of the crust and ocean of Europa11 suggest the formation of pyrite-like materials, which are a major component. It was assumed that the niches of radioactive materials contain 238U and 232Th and that 40K is present in the ocean in concentrations that are expected to be higher than what is found in modern terrestrial oceans34.
Figure 2.
Model of Europa emphasizing the radiogenic material and the gamma-ray radiolysis phenomena occurring in the seabed. We assume 238U and 232Th are within the rocks of the Jovian moon and 40K is dissolved in the water. Adapted from Chyba & Hand (2001).
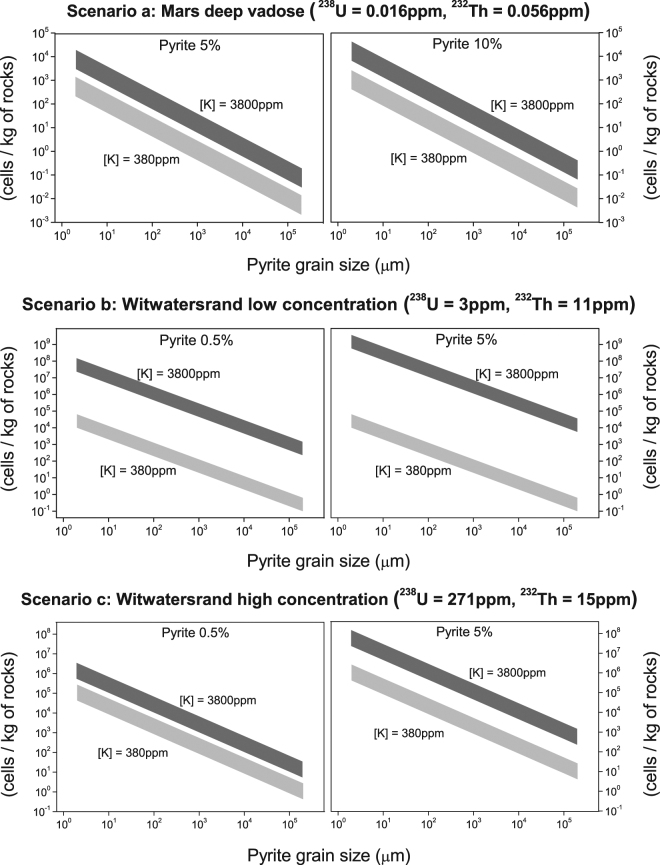
For uranium and thorium, the concentration from three subsurface scenarios17 was used because the actual concentrations on the Europa seabed are still unknown: a) Martian deep vadose (water-unsaturated zone); b) rocks from non-mineralized strata having low concentrations of radioactive elements from the Witwatersrand basin; and c) rocks from mineralized strata having high concentrations of radioactive elements on the Witwatersrand basin. For potassium, it was assumed that concentrations were in the range of 380 ppm (as in Earth’s ocean34) to 3800 ppm (which may be closer to that of the Europan ocean8), with the unstable 40K isotope35 accounting for 0.0117%.
For the pyrite sites, the presence of homogeneous pyrite grains covering parts of the niches was assumed. We use the experimental value of the surface area of pyrite, Spy = 226 cm2/g17, related to grains in the range of 100 to 150 μm in the size as a base to calculate other plausible scenarios for the granulometry that could possibly exist on Europa. These grains of pyrite were modeled as small spheres that fill a cubic space, just as a typical sphere-packing model. Varying the spherical grain size, i.e., the diameter of the sphere (φ), implies a variation of the surface area of the grain, Spy(φ). Considering this model, the total surface area of the sphere packing is inversely proportional to the sphere radius. Thus, the sulfate production (Ps) was estimated for different possible grain sizes based on the Wentworth36 grade scale, considering the concentrations of radionuclides and pyrite for the different scenarios described earlier. For each type of aggregate, a homogeneous grain size distribution was considered. In other words, every grain has the same average size.
Results
Table 1 shows the calculated total surface area of pyrite for the different types of aggregates. Based on the values in Table 1, the sulfate production per year as a function of the pyrite rock mass (presented on Table S1) was calculated as well as the cell-carrying capacity (the quantity of cells that could survive) of Ca. D. audaxviator per kilogram of rock present on the local site for the proposed analogue scenarios (summarized in Fig. 3 and further described in Table S2). The radiolytic sulfate production rates were based on the model of Lefticariu et al.17, but greater values for the rates were obtained, not only due to the addition of the 40K source but also due to a revision of the previous calculations.
Table 1.
Sphere-packing model results for the surface area of pyrite from different types of aggregates, based on the Wentworth scale36.
| Type of aggregate | Grain φ(μm) | Spy(φ) (m2.kg−1) |
|---|---|---|
| Clay | 2 | 1.41 × 103 |
| Silt | 10 | 2.83 × 102 |
| 60 | 4.71 × 101 | |
| Sand | 125 | 2.26 × 101 |
| 500 | 5.65 | |
| 1000 | 2.83 | |
| Pebbles | 10000 | 2.83 × 10−1 |
| 50000 | 5.65 × 10−2 | |
| Cobbles | 100000 | 2.83 × 10−2 |
| 200000 | 1.41 × 10−2 |
Figure 3.
Log-Log plot of the cell-carrying capacity per mass of rocks that contains pyrite compared to the results for the different uranium and thorium scenarios (a,b and c, as described in section 4) and the assumed minimum (light gray) and maximum (dark gray) potassium concentrations. The X-axis represents the variation in grain size of pyrite based on the classification and based on the Wentworth scale (see Table S2), which is inversely proportional to the surface area available for oxidation.
The difference in the K concentration for the Europan and the terrestrial ocean had an important outcome. Figure 3 shows that a 10 times greater concentration of K can provide enough sulfate for a 1000-fold increase in cell number. Table S2 shows that if we consider 1 kg of rocky material with an aqueous medium as small as 2 ml, as in the samples in the experimental work of water radiolysis17, scenarios b and c (described on section 4) significantly exceed the necessity to maintain a cell density of 4 × 107 cells per liter, which is the average density that was present in samples of fracture water from the Witwatersrand basin region17.
Once more information regarding the existence, concentration and granulometry of pyrite and the presence of radioactive isotopes on the seabed of Europa is obtained from models, experiments or direct/indirect measurements by space missions, the results presented in Fig. 3 may be useful to estimate the habitability of the moon in terms of biologically useful energy sources.
Discussion
In this work, deep terrestrial environments, such as the Mponeng gold mine, and selected Martian geological settings were evaluated as reasonable analogues for the under-crust oceanic non-illuminated and non-photosynthetic environment of Europa. In this context, the Candidatus Desulforudis audaxviator extremophile was used as a model organism because it is prominent from fracture water sampled from depths greater than 1.5 km across the Witwatersrand basin and it dominates the biota discovered 2.8 km below the land surface. The environmental conditions of Mponeng mine as a deep subsurface environment considering the lack of O2 and high temperatures can be considered similar to those of the seabed of Europa37, which is heated by tidal interaction with Jupiter. Because it is chemoautotrophic, this bacterium candidate species has the capacity to fix its own carbon (thus reducing the necessity of reduced organics) and to thrive in regions with a chemical disequilibrium produced by water radiolysis.
Our calculation assumes that there is enough radiolytic endogenous sulfate production to enter the microorganism metabolism even if we restrict the results to gamma-ray radiolysis. This simplification was based on the fact that the rate of radiolytic production of from water, which reacts with pyrite to form sulfate, is at least one order of magnitude higher than the rate due to other decay channels, such as alphas (per electron-Volt)32. Despite the fact that rock porosity and space constraints were not considered in our calculations, those parameters were included in models to calculate the production far from the solid-water interface of radioactive mineral28,38. Here, a simple model considered local radiolysis in the aqueous environment, and this model showed enough to provide some reference to the objectives established for this work. In addition, considering the possibility for existence of hydrothermal systems on the Europa seabed11, these can be another endogenous source of sulfate39. These sources were assumed to exist far from our radiolytic system, and for the survival of a species such as Ca. D. audaxviator, it is necessary that the environment is depleted of oxygen and has a high pH20 – a condition normally not matched by nearby hydrothermal vents present on Earth39. More observational data from space missions are needed to constrain this information for the case of Europa. It has also been proposed that sulfate could have an exogenous source3,40, namely, from Io40. We argue that although this could be a source, the icy crust would prevent efficient mixing, made even less probable when associated with the abyssal depths of the ocean. It was assumed that sulfate is not used in other reactions that could prevent its availability for the microorganism. The main route for the depletion of sulfate could be, under Europan conditions, the precipitation of sulfate in the form of non-soluble salts such as BaSO4 and/or CaSO4. However, to study these possible sinks, more information is needed regarding the abundances of Ca+ and Ba+ and their equilibrium reactions under Europan conditions, especially pH and temperature. Additionally, reactions with silica can occur depending on the temperature, for example,, releasing sulfur in the form of gas. Thus, as the dynamics of cryotectonism is not completely understood and the rate of sulfate delivery or sinking remains poorly constrained, these effects were not taken into account in the present work. Future direct measurements and models are still needed to better constrain the habitability of Europa.
Other open questions arise, such as the unknown distribution of 40K on the ocean. If it is uniformly distributed, then the release of O2 by the radiolysis of water would have oxidized the ocean over time, which could halt sulfate production. However, a non-uniform distribution would place some restrictions on the present results, and although it is easy to imagine niches of uranium and thorium, it is difficult to imagine that there would also be a clump of 40K together with the niches of 238U and 232Th. An example of this clumping would be due to the presence of potassium minerals with low solubility such as jarosite (KFe3(OH)6(SO4)2).
Another product of radiolysis is H2O2, which is formed according to the reaction . The sterilizing power of the peroxide could be a caveat. Too much peroxide near the microorganisms could minimize the habitability of the environment when considering the case of Ca. D. audaxviator, whose genes lack functional peroxidase homologs20. However, we suppose that this situation would not occur in a system such as the one modeled here, since H2O2 is a kinetically unstable chemical species and the rate of water formation from the same reagent is double the hydrogen peroxide formation rate16,17. Similarly, the presence of O2 as a product of radiolysis could be important considering its sterilizing power for Ca. D. audaxviator, as it also lacks a complete system for oxygen resistance20. However, deep environments such as the Mponeng gold mine and others are depleted of O2 and are reductant-rich19,20,22,23, which suggests that molecular oxygen and H2O2 have a relevant sink that may be the pyrite mineral itself, and the same may occur for the deep subsurface of Europa.
The results for the Europan framework are also useful for studies involving other Solar System icy moons that present similar geological activity and planetary formation history, such as Enceladus30,41. The Cassini mission showed a local chemical disequilibrium and evidence of the existence of hydrothermal systems as well as possible radiolysis under the icy shell of Enceladus13. In addition, like Europa, it is possible that Enceladus hosts minerals such as pyrite30,42. Thus, this moon is also a propitious celestial body to host life43. In other words, the habitability question addressed here could provide an analogue application for Enceladus, which is another promising target for astrobiology studies.
Conclusions
Our results contribute to the evolving picture of Europa and other icy moons, such as Enceladus, as promising habitable environments. Sulfate production via γ-ray radiolysis was shown to be enough to supply the minimum energy required to maintain a considerable cell mass of the sulfate-reducing bacterium Ca. D. audaxviator used as a model organism. The cell quantity was shown to be comparable to that found in deep terrestrial environments if one assumes conditions similar to that in experimental work on radiolysis-produced sulfate. The total absence of sulfate sinks that could compete with a bacterial single-species ecosystem was assumed. However, as uranium also decays by α and β decay, the released energy should be greater than that calculated here, more water radiolysis would occur, and more pyrite would suffer oxidation. Therefore, this result can represent a lower limit for our sulfate-dependent ecosystem energy requirement. Otherwise, there would be no bacterial activity living in a subsurface environment such as that of the Mponeng gold mine. In fact, these and other deep and inhabited environments on Earth represent good analogues for Europa and could be further explored for this application, including serving as the basis for future space missions.
Our model for Europa can provide more energy than necessary to sustain the modeled microbial life even only by the gamma decay of 40K, since its abundance can be 10 times (or more) greater than that found in Earth’s oceans. This result makes Europa a propitious place for the development of an ecosystem that sustains forms of life such as the sulfate-reducing bacteria Candidatus Desulforudis audaxviator, although this estimate needs more constraints from experimental data. An important observation based on our results may be the relevance of the 40K concentration in the Europan ocean. As shown in Fig. 1, it creates a considerable difference in the range of the cell-carrying capacity based on the sulfate metabolism. The same dependency exists with pyrite grain size, and direct or indirect measurements of this characteristic are important to better constrain the model.
Electronic supplementary material
Acknowledgements
The authors thank FAPESP (project 2016/06114-6), CAPES and CNPq (project 424367/2016-5) for the financial support and the Research Unit in Astrobiology (NAP/Astrobio – PRP/USP) for the institutional support. MGBA also acknowledges FAPESP project 2011/23996-9 and FAPESP Thematic Project 2013/26258-4. The authors also thank Serrapilheira Project number G-1709-20205.
Author Contributions
T.A. reviewed and expanded the calculations and was responsible for the writing of the main body of the manuscript; M.G.B.A. was responsible for the first version of the calculations and manuscript writing and revision; F.R. contributed to the discussion of the physicochemical models, a discussion of the results and final review of the manuscript and figures; D.G. was responsible for the initial idea for the article, proposed the model for the reactions and the use of the terrestrial analogue for Europa, and contributed to the writing of the manuscript and discussion of the implication of the results.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/7/2020
The version of this Article previously published quoted an incorrect email address for Márcio Avellar. Correspondence and requests for materials should also be addressed to mgb.avellar@gmail.com. This has now been corrected in the HTML and PDF versions of the Article.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18470-z.
References
- 1.Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- 2.Russell MJ, et al. The Drive to Life on Wet and Icy Worlds. Astrobiology. 2014;14:308–343. doi: 10.1089/ast.2013.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hand KP, Carlson RW, Chyba CF. Energy, Chemical Disequilibrium, and Geological Constraints on Europa. Astrobiology. 2007;7:1006–1022. doi: 10.1089/ast.2007.0156. [DOI] [PubMed] [Google Scholar]
- 4.Blair CC, D’Hondt S, Spivack AJ, Kingsley RH. Radiolytic hydrogen and microbial respiration in subsurface sediments. Astrobiology. 2007;7:951–970. doi: 10.1089/ast.2007.0150. [DOI] [PubMed] [Google Scholar]
- 5.Cockell CS, et al. Habitability: A Review. Astrobiology. 2016;16:89–117. doi: 10.1089/ast.2015.1295. [DOI] [PubMed] [Google Scholar]
- 6.Vance S, et al. Hydrothermal systems in small ocean planets. Astrobiology. 2007;7:987–1005. doi: 10.1089/ast.2007.0075. [DOI] [PubMed] [Google Scholar]
- 7.Hoehler TM. An Energy Balance Concept for Habitability. Astrobiology. 2007;7:824–838. doi: 10.1089/ast.2006.0095. [DOI] [PubMed] [Google Scholar]
- 8.Chyba CF. PLANETARY SCIENCE: Enhanced: Life Without Photosynthesis. Science (80-.). 2001;292:2026–2027. doi: 10.1126/science.1060081. [DOI] [PubMed] [Google Scholar]
- 9.Zahnle K, Schenk P, Levison H, Dones L. Cratering rates in the outer solar system. Icarus. 2003;163:263–289. doi: 10.1016/S0019-1035(03)00048-4. [DOI] [Google Scholar]
- 10.Grasset O, et al. JUpiter ICy moons Explorer (JUICE): An ESA mission to orbit Ganymede and to characterise the Jupiter system. Planet. Space Sci. 2013;78:1–21. doi: 10.1016/j.pss.2012.12.002. [DOI] [Google Scholar]
- 11.Kargel J, et al. Europa’s Crust and Ocean: Origin, Composition, and the Prospects for Life. Icarus. 2000;148:226–265. doi: 10.1006/icar.2000.6471. [DOI] [Google Scholar]
- 12.McKay CP, Anbar AD, Porco C, Tsou P. Follow the Plume: The Habitability of Enceladus. Astrobiology. 2014;14:352–355. doi: 10.1089/ast.2014.1158. [DOI] [PubMed] [Google Scholar]
- 13.Waite, J. H. et al. Cassini finds molecular hydrogen in the Enceladus plume: Evidence for hydrothermal processes. 356, 155–159 (2017). [DOI] [PubMed]
- 14.Draganic, I. G. & Draganic, Z. D. The Radiation Chemistry of Water. 26 (1971).
- 15.Draganic, I. G. Radiolysis of water: a look at its origin and occurrence in the nature. 72, 181–186 (2005).
- 16.Pastina, B. & Laverne, J. A. Effect of Molecular Hydrogen on Hydrogen Peroxide in Water Radiolysis. 9316–9322 (2001).
- 17.Lefticariu L, Pratt LA, LaVerne JA, Schimmelmann A. Anoxic pyrite oxidation by water radiolysis products - A potential source of biosustaining energy. Earth Planet. Sci. Lett. 2010;292:57–67. doi: 10.1016/j.epsl.2010.01.020. [DOI] [Google Scholar]
- 18.Draganic IG, et al. 1983. Natural nuclear reactors and ionizing radiation in the Precambrian. Precambrian Res., 20: 283–298. 1983;20:283–298. doi: 10.1016/0301-9268(83)90077-3. [DOI] [Google Scholar]
- 19.Lin LH, et al. Radiolytic H2 in continental crust: Nuclear power for deep subsurface microbial communities. Geochemistry, Geophys. Geosystems. 2005;6:1–13. [Google Scholar]
- 20.Chivian D, et al. Environmental Genomics Reveals a Single-Species Ecosystem Deep Within Earth. Science (80-.). 2008;322:275–278. doi: 10.1126/science.1155495. [DOI] [PubMed] [Google Scholar]
- 21.Lin L-H, et al. Long-Term Sustainability of a High-Energy, Low-Diversity Crustal Biome. Science (80-.). 2006;314:479–482. doi: 10.1126/science.1127376. [DOI] [PubMed] [Google Scholar]
- 22.Dubessy, J. et al. Radiolysis evidenced by Hz-O2 and Hz-bearing fluid inclusions in three uranium deposits. 52 (1988).
- 23.Savary V, Pagel M. The effects of water radiolysis on local redox conditions in the Oklo, Gabon, natural fission reactors 10 and 16. Geochim. Cosmochim. Acta. 1997;61:4479–4494. doi: 10.1016/S0016-7037(97)00261-5. [DOI] [Google Scholar]
- 24.Atri, D. On the possibility of galactic cosmic ray-induced radiolysis-powered life in subsurface environments in the Universe. J. R. Soc. Interface13, 20160459 (2016). [DOI] [PMC free article] [PubMed]
- 25.Melott AL. A Possible Role for Stochastic Astrophysical Ionizing Radiation Events in the Systematic Disparity between Molecular and Fossil Dates. Astrobiology. 2017;17:87–90. doi: 10.1089/ast.2016.1527. [DOI] [PubMed] [Google Scholar]
- 26.Marinho F, Paulucci L, Galante D. Propagation and energy deposition of cosmic rays’ muons on terrestrial environments. Int. J. Astrobiol. 2014;13:1–5. doi: 10.1017/S1473550414000160. [DOI] [Google Scholar]
- 27.Bouquet A, Glein CR, Wyrick D, Waite JH. Alternative Energy: Production of H 2 by Radiolysis of Water in the Rocky Cores of Icy Bodies. Astrophys. J. 2017;840:L8. doi: 10.3847/2041-8213/aa6d56. [DOI] [Google Scholar]
- 28.Dzaugis ME, et al. Radiolytic Hydrogen Production in the Subseafloor Basaltic Aquifer. 2016;7:1–12. doi: 10.3389/fmicb.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onstott TC. Proc. SPIE. 1997. Deep gold mines of South Africa: windows into the subsurface biosphere; pp. 344–357. [Google Scholar]
- 30.Sohl F, et al. Subsurface water oceans on icy satellites: Chemical composition and exchange processes. Space Sci. Rev. 2010;153:485–510. doi: 10.1007/s11214-010-9646-y. [DOI] [Google Scholar]
- 31.Hazen RM, et al. Mineral evolution. Am. Mineral. 2008;93:1693–1720. doi: 10.2138/am.2008.2955. [DOI] [Google Scholar]
- 32.Pastina B, LaVerne JA. Effect of Molecular Hydrogen on Hydrogen Peroxide in Water Radiolysis. J. Phys. Chem. A. 2001;105:9316–9322. doi: 10.1021/jp012245j. [DOI] [Google Scholar]
- 33.Le Caër S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water. 2011;3:235–253. doi: 10.3390/w3010235. [DOI] [Google Scholar]
- 34.Draganić IG, Bjergbakke E, Draganić ZD, Sehested K. Decomposition of ocean waters by potassium-40 radiation 3800 Ma ago as a source of oxygen and oxidizing species. Precambrian Res. 1991;52:337–345. doi: 10.1016/0301-9268(91)90087-Q. [DOI] [Google Scholar]
- 35.Lide, D. R. CRC Handbook of Chemistry and Physics. eBook 3485 978-1466571143 (2003).
- 36.Wentworth CK. A scale of grade and class terms for clastic sediments. J. Geol. 1922;30:377–392. doi: 10.1086/622910. [DOI] [Google Scholar]
- 37.Marion GM, Fritsen CH, Eicken H, Payne MC. Factors, Potential Habitats, and Earth Analogues. Astrobiology. 2003;3:785–811. doi: 10.1089/153110703322736105. [DOI] [PubMed] [Google Scholar]
- 38.Dzaugis ME, Spivack AJ, Hondt SD. A quantitative model of water radiolysis and chemical production rates near radionuclide-containing solids x. Radiat. Phys. Chem. 2015;115:127–134. doi: 10.1016/j.radphyschem.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell MJ, Arndt NT. Geodynamic and metabolic cycles in the Hadean. Biogeosciences Discuss. 2005;2:97–111. doi: 10.5194/bg-2-97-2005. [DOI] [Google Scholar]
- 40.Pasek Ma, Greenberg R. Acidification of Europa’s subsurface ocean as a consequence of oxidant delivery. Astrobiology. 2012;12:151–9. doi: 10.1089/ast.2011.0666. [DOI] [PubMed] [Google Scholar]
- 41.Čadek O, et al. Enceladus’s internal ocean and ice shell constrained from Cassini gravity, shape, and libration data. Geophys. Res. Lett. 2016;43:5653–5660. doi: 10.1002/2016GL068634. [DOI] [Google Scholar]
- 42.Zolotov MY. An oceanic composition on early and today’s Enceladus. Geophys. Res. Lett. 2007;34:1–5. doi: 10.1029/2007GL031234. [DOI] [Google Scholar]
- 43.McKay CP, Porco CC, Altheide T, Davis WL, Kral TA. The Possible Origin and Persistence of Life on Enceladus and Detection of Biomarkers in the Plume. Astrobiology. 2008;8:909–919. doi: 10.1089/ast.2008.0265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.