Abstract
Embodied theories of emotion assume that emotional processing is grounded in bodily and affective processes. Accordingly, the perception of an emotion re-enacts congruent sensory and affective states; and conversely, bodily states congruent with a specific emotion facilitate emotional processing. This study tests whether the ability to process facial expressions (faces having a neutral expression, expressing fear, or disgust) can be influenced by making the participants’ body state congruent with the expressed emotion (e.g., high heart rate in the case of faces expressing fear). We designed a task requiring participants to categorize pictures of male and female faces that either had a neutral expression (neutral), or expressed emotions whose linkage with high heart rate is strong (fear) or significantly weaker or absent (disgust). Critically, participants were tested in two conditions: with experimentally induced high heart rate (Exercise) and with normal heart rate (Normal). Participants processed fearful faces (but not disgusted or neutral faces) faster when they were in the Exercise condition than in the Normal condition. These results support the idea that an emotionally congruent body state facilitates the automatic processing of emotionally-charged stimuli and this effect is emotion-specific rather than due to generic factors such as arousal.
Introduction
Embodied theories of emotion propose that emotional processing is grounded in situated conceptualizations and the re-enactment of the same affective, sensory and motor states that are activated when the emotion is experienced1–3. In this perspective, shared brain and bodily resources support the feeling of an emotion, its display, the ability to perceive the emotion when displayed by others, and to perform other related tasks such as processing emotional language, images or faces. Support for this view comes, for example, by evidence showing that the perception of emotion involves areas for feeling the same emotions4. This and other studies show that a key role in emotional processing is played by neuronal structures that receive interoceptive feedback on the physiological condition of the body such as the insular cortex, which is considered a key locus of subjective feelings5–10 and which is important for adaptive regulation of energy and allostasis11.
However, all these studies measure central nervous representations of body state, not physiological aspects of body state. It is less clear which role the body itself plays in emotional processing. More than one century ago, William James argued that physiological changes are able to cause specific emotions rather than being their consequences12,13. The so-called James-Lange hypothesis has generated controversy and has been re-proposed and extended multiple times. For example, Damasio & Carvalho14 suggested that “changes in body state cause automatic physiological reactions as well as mental experiences — feelings — such as hunger, thirst, pain or fear” (p. 143). Along similar lines, Craig15 suggested that changes in body state trigger an interoceptive process that gives rise to feelings and emotions in cortical brain areas such as the insula. This theory offers an integrative perspective on emotional processing, which emphasizes a circular causality between somatic/physiological processes and central representations of these processes.
This latter theoretical perspective links well to anatomical evidence that interoceptive afferent fibers monitor the state of all body organs, and report interoceptive signals to cortical structures, thus producing a central representation of homeostatic state and the physiological condition of body tissues, including behaviourally relevant parameters such as bodily temperature and pain5,15,16. Importantly, this process culminates in the insula, which is in an excellent position to integrate multimodal information about motivationally salient, hedonic and cognitive/social signals from other parts of the brain, and to use this multimodal information to exert control over the autonomic system it monitors (aka allostasis11). In this framework, emotional experiences and feeling states would be associated to the functioning of the cortical system that monitors (and controls) the physiological and homeostatic condition of the body, and in particular to the anterior insula5. Support for this idea comes from studies that imply the insula in the processing and awareness of various emotions and feelings9,17–19. Hence, in this framework, emotional processing - e.g., feeling an emotion, perceiving or recognizing an emotion - would be inextricably linked (and permeable to) bodily signals as monitored by interoceptive streams.
The aforementioned perspective on allostasis and emotional processing has been recently cast in more formal terms, by appealing to the idea that the brain uses interoceptive signals to implement a form of interoceptive or embodied inference, in the same way it uses sensory signals to implement perceptual inference20–24. Interoceptive inference exploits bottom-up (afferent) and top-down (efferent) interoceptive dynamics in order to maintain a central estimate of physiological and homeostatic parameters of the body, and to take corrective actions (e.g., trigger autonomic reflexes) to control these parameters if they are, or are expected to be, outside safe ranges - thus complying to the overall imperative of minimizing prediction error (or free energy), which is crucial for the survival of the organism25–27. Importantly, the internal estimate of bodily parameters formed during this process (and culminating in the insula) would also constitute part and parcel of emotional experience. This perspective is thus coherent with the idea that emotional experience and feelings derive from central representations of the physiological condition of the body and of changes in bodily state6. If this hypothesis holds, manipulating interoceptive signals by changing body state should influence emotional processing. Furthermore, this change should be specific; for example, inducing a body state that is congruent with a given emotion (e.g., high heart rate for fear) should facilitate the processing of that emotion, but not of other emotions that are not (or significantly less strongly) associated to the same bodily states (e.g., disgust).
The aim of this study is testing if the ability to process other’s facial expressions (faces expressing fear vs. disgust or neutral faces) can be influenced by manipulating the participants’ heart rate to make it “emotionally congruent” with the observed facial expression (i.e., higher heart rate for faces expressing fear vs. disgust or neutral faces). In keeping with the view that emotional processing is an embodied process, we hypothesize that participants with (experimentally manipulated) increased heart rate are facilitated in processing faces expressing congruent physiological/emotional state (in our study, fear, which is physiologically associated with high heart rate). Comparing one emotion that is strongly associated with high heart rate (fear) and one emotion that is weakly or not associated with it (disgust) will permit us to assess whether a facilitatory effect is specific to a body state (and its associated interoceptive signals) or is due to a more generic process that results from physical activity such as arousal - intended here as a generic state of physical/mental alertness and readiness to move or to process stimuli, which is not univocally associated to a particular emotion28.
To test whether our “embodiment of fear” hypothesis holds, we designed an experiment in which participants were tested into two conditions: an Exercise condition in which they performed physical exercise before the experiment, causing an acceleration of heart rate; and a Normal condition, in which they performed no physical exercise before the experiment. Participants were presented with pictures of male and female faces with fear, disgust or neutral expressions and performed a gender categorization task. Our central hypothesis was that high heart rate (induced by physical exercise) would have a facilitatory effect on the processing of fearful faces (because fear is congruent with high heart rate) but not of disgusted or neutral faces.
We follow a consolidated tradition, especially in the human neuroimaging literature, of studying emotional processing by using incidental tasks - and especially gender categorization tasks - whose demands are orthogonal to the real goals of the experiment, rather than by asking participants to process emotional content explicitly29–31, in order to avoid confounds with other (e.g., metacognitive) processes or methodological problems such as demand biases32. Studies comparing direct and incidental processing of emotional content have validated this methodology by showing that both tap emotion-related brain circuits33–35. The argument underlying our incidental task is that, if high heart rate increases the sensitivity to fear-related stimuli, it will influence both emotional and non-emotional judgments. Measuring participants’ speed and uncertainty during gender categorization would thus offer an unbiased window over our variable of interest: namely, the congruency between their body state and different emotions displayed during the incidental task. In other words, using an incidental task would provide a strong proof of automatic influences of physiological state on emotional processing (and excitation transfer 36) that are unconfounded by other, more explicit (e.g., metacognitive) processes or explicit attention regulation37.
Subjects provided their responses by moving a computer mouse on the selected category. Recent studies using mouse movements (or other continuous kinematic measures) permitted to shed light into the dynamic properties of the moment-to-moment decision process and have been applied to a number of paradigms, such as numerical and color comparisons, categorization of ambiguous figures, and semantic categorization38–46. Similarly, in this study we collected two kinematic measures in order to reveal the online dynamics of the decision process, and in particular how the choice uncertainty is reflected in the trajectory curvature47,48. In particular, we focused on two kinematic measures: Maximum Deviation (MD) and Area Under the Curve (AUC). MD is the length of a perpendicular line between the idealized straight-line trajectory and the farthest point from that straight-line in the observed trajectory. AUC is the geometric area between the observed mouse-trajectory and an idealized straight-line trajectory drawn from the start and end points. Both measures assess the degree of attraction toward an unselected response49. We expect faster responses and more direct mouse trajectories (assessed by MD and/or AUC) when the presented stimulus was congruent with the body state of the participant (e.g. fearful faces after exercise) compared to incongruent situation (e.g. neutral faces or with expression of disgust after exercise).
Methods
A group of 24 male students of the G. D’Annunzio University of Chieti (Italy) with ages ranged from 20 to 26 years were recruited for participation. Participants were members of the same cultural group (i.e., Caucasian). All were right-handed with normal or corrected to normal vision. Our sample was composed only by male subjects to avoid confounds due to gender related factors (for example, many studies have found women to be more emotionally responsive than men, particularly when processing facial expressions50–52). Informed consent was obtained from each participant and the study protocol conforms to the ethical guidelines of the Declaration of Helsinki (BMJ 1991; 302; 1194) as reflected in prior approval by the Institution’s human research committee (ISTC-CNR, Rome - N.0003971/04/12/2015).
Experimental stimuli comprised 120 pictures of male or female faces, varied for facial expression (neutral, disgust and fear). We used 60 male faces (20 neutral, 20 fearful, 20 disgust emotional expressions) and 60 female faces (20 neutral, 20 fearful, 20 disgust emotional expressions). The same facial identities were used to depict each of the three expressions. The stimuli used came from the Karolinska Directed Emotional Faces (KDEF) database. Color images were transformed to 256 gray-level scale. We then normalized the luminance of the images and applied a Hann window in order to remove the hair and the peripheral information of the faces53–55. This was done in order to avoid a categorization of the gender based on the hair of the faces56. Different combinations of facial expression produce the following categories: female neutral, female fear, female disgust, male neutral, male fear, and male disgust.
On each of the 120 trials, participants clicked on the/START/button located at the bottom-center of the PC screen. Then a face appeared centrally and, in order to perform the gender categorization task, participants were asked to click on the ‘male’ or ‘female’ response in the top-left and top-right corner of the screen. Rightward and leftward responses were counterbalanced across participants. A time deadline of 1400 ms was used, and responses exceeding it resulted in the appearance of a ‘time out’ message. Trials were presented in two blocks with a pause in between. A practice session of 8 trials familiarized the participants with the procedure. During participants’ responses, the x and y coordinates of the mouse trajectories were recorded (sampling rate of approximately 70 Hz) using the MouseTracker software47.
All participants (n. 24) performed the experiment in two conditions: in the Exercise condition they were asked to perform physical exercise (i.e., stepper) for 3 minutes, at their own rhythm, before the actual experiment, and during the pause between the experimental blocks. This procedure permitted to control that the heartbeat of participants of the Exercise condition did not come down to a normal rhythm during the execution of the experimental task (see below). In the Normal condition, participants performed the experiment without any preceding exercise. Rather, before the experiment and after each experimental block, they were asked to relax on their chair for 3 minutes (in order to make the timing of two conditions as similar as possible). The order of conditions was counterbalanced: 12 subjects performed the experiment in the Exercise condition first, and in the Normal condition one week later, at approximately the same hour of the day; the other 12 subjects followed the opposite order. The position of the two response buttons (male and female) was counterbalanced, too.
During the experiment, a Polar RS800CX (which records HR beat by beat at +/−1 ms) was used to record variations in participants’ Heart Rate (HR). The experimenter placed new ECG electrodes to measure heart rate before and during each block of the experiment. Participants in the Exercise condition have average heart rate of 118 ± 8 bpm during the experiment. Participants in the Normal condition have average rate of 71 ± 8 bpm.
The dependent variables were participants’ overall response time (RT), two kinematic parameters of movement (velocity and acceleration peaks) and three parameters that measure movement trajectories during the response: Area Under the Curve (AUC) and Maximum Deviation (MD) of the trajectories. Following standard procedures used in MouseTracker studies, response trajectories were first rescaled into a standard coordinate space, and the duration of the movements were normalized by re-sampling the time vector into 101 time-steps using linear interpolation to allow averaging across multiple trials47. Responses exceeding the time deadline (0.47% of the total data), with standard deviations (SD) greater than 3 times average of reaction time (5.4%) and incorrect categorization (11% of trials) were discarded from analysis.
Linear mixed-effects models (LMMs)57 were used to analysed response time data, with the ‘lmerTest’ package58. Backward elimination of non-significant effects was performed with the step function (backward elimination of the random part is performed first, followed by backward elimination of the fixed part. Finally, least squares means and their differences for the fixed part of the model are calculated. The p-values for the fixed effects are calculated from an F test and t test based on Sattethwaite’s approximation). The model included random intercept for Subjects and Items, with maximal by-subject random structure as a baseline model, and Facial Expression (Neutral, Disgust, Fear) and Session (Exercise, Normal) as fixed effects.
Results
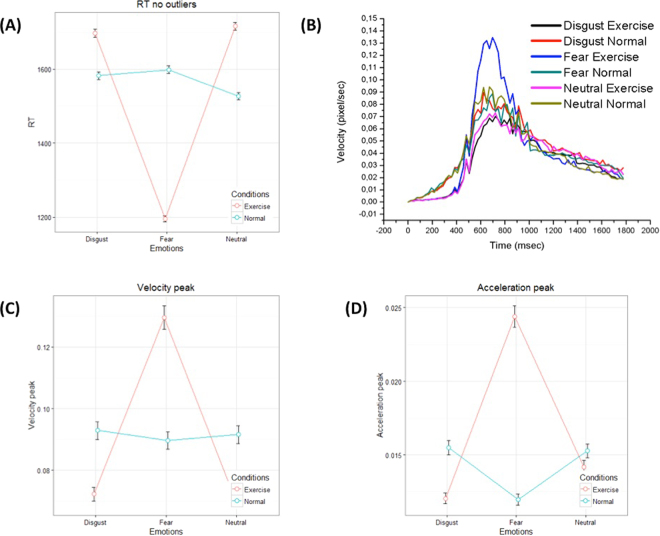
About 3.7% of total data points were removed as response outliers (identified with the Turkey’s method, which identifies the outliers ranged above and below the 1.5* interquartile range of response time). The analysis of correct response time showed a significant main effect of Facial Expression (F (2, 22.12) = 213.3, p-value < 0.001), and a significant Facial Expression by Session interaction (F (2, 8229.59) = 783.41, p-value < 0.001); see Fig. 1A. The main effect of Session was kept in the model but was not significant (F (1, 22.93) < 1, ns). A significant difference emerged between Fear Exercise and Fear Neutral (estimate of differences in LSMEANS = −408.2, SE = 41.7, DF = 25.6, t-value = −8.76, p-value < 0.001), with faster response to fearful faces from the Exercise group. Differences among groups emerged also for neutral and disgust-related faces, but in the opposite direction, that is, slower responses for the Exercise group (estimate of differences in LSMEANSNeutral = 206.6, SE = 41.7, DF = 25.7, t-value = −1.09, p-value < 0.001; estimate of differences in LSMEANSDisgust = 132.6, SE = 41.8, DF = 25.8, t-value = 3.18, p-value < 0.005, respectively).
Figure 1.
Mouse tracking kinematic data. (A) Correct response time as a function of Session (Exercise, Normal) by Facial Expression (Neutral, Disgust, Fear). Error bars depict Standard Error of the Mean. (B) X-velocity profile of the mouse movements of participants processing faces with disgust (exercise, black; rest orange), fear (exercise, violet; rest, green) and neutral (exercise, light violet; rest, ochre) expressions. (C,D) Velocity peaks (C) and acceleration peaks (D) of mouse movements as a function of Session (Exercise, Normal) by Facial Expression (Neutral, Disgust, Fear). Peaks are reported in Table 1.
The analysis of velocity and acceleration peaks in the x-axis during the movement (see Table 1 and Fig. 1) shows almost the same pattern as reaction time. Analysis of velocity peak data showed a significant main effect of Facial Expression (F(2, 8528.93) = 75.29, p-value < 0.001) and the interaction Session by Facial Expression (F(2, 8469.2) = 89.51, p-value < 0.001); see Fig. 1B,C. Main effect of Session was not significant (F(1, 23) < 1, ns). A significant difference emerged between Fear Exercise and Fear Neutral (SE = 0.014, DF = 25.3, t-value = 2.88, p-value < 0.05), with a higher peak of velocity in the response to fearful faces from the Exercise group. No other differences were significant (SENeutral = 0.014 DF = 25.3, t-value = −1.5, ns; = 132.6; SEDisgust = 0.014, DF = 25.3, t-value = −1.48, ns).
Table 1.
Velocity and acceleration peaks. Velocity peak is calculated as the maximal value in the velocity profile, in the x-axis. Acceleration peak is calculated as the maximal value in the acceleration profile. Peak latencies were defined as the time elapsed between movement onset and maximum peak amplitude.
| Velocity Peak | Acceleration Peak | |||
|---|---|---|---|---|
| Time (ms) | Mean amplitude (pixel/sec) | Time (ms) | Mean amplitude (pixel/sec) | |
| Neutral rest | 672 | 0,09414 | 625–648 | 0,01675 |
| Neutral Exercise | 720 | 0,07301 | 553–576 | 0,01464 |
| Fear Rest | 696 | 0,08862 | 553–576 | 0,01174 |
| Fear Exercise | 696 | 0,13451 | 553–576 | 0,02442 |
| Disgust Rest | 672 | 0,08962 | 625–648 | 0,01884 |
| Disgust Exercise | 720 | 0,07014 | 553–576 | 0,01085 |
The analysis of acceleration peak showed significant effect of Facial Expression (F(2,8588.7) = 53.29, p-value < 0.001) and interaction (F(1,8588.7) = 181.53, p-value < 0.001); the main effect of Session was not significant (F(1,23) = 1.37, ns); see Fig. 1D. Like in the analysis of velocity peak, the analysis reveals a higher acceleration in the Exercise session with fearful faces compared to neutral faces (SE = 0.0006, DF = 25.5, t-value = 5.38, p-value < 0.001). No differences emerged with disgust-related faces (SE = 0.0023, DF = 25.5, t-value = −1.49, ns) or neutral faces (SE = 0.0023, DF = 25.5, t-value = −0.47, ns).
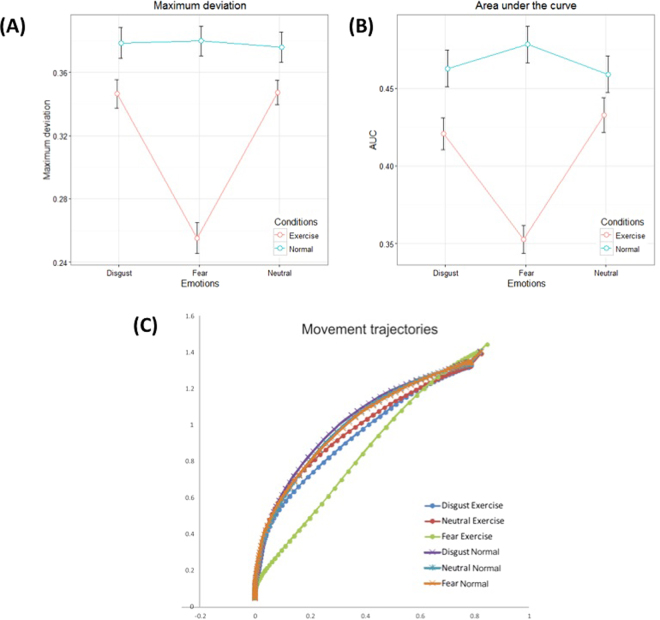
On maximum deviation (MD), we found a significant main effect of Session (F(1,23) = 9.47, p-value < 0.05), Facial Expression (F(2,8572.17) = 15.54, p-value < 0.001), and their interaction (F(2,8539) = 18.565, p-value < 0.001). Significant differences of LSMEANS emerged between Exercise vs. Normal sessions with fear-related faces (SE = 0.0226, DF = 37, t-value = −5.54, p-value < 0.001). No differences emerged with disgust-related faces (SE = 0.0226, DF = 37.1, t-value = −1.41, ns) or neutral faces (SE = 0.0226, DF = 37, t-value = −1.25, ns), see Fig. 2A.
Figure 2.
Mouse tracking trajectory data. (A) Maximum deviation MD and (B) Area Under the Curve AUC as a function of Session (Exercise vs. Normal) by Facial Expression (Neutral, Disgust, Fear) interaction. Error bars depict Standard Error of the Mean. (C) Average spatial trajectories of the responses of disgust (Exercise, blue; Normal, violet), neutral (Exercise, red; Normal, light blue) and fear (Exercise, green; Normal, orange).
A similar patter of results emerged on the measure of area under the curve (AUC), with significant fixed-effect of Session (F(1,8431.98) = 56.93, p-value < 0.001), Facial Expression (F(2,8600.56) = 3.72, p-value < 0.05) and interaction (F(2,8601.27) = 12.48, p-value < 0.001). Significant differences of LSMEANS emerged between Exercise vs. Normal sessions in fear-related faces (SE = 0.0150, DF = 8582.3, t-value = −8.37, p-value < 0.001), and to a lesser extent, disgust-related faces (SE = 0.0150, DF = 8588.3, t-value = −2.77, p-value < 0.05). No differences emerged with neutral faces (SE = 0.0149, DF = 8556.5, t-value = −1.81, ns); see Fig. 2B. See also Fig. 2C for an illustration of average mouse trajectories during the task.
We conducted an additional analysis to assess if the effects we found were gender-specific, i.e., specific for male versus female faces. In the analysis of reaction time, there was no significant effect of gender (F(1, 24.23) < 1, ns), no interaction condition by gender (F(1,8160.8) < 1, ns) or facial expression by gender (F(2,8153) < 1, ns). The same pattern emerged also for peak velocity (Gender: F(1, 8532.21) = 1.23, ns; Condition by Gender: F(1,8564.82) < 1, ns; Facial Expression by Gender: F(2,8542.54) = 1.45, ns) and peak acceleration (Gender: F(1, 8582.7) = 1.33, ns; Condition by Gender: F(1,8582.7) < 1, ns; Facial Expression by Gender: F(2,8582.7) = 1.86, ns).
Discussion
Our results indicate that participants whose heart rate was experimentally manipulated (raised) recognized faster faces expressing fear - an emotion that is strongly congruent with high heart rate - compared to faces expressing disgust - an emotion having significantly weaker linkage (or no linkage) with high heart rate - or neutral faces. This conclusion is supported by convergent evidence obtained by measures of reaction time and peak velocity and acceleration during the response. Furthermore, two measures of choice uncertainty during the choice, maximum deviation (MD) and area under the curve (AUC), support the conclusion that participants whose heart rate is higher are not only faster in recognizing faces expressing fear compared to a control group, but also less uncertain during the choice. In sum, these results indicate that a rather mundane manipulation of physiological and interoceptive state is sufficient to produce an “embodied congruency” effect between body and interoceptive state (high heart rate) and stimuli charged with congruent emotional content (faces expressing fear).
Importantly, the facilitation in perceptual processing was only evident for faces expressing fear, but not faces expressing disgust or neutral faces, highlighting the specificity of the effect. Reassuringly, our results are coherent across different measures: reaction time, kinematic measures of peak velocity and acceleration, and measures of maximum spatial deviation from an ideal straight trajectory and area under the curve of this deviation – where the two latter measures are associated with choice uncertainty. This coherent pattern of results indicates that the facilitating effect of high heart rate depends on its congruency with the emotion of fear, not on more generic factors (e.g., a generic emotional or arousal state that may facilitate the recognition of all emotional stimuli, or speed up overall response time independent of the stimuli). This study thus replicates and extends previous findings that facial expressions and body arousal can influence the recognition of emotionally charged stimuli28,59–64, highlighting the importance of congruent bodily states. A further indication of that the Exercise condition did not produce a generic facilitatory effect comes from the fact that participants in this condition were generally slower (not faster) in recognizing disgusted and neutral faces. At the same time, the Exercise condition determined trajectories that deviated less from an ideal trajectory - although this effect was only significant for fearful faces in the case of maximum deviation, and more pronounced for fearful compared to disgusted faces in the case of area under the curve (see also Fig. 2C). A careful analysis of the velocity profiles of the spatial trajectories (Fig. 1B) helps understanding this pattern of results. As shown in Fig. 1B, mouse movements were initially slower in the Exercise condition, which would in general increase reaction time but produce less choice uncertainty65. A slower initial phase of movement would thus explain why we observed both slower reaction times and decreased choice uncertainty in the Exercise condition. Figure 1B shows that when participants in the Exercise condition processed fearful faces, their velocity peak was higher - which explains their faster reaction time. Interestingly, in this case latter decrease in uncertainty goes hand in hand with faster (not slower) reaction time, which is the hallmark of a facilitatory effect.
The fact that the processing of fearful faces is only facilitated in the Exercise (but not the Normal) condition rules out the possibility that fearful faces where easier to discriminate than disgusted or neutral faces. This comparison allows us to rule out other possible alternative explanations, too, such as the possibility that showing people fearful faces could in itself increase heart rate, which in turn could enhance their processing. If that was the case, we should have observed faster processing of fearful faces in both Exercise and Normal conditions; but we do not. This pattern of results thus indicates that the facilitatory effect was due to congruence between emotional stimuli and experimentally-manipulated bodily state, not to the mere presence of an emotionally-charged stimulus (e.g. a fearful face).
Finally, the fact that the facilitation was present in an incidental cognitive task (a gender categorization task) that did not require subjects to evaluate explicitly the emotional content of the stimuli suggests that it rests on low-level inferential mechanisms rather than higher (e.g., metacognitive) processing stages of emotional content - which is in keeping with the results of other recent experiments29–31. Our study adds to this body of work by shedding light on the possible interactions between physiological state and the processing of emotional stimuli. A widespread debate concerns the “direction of causality” between emotional and bodily processes: does the emotion of fear elicit high heart rate, or does high heart rate elicit emotion - or both? The idea that changes in bodily state are part and parcel of emotional processing can be traced back at least to the James-Lange hypothesis 12,13 and is key to many modern theories of embodied emotion and interoception14,15,66. One central claim of this approach is that changes in body state can produce emotional states and thus plausibly influence emotional processing at large. Our results support this view, by showing that the modification of the physiological state of the participants’ bodies (increasing heart rate) facilitated the implicit processing of congruent stimuli (faces expressing fear) but not of other emotional (disgust) or neutral stimuli. While changes in arousal measures during emotional processing have been extensively reported67 here we report the reverse relation: manipulating the subjects’ body state can influence emotional processing, even in an incidental task.
The empirical basis for an association of fear and high heart rate is large, and several studies report unchanged or even lowered heart rate during the processing of disgusting stimuli68–71. However, a case has been made also for a linkage between disgust and increased heart rate under specific circumstances72. Specifically, the literature distinguishes between two disgust-related body states - core disgust (related to, e.g., food contamination) and body-boundary violation (BBV) disgust (related to, e.g., mutilation and blood) - and a case has been made that HR may remain stable or sometimes increase in the former (core disgust) case and decrease in the latter (BBV) case72. Interestingly, core and BBV disgust also correspond to partially different facial expressions73. More than 63% (38 out of 60) of the stimuli in our study show the most characteristic features of faces expressing BBV disgust (upper lip retraction), while no stimuli in our study show the two most characteristic features of faces expressing core disgust (mouth gape or tongue protrusion)73. According to the taxonomy of 73, our stimuli thus largely fall within the sub-category of facial expressions related to BBV disgust, for which there is no potential confound with high heart rate. Finally, and most importantly in this context, there is ample evidence from studies comparing fear and disgust that heart rate is significantly higher in the former case, hence providing a rationale for our comparison71,74,75. In other words, even if one places fear- and disgust-related stimuli along a continuum (in relation to high heart rate), our experimental manipulation would be expected to affect the processing of fear-related stimuli significantly more than disgust-related stimuli - which is exactly what we report.
The findings reported in this paper may be also explained by assuming that energy expenditure (i.e., exercise) leads to quicker processing of facial expressions related to resource and behavioral mobilization. Since exercising is an act of resource expenditure and mobilization (similar to “running away”), perceivers who performed an exercise very recently may be able to more quickly perceive facial expressions of fear, which (compared to disgust or neutral expressions) signal a threat in the environment and the necessity of bodily action. This explanation is not incoherent with our arguments, if one considers that the plausible evolutionary basis for high heart rate in fear processing is exactly the fast mobilization of resources in case of threat; and that the embodiment of fear plausibly encompasses a general state of bodily resources mobilizations, not just heart rate acceleration. In other words, while this alternative explanation highlights the link between energy expenditure (i.e., exercise) and fear processing rather than between high heart rate and fear processing, all these phenomena are tightly linked at the physiological level (e.g., exercise causes high heart rate) and from an adaptive viewpoint.
This study adds to a large body of literature on emotional processing and its embodiment. This body of literature is sometimes fragmented or even contradictory; indeed, despite its pervasiveness in human life, emotion has revealed to be an elusive phenomenon to define and to assess empirically14,76. One recurrent question in this literature is whether it is possible to find emotion-specific patterns in peripheral autonomic and central neural responses77,78. A meta-analysis of neuroimaging studies indicates that consistent neural correlates of specific emotions (e.g., happiness and sadness) can be found across studies79. Furthermore, a recent study highlights that it is possible to clearly dissociate two disgust forms by looking at their distinct patterns of autonomic and central responses66; and another study reports that fearful faces were detected more easily and were rated as more intense when the timing of their presentation was congruent to the subjects’ individual heartbeats80. At the same time, there have been some failed attempts to induce specific emotional states via electrical stimulation81, and this has lead to the suggestion that several other dimensions such as social aspects should be considered to fully understand emotional processing, besides primary emotion circuits. Embodied theories of cognition have the potential to play an integrative role, by suggesting that emotion is a multifarious phenomenon and it links to a multitude of situational, affective, perceptual and bodily states, all of which play a role in emotional processing1–3. In this perspective, emotional processing can be conceptualized as an embodied simulation that elicits modal circuits for sensory and affective states that are active whenever an emotion is experienced - consistent with the general claim of embodied theories that cognition is based on embodied simulation and the re-enactment of modal brain circuits3,82–87.
This claim has been addressed by studies that manipulated proprioceptive and motor circuits (e.g., face muscles) to make them more or less congruent with emotional stimuli (e.g., faces expressing emotions) and which showed an influence of peripheral events (subjects’ facial expressions) on emotional processing59–64,88,89. At difference with those studies, which focused on muscular movements and motor (or proprioceptive) channels, here we targeted interoceptive channels - which are increasingly recognized to be key to emotional experience, feeling, and consciousness8,14,20,22,23,90. Our study thus constitutes a more direct test of the idea that bodily state influences emotional processing through interoceptive channels. A possible explanation of the relations between bodily state and the perception of emotional content is offered by theories of emotion resonance 91. In this perspective, feeling an emotion can influence emotion perception via direct, resonant mechanisms in the brain, much like performing an action influences action perception92,93. An alternative hypothesis stems from recent theories of interoceptive predictive coding 22,27,94, embodied predictive coding 21,26,95 and Embodied Predictive Interoception Coding (EPIC) 20,96, all of which describe the processing of interoceptive events, or of combined perceptual and interoceptive events, in terms of surprise (or free energy) minimization25. For example, the theory of embodied predictive coding21 suggests that perceptual inference combines sensory and interoceptive streams of evidence - more specifically, it tries to simultaneously minimize prediction errors between hypotheses and sensations in both streams - and so it is considerably faster when the emotional state of the participant is congruent with that of the to-be-recognized figure (as the prediction error to be minimized is smaller). The error-minimization mechanism implicit in predictive coding and interoceptive inference is analogous to a situated simulation of emotionally charged events, when the current bodily state is considered as part of the overall experience to be re-enacted during perceptual processing3. The specific hypotheses proposed here remain to be tested empirically; however, they all highlight the importance of fusing information processing streams in “the brain” and “the (rest of the) body” and all assign the body a central stage in perceptual and conceptual processing, as James and Lange hypothesized long time ago.
This result also links to a body of literature showing bidirectional interactions between “emotion” and “cognition”; for example, a recent meta-analysis discusses several studies where emotions (e.g., happiness and sadness) were elicited that caused correlated changes in behavior, experience, and physiology97. However, it is less clear what is the adaptive value of these interactions; for example, several so-called “dual theories” challenge that so-called “emotional” (or “hot”) forms of processing can be deleterious for an otherwise supposedly “rational” (or “cold”) cognition98. Contrary to the idea that emotional processing makes so-called “rational” behaviour less efficacious, several authors have argued that these bidirectional interactions can have adaptive value76,99,100. In this vein, it is possible to speculate that increased arousal and faster emotional processing revealed in our study can be linked to the adaptive value of responding faster to dangerous situations. This idea is in keeping with recent studies showing that also perception is modulated by valence; for example, threatening objects such as spiders appear to move faster101 and be closer and larger102 than non-threatening objects; see also103. Taken together, this body of evidence suggests that both the exteroceptive (sensory) and interoceptive (including affect-related) dimensions are not neutral but tuned to facilitate action and a cost-benefit analysis of the situation104,105.
Conclusion
The ways emotional and perceptual processing interact have always fascinated scholars and laymen, but our current understanding of these problems is still incomplete. In the current experiment, we asked whether inducing a physiological state that was congruent with to-be-recognized emotional stimuli facilitated their processing. Participants in the high-heart-rate group (Exercise condition) showed a faster gender categorization of faces depicting emotional expressions that were congruent with their body state (fearful faces) but not of faces depicting incongruent emotional expressions (disgusted faces) or of neutral faces. In keeping with our main hypothesis, these results suggest that the induction of an emotionally congruent bodily state influences the perception of other’s congruent facial expressions, even in the case of an incidental cognitive task. These results thus provide support for embodied theories of emotion, which suggest that bodily processes are part and parcel of emotional processing and not just irrelevant byproducts – or, in other words, that somatic and interoceptive states such as high heart rate participate in the embodiment of fear.
Acknowledgements
Research funded by the EU’s FP7 under grant agreement no FP7-ICT-270108 (Goal-Leaders) to GP and the LabEx Project (IMOBS3) to GP, LB, and MM, and a grant from the Institut Universitaire de France to MM.
Author Contributions
G. Pezzulo developed the study concept. G. Pezzulo, L. Barca and M. Mermillod provided an initial design of the study, which all the authors then contributed to revise and finalize. M. Mermillod designed the experimental stimuli. P. Iodice, P. Chausse and S. Monceau performed the piloting, testing and data collection. P. Iodice and L. Barca performed the data analysis. G. Pezzulo and M. Mermillod wrote the initial draft of the manuscript. All the authors revised the manuscript and approved the final version for submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson-Mendenhall CD, Barrett LF, Barsalou LW. Neural evidence that human emotions share core affective properties. Psychol. Sci. 2013;24:947–956. doi: 10.1177/0956797612464242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson-Mendenhall, C. D., Barrett, L. F. & Barsalou, L. W. Situating emotional experience. Front. Hum. Neurosci. 7 (2013). [DOI] [PMC free article] [PubMed]
- 3.Wilson-Mendenhall CD, Barrett LF, Simmons WK, Barsalou LW. Grounding emotion in situated conceptualization. Neuropsychologia. 2011;49:1105–1127. doi: 10.1016/j.neuropsychologia.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botvinick M, et al. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 5.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 7.Craig ADB. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 8.Craig ADB. The sentient self. Brain Struct. Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 9.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 10.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Sterling P. Allostasis: a model of predictive regulation. Physiol. Behav. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 12.James, W. The Principles of Psychology. (Dover Publications, 1890).
- 13.James, W. What is an emotion? Mind os-IX 188–205 (1884).
- 14.Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 15.Craig, A. D. How do you feel?: An interoceptive moment with your neurobiological self. (Princeton University Press, 2015).
- 16.Andrew D, Craig AD. Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J. Physiol. 2001;537:489–495. doi: 10.1111/j.1469-7793.2001.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damasio, A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. (Harvest Books, 2000).
- 18.Phillips ML, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 19.Damasio AR, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 20.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezzulo, G. Why do you fear the Bogeyman? An embodied predictive coding model of perceptual inference. Cognitive, Affective, and Behavioral Neuroscience (2013). [DOI] [PubMed]
- 22.Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Seth, A. K., Suzuki, K. & Critchley, H. D. An Interoceptive Predictive Coding Model of Conscious Presence. Front. Psychol. 2 (2012). [DOI] [PMC free article] [PubMed]
- 24.Gu X, FitzGerald TH. Interoceptive inference: homeostasis and decision-making. Trends Cogn Sci. 2014;18:269–270. doi: 10.1016/j.tics.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 26.Pezzulo G, Rigoli F, Friston KJ. Active Inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 2015;136:17–35. doi: 10.1016/j.pneurobio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seth AK, Friston KJ. Active interoceptive inference and the emotional brain. Phil Trans R Soc B. 2016;371:20160007. doi: 10.1098/rstb.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kever A, et al. The body language: The spontaneous influence of congruent bodily arousal on the awareness of emotional words. J. Exp. Psychol. Hum. Percept. Perform. 2015;41:582. doi: 10.1037/xhp0000055. [DOI] [PubMed] [Google Scholar]
- 29.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat. Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- 30.Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: time course and topographic evoked-potentials mapping. Hum. Brain Mapp. 2005;26:65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFadyen J, Mermillod M, Mattingley JB, Halász V, Garrido MI. A Rapid Subcortical Amygdala Route for Faces Irrespective of Spatial Frequency and Emotion. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:3864–3874. doi: 10.1523/JNEUROSCI.3525-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orne MT. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. Am. Psychol. 1962;17:776. doi: 10.1037/h0043424. [DOI] [Google Scholar]
- 33.Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage. 2003;20:84–97. doi: 10.1016/S1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 34.Critchley H, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum. Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorno-Tempini ML, et al. Explicit and Incidental Facial Expression Processing: An fMRI Study. NeuroImage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 36.Oosterwijk S, et al. States of mind: Emotions, body feelings, and thoughts share distributed neural networks. NeuroImage. 2012;62:2110–2128. doi: 10.1016/j.neuroimage.2012.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 38.Barca, L. & Pezzulo, G. Unfolding Visual Lexical Decision in Time. PLoS ONE (2012). [DOI] [PMC free article] [PubMed]
- 39.Barca L, Pezzulo G. Tracking Second Thoughts: Continuous and Discrete Revision Processes during Visual Lexical Decision. PLOS ONE. 2015;10:e0116193. doi: 10.1371/journal.pone.0116193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman JB. Abrupt category shifts during real-time person perception. Psychon. Bull. Rev. 2014;21:85–92. doi: 10.3758/s13423-013-0470-8. [DOI] [PubMed] [Google Scholar]
- 41.Freeman JB, Dale R, Farmer TA. Hand in motion reveals mind in motion. Front Psychol. 2011;2:59. doi: 10.3389/fpsyg.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKinstry C, Dale R, Spivey MJ. Action dynamics reveal parallel competition in decision making. Psychol Sci. 2008;19:22–24. doi: 10.1111/j.1467-9280.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 43.Quinton, J. C., Catenacci Volpi, N., Barca, L. & Pezzulo, G. The cat is on the mat. Or is it a dog? Dynamic competition in perceptual decision making. IEEE Trans. Syst. Man Cybern. Part Syst. (2013).
- 44.Song J-H, Nakayama K. Hidden cognitive states revealed in choice reaching tasks. Trends Cogn Sci. 2009;13:360–366. doi: 10.1016/j.tics.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Flumini A, Barca L, Borghi AM, Pezzulo G. How do you hold your mouse? Tracking the compatibility effect between hand posture and stimulus size. Psychol. Res. 2015;79:928–938. doi: 10.1007/s00426-014-0622-0. [DOI] [PubMed] [Google Scholar]
- 46.Smeding A, Quinton J-C, Lauer K, Barca L, Pezzulo G. Tracking and simulating dynamics of implicit stereotypes: A situated social cognition perspective. J. Pers. Soc. Psychol. 2016;111:817–834. doi: 10.1037/pspa0000063. [DOI] [PubMed] [Google Scholar]
- 47.Freeman JB, Ambady N. MouseTracker: software for studying real-time mental processing using a computer mouse-tracking method. Behav Res Methods. 2010;42:226–241. doi: 10.3758/BRM.42.1.226. [DOI] [PubMed] [Google Scholar]
- 48.Freeman JB, Pauker K, Apfelbaum EP, Ambady N. Continuous dynamics in the real-time perception of race. J. Exp. Soc. Psychol. 2010;46:179–185. doi: 10.1016/j.jesp.2009.10.002. [DOI] [Google Scholar]
- 49.Hehman E, Stolier RM, Freeman JB. Advanced mouse-tracking analytic techniques for enhancing psychological science. Group Process. Intergroup Relat. 2015;18:384–401. doi: 10.1177/1368430214538325. [DOI] [Google Scholar]
- 50.Buck RW, Savin VJ, Miller RE, Caul WF. Communication of affect through facial expressions in humans. J. Pers. Soc. Psychol. 1972;23:362–371. doi: 10.1037/h0033171. [DOI] [PubMed] [Google Scholar]
- 51.Greenwald MK, Cook EW, Lang PJ. Affective judgment and psychophysiological response: Dimensional covariation in the evaluation of pictorial stimuli. J. Psychophysiol. 1989;3:51–64. [Google Scholar]
- 52.Schwartz GE, Brown SL, Ahern GL. Facial muscle patterning and subjective experience during affective imagery: sex differences. Psychophysiology. 1980;17:75–82. doi: 10.1111/j.1469-8986.1980.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 53.Mermillod M, Vermeulen N, Lundqvist D, Niedenthal PM. Neural computation as a tool to differentiate perceptual from emotional processes: The case of anger superiority effect. Cognition. 2009;110:346–357. doi: 10.1016/j.cognition.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Mermillod M, Vuilleumier P, Peyrin C, Alleysson D, Marendaz C. The importance of low spatial frequency information for recognising fearful facial expressions. Connect. Sci. 2009;21:75–83. doi: 10.1080/09540090802213974. [DOI] [Google Scholar]
- 55.Mermillod M, Bonin P, Mondillon L, Alleysson D, Vermeulen N. Coarse scales are sufficient for efficient categorization of emotional facial expressions: Evidence from neural computation. Neurocomputing. 2010;73:2522–2531. doi: 10.1016/j.neucom.2010.06.002. [DOI] [Google Scholar]
- 56.Kaminski G, Méary D, Mermillod M, Gentaz E. Is it a he or a she? Behavioral and computational approaches to sex categorization. Atten. Percept. Psychophys. 2011;73:1344–1349. doi: 10.3758/s13414-011-0139-1. [DOI] [PubMed] [Google Scholar]
- 57.Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013;68:255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. Package ‘lmerTest’. R Package Version2 (2015).
- 59.Beffara B, et al. Enhanced embodied response following ambiguous emotional processing. Cogn. Process. 2012;13:103–106. doi: 10.1007/s10339-012-0468-6. [DOI] [PubMed] [Google Scholar]
- 60.Havas, et al. Emotion simulation during language comprehension. Psychon. Bull. 38 Rev. 2007;14:436–441. doi: 10.3758/BF03194085. [DOI] [PubMed] [Google Scholar]
- 61.Havas DA, Glenberg AM, Gutowski KA, Lucarelli MJ, Davidson RJ. Cosmetic use of botulinum toxin-a affects processing of emotional language. Psychol. Sci. 2010;21:895–900. doi: 10.1177/0956797610374742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCanne TR, Anderson JA. Emotional responding following experimental manipulation of facial electromyographic activity. J. Pers. Soc. Psychol. 1987;52:759–768. doi: 10.1037/0022-3514.52.4.759. [DOI] [PubMed] [Google Scholar]
- 63.Niedenthal PM, Mermillod M, Maringer M, Hess U. The Simulation of Smiles (SIMS) model: Embodied simulation and the meaning of facial expression. Behav. Brain Sci. 2010;33:417–433. doi: 10.1017/S0140525X10000865. [DOI] [PubMed] [Google Scholar]
- 64.Pitcher D, Garrido L, Walsh V, Duchaine BC. Transcranial Magnetic Stimulation Disrupts the Perception and Embodiment of Facial Expressions. J. Neurosci. 2008;28:8929–8933. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lepora, N. F. & Pezzulo, G. Embodied Choice: How action influences perceptual decision making. PLoS Comput Biol (2015). [DOI] [PMC free article] [PubMed]
- 66.Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The Embodiment of Emotional Feelings in the Brain. J. Neurosci. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee GP, Meador KJ, Loring DW, Bradley KP. Lateralized changes in autonomic arousal during emotional processing in patients with unilateral temporal lobe seizure onset. Int. J. Neurosci. 2002;112:743–757. doi: 10.1080/00207450290025743. [DOI] [PubMed] [Google Scholar]
- 68.Gilchrist PT, Vrinceanu T, Béland S, Bacon SL, Ditto B. Disgust stimuli reduce heart rate but do not contribute to vasovagal symptoms. J. Behav. Ther. Exp. Psychiatry. 2016;51:116–122. doi: 10.1016/j.jbtep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Shenhav A, Mendes WB. Aiming for the stomach and hitting the heart: Dissociable triggers and sources for disgust reactions. Emotion. 2014;14:301. doi: 10.1037/a0034644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stark R, Walter B, Schienle A, Vaitl D. Psychophysiological correlates of disgust and disgust sensitivity. J. Psychophysiol. 2005;19:50. doi: 10.1027/0269-8803.19.1.50. [DOI] [Google Scholar]
- 71.Levenson RW. Autonomic Nervous System Differences among Emotions. Psychol. Sci. 1992;3:23–27. doi: 10.1111/j.1467-9280.1992.tb00251.x. [DOI] [Google Scholar]
- 72.Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol. Psychol. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Rozin P, Lowery L, Ebert R. Varieties of disgust faces and the structure of disgust. J. Pers. Soc. Psychol. 1994;66:870–881. doi: 10.1037/0022-3514.66.5.870. [DOI] [PubMed] [Google Scholar]
- 74.Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- 75.Woody SR, Teachman BA. Intersection of Disgust and Fear: Normative and Pathological Views. Clin. Psychol. Sci. Pract. 2006;7:291–311. doi: 10.1093/clipsy.7.3.291. [DOI] [Google Scholar]
- 76.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 77.Cacioppo JT, et al. The psychophysiology of emotion. Handb. Emot. 2000;2:173–191. [Google Scholar]
- 78.Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int. J. Psychophysiol. 2006;61:5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 79.Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 2010;22:2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- 80.Garfinkel SN, et al. Fear from the Heart: Sensitivity to Fear Stimuli Depends on Individual Heartbeats. J. Neurosci. 2014;34:6573–6582. doi: 10.1523/JNEUROSCI.3507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrett LF. Emotions are real. Emotion. 2012;12:413–429. doi: 10.1037/a0027555. [DOI] [PubMed] [Google Scholar]
- 82.Vermeulen N, Chang B, Corneille O, Pleyers G, Mermillod M. Verifying properties of concepts spontaneously requires sharing resources with same-modality percept. Cogn. Process. 2013;14:81–87. doi: 10.1007/s10339-012-0533-1. [DOI] [PubMed] [Google Scholar]
- 83.Vermeulen, N., Chang, B., Mermillod, M., Pleyers, G. & Corneille, O. Memory for words representing modal concepts. Exp. Psychol. (2013). [DOI] [PubMed]
- 84.Barsalou LW. Perceptual symbol systems. Behav. Brain Sci. 1999;22:577–600. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- 85.Pezzulo G, et al. The Mechanics of Embodiment: A Dialogue on Embodiment and Computational Modeling. Front. Cogn. 2011;2:1–21. doi: 10.3389/fpsyg.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pezzulo G, et al. Computational Grounded Cognition: a new alliance between grounded cognition and computational modeling. Front. Psychol. 2013;3:612. doi: 10.3389/fpsyg.2012.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson-Mendenhall, C. D. Constructing Emotion Through Simulation. Curr. Opin. Psychol. 10.1016/j.copsyc.2017.07.015 (2017). [DOI] [PubMed]
- 88.Hennenlotter A, et al. The link between facial feedback and neural activity within central circuitries of emotion–new insights from botulinum toxin-induced denervation of frown muscles. Cereb. Cortex N. Y. N 1991. 2009;19:537–542. doi: 10.1093/cercor/bhn104. [DOI] [PubMed] [Google Scholar]
- 89.Lanzetta JT, Cartwright-Smith J, Kleck RE. Effects of nonverbal dissimulation on emotional experience and autonomic arousal. J. Pers. Soc. Psychol. 1976;33:354–370. doi: 10.1037/0022-3514.33.3.354. [DOI] [PubMed] [Google Scholar]
- 90.Wiens S. Interoception in emotional experience. Curr. Opin. Neurol. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- 91.Decety J, Meyer M. From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev. Psychopathol. 2008;20:1053–1080. doi: 10.1017/S0954579408000503. [DOI] [PubMed] [Google Scholar]
- 92.Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 93.Donnarumma, F., Costantini, M., Ambrosini, E., Friston, K. & Pezzulo, G. Action perception as hypothesis testing. Cortex 89, 45–60 (2017). [DOI] [PMC free article] [PubMed]
- 94.Seth, A. K. The cybernetic Bayesian brain. In OpenMind (Open MIND. Frankfurt am Main: MIND Group, 2014).
- 95.Pezzulo G, Barca L, Friston KJ. Active inference and cognitive-emotional interactions in the brain. Behav. Brain Sci. 2015;38:e85. doi: 10.1017/S0140525X14001009. [DOI] [PubMed] [Google Scholar]
- 96.Barrett LF, Quigley KS, Hamilton P. An active inference theory of allostasis and interoception in depression. Phil Trans R Soc B. 2016;371:20160011. doi: 10.1098/rstb.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lench HC, Flores SA, Bench SW. Discrete emotions predict changes in cognition, judgment, experience, behavior, and physiology: a meta-analysis of experimental emotion elicitations. Psychol. Bull. 2011;137:834–855. doi: 10.1037/a0024244. [DOI] [PubMed] [Google Scholar]
- 98.Kahneman D. A perspective on judgment and choice: Mapping bounded rationality. Am. Psychol. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- 99.Damasio, A. R. Descartes’ Error: Emotion, Reason and the Human Brain. (Grosset/Putnam, 1994).
- 100.Inzlicht M, Bartholow BD, Hirsh JB. Emotional foundations of cognitive control. Trends Cogn. Sci. 2015;19:126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Witt JK, Sugovic M. Spiders appear to move faster than non-threatening objects regardless of one’s ability to block them. Acta Psychol. (Amst.) 2013;143:284–291. doi: 10.1016/j.actpsy.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 102.Vasey MW, et al. It was as big as my head, I swear! Biased spider size estimation in spider phobia. J. Anxiety Disord. 2012;26:20–24. doi: 10.1016/j.janxdis.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barrett LF, Bar M. See it with feeling: affective predictions during object perception. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gibson, J. J. The ecological approach to visual perception. (Lawrence Erlbaum Associates, Inc, 1979).
- 105.Proffitt DR. Embodied Perception and the Economy of Action. Perspect. Psychol. Sci. 2006;1:110–122. doi: 10.1111/j.1745-6916.2006.00008.x. [DOI] [PubMed] [Google Scholar]