TO THE EDITOR: Humanized mice carrying human hematopoietic and immune systems are considered as ideal tools for studying hematopoiesis, infectious disease, and immunology [1]. Various strains of immunodeficient mice have been developed to closely recapitulate human biological systems. Currently, NOD.Cg-PrkdcscidIl2rtm1Wjl/SzJ (NOD-scid IL2rnull, NSG) mice, which lack T-, B-, and NK cell activity, are considered as ideal candidates to establish humanized mice [1]. Humanized mouse models are still under development in order to improve human cell engraftment and function [1]. Various humanization protocols exist, including those pertaining to the age of the recipient mice, conditioning, cell source, and route of cell administration. As in human hematopoietic stem cell transplantation, systemic (vein) administration is predominantly used to create humanized mice [1]. Here, we report that intrahepatic injection of human umbilical cord blood hematopoietic stem cells into the liver of newborn NSG mice after busulfan conditioning allowed significantly high human CD3+ T-cell engraftment.
NSG mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions at Laboratory of Animal Research Center in Korea Institute of Radiological Medical Sciences (Seoul, Korea). All experiments were performed according to guidelines of Institutional Animal Care and Use Committee (IACUC). Human umbilical cord blood CD34+ cells (StemPro CD34+ Cell Kit) were purchased from Life Technologies (Carlsbad, CA, USA). Newborn NSG mice (≤ 48 hours after birth) were injected with busulfan (Korea Otsuka Pharmaceutical, Korea) in their retro-orbital sinus (25 mg/kg, 50–100 g per dose) 24 hours prior to transplantation. The next day, 3×104 hCD34+ cells were injected into the liver. Twelve weeks later, mice were sacrificed and mononuclear cells were isolated from bone marrow, liver, spleen, and peripheral blood. Single-cell suspensions were prepared by standard procedures and stained with the following antibodies: hCD45-allophycocyanin (APC), hCD3-fluorescein isothiocyanate (FITC), and hCD19-phycoerythrin (PE) (BD Biosciences, San Jose, CA, USA). Flow cytometry was performed on FACSCanto II (BD Biosciences, San Jose, CA, USA).
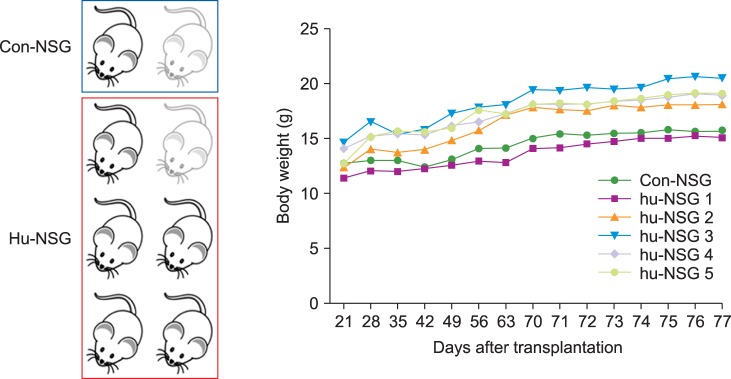
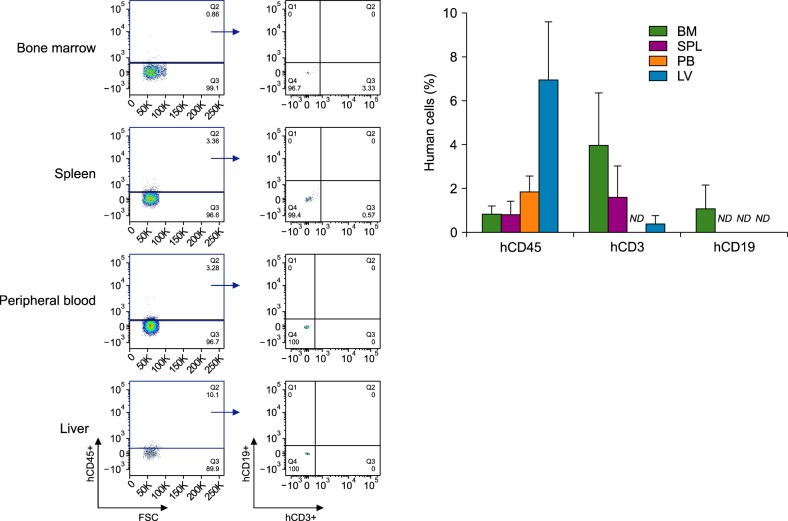
Among the 8 NSG mice (including 2 control mice), 2 showed features suggesting graft versus host disease (weight loss, hunched posture, and diminished activity) and died on Day 26 of transplantation (Fig. 1). In the 12th week, the percentages of hCD45+ cells in the NSG mouse systems were 6.96% in the liver, 1.84% in the peripheral blood, 0.81% in the bone marrow, and 0.8% in the spleen. Unexpectedly, CD19+ B-cell population was barely detected in mouse tissues, whereas significantly high human CD3+ T-cell population was observed. The population of hCD19+ B-cells was 1.09% in the bone marrow, and was not detected in any other tissue. The populations of hCD3+ T-cells were 3.98% in the bone marrow, 1.61% in the spleen, and 0.39% in the liver (Fig. 2).
Fig. 1. Survival and weight changes of newborn NSG mice after hCD34+ cell injection. Humanized NSG mice were monitored daily from the 3rd week of transplantation. Most of the newborn NSG mice did well, but two showed features suggesting graft versus host disease (weight loss, hunched posture, and diminished activity) and died on the 26th day of transplantation.
Fig. 2. Reconstitution of human cells in newborn NSG mice after intrahepatic transplantation of CD34+ cells. Mononuclear cells derived from bone marrow, spleen, peripheral blood and liver tissues of humanized NSG mice were isolated in the 12th week of transplantation and were stained with anti-hCD45, anti-hCD3, and anti-hCD19 antibodies. Data are means±SEM and representative of four mice per group, excluding lowest values (N=5).
Abbreviations: BM, bone marrow; LV, liver; ND, not detected; PB, peripheral blood; SPL, spleen.
Even 12 weeks after the intrahepatic injection, human CD45+ cell reconstitution rate was high in the liver of NSG mice. The liver is the primary site of hematopoiesis during the embryo and neonatal period [2]. As other liver functions increase several weeks after birth, the site of hematopoiesis gradually switches to the bone marrow [2]. It has been reported that the fetal liver provides a favorable microenvironment for hematopoiesis, and that macrophages were one of the major components comprising the early embryonic hematopoietic microenvironment in mice [3]. The microenvironment of the fetal liver enhances cell cycle progression and proliferation of hematopoietic stem cells, with activation of Wnt signaling pathway [4]. On the contrary, microenvironment of the adult liver maintains hematopoietic stem cells in a quiescent state, due to the preferential role of Notch signaling pathway [4]. In adults, local damage of the liver stimulates liver regeneration and increases growth factors [5]. It was assumed that CD34+ cells from cord blood could be stimulated by stem cell factor or hepatocyte growth factor [6]. However, a significantly low engraftment of human CD34+ cord blood stem cells was found after intrahepatic transplantation in adult NOD/SCID mice compared to newborn mice [6].
Limited data exist regarding humanized NSG mice generated by intrahepatic injection of human hematopoietic stem cells. Organ-specific transplantation of hematopoietic stem cells is a useful method to study hematopoiesis and immune reconstitution [6]. It is reported that the differentiation pattern seems to differ between intrahepatic and intravenous transplanted CD34+ cells in NOD/SCID mice [6,7,8]. Intrahepatic transplantation of CD34+ cord blood stem cells into newborn NOD/SCID mice induced successful engraftment of human cells. A high percentage of engrafted human cells was CD19+ B-cells, but lacked T-cell differentiation [6]. Others also reported that intrahepatic transplantation of cord blood CD34+ cells into newborn NSG allowed efficient multi-organ and multi-lineage hematopoietic engraftment, predominantly of B-cells [7]. The two studies conditioned their newborn mice with irradiation and analyzed human cell engraftment earlier (≤10 wk). Meanwhile, Choi et al. [8] reported that human T-cells developed in the liver of humanized NSG mice on intrahepatic injection of human cord blood CD34+ cells. They used busulfan conditioning and analyzed human cell engraftment for a prolonged period - until 20 weeks. Our study adopted busulfan conditioning and analyzed human cell engraftment during the 12th week after intrahepatic injection. Although this might explain the T-cell differentiation observed in our study, the reason we could not observe any B-cells in any of the mouse tissues is still elusive.
The peculiar finding of our study is that a significantly high human CD3+ T-cell population was detected in the bone marrow and spleen of the NSG mice, with barely detectable CD19+ B-cell population in all tissues. The extent to which the transplanted human stem cells would reconstitute the hematopoietic system in NSG mice is still uncertain. The microenvironment and cytokines required for hematopoietic system development differs in human and mouse systems [1,9]. NSG mice lack HLA molecules for human T-cell education, and have poorly organized lymphoid architecture and deficiencies in development of lymph nodes [9]. Previous studies reported that most of the initially engrafted human cells in NSG mice were B-cells [10]. The engraftment level of human T-cells was lower than that of B-cells, and appeared 12–16 weeks after hematopoietic stem cell transplantation [10]. It is presumed that T-cell development predominantly occurs in the thymus of NSG mice. However, only minute evidence exists supporting this presumption. The transplanted human cord blood stem cells are detectable in mouse organs. However, in the thymus at different time intervals after long-term engraftment, no CD3 expression was found [11]. Moreover, marginal enlargement of the thymus and minute increases in cellular number of the thymus were observed in humanized NSG mice, compared to normal NSG mice [8]. Various studies tried to increase reconstitution of human hematopoietic cells. The bone marrow, liver, thymus (BLT) model showed robust and stable engraftment of multiple human hematopoietic lineages, including T-cells [12]. Administration of recombinant human IL-7 improved T-cell development in humanized mice [13]. Currently, development of new generation of immunodeficient mice strains, which express human hematopoietic growth factors, is underway [14,15].
In this experiment, intrahepatic injection of human hematopoietic stem cells into the liver of newborn NSG mice resulted in a significantly higher human CD3+ T-cell population in the bone marrow and spleen, whereas CD19+ B-cell population was barely detectable in all tissues. We assume that intrahepatic injection of CD34+ cells in newborn NSG mice could facilitate T-cell reconstitution and unidentified factors in the fetal/newborn liver might contribute to T-cell development. Further studies are necessary to explore the detailed cellular and molecular mechanisms regarding the role of the liver in the reconstitution of human hematopoietic cells.
Acknowledgments
This study was supported by a grant of the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by Ministry of Science, ICT and Future Planning, Republic of Korea (1711021931).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki K, Iwatsuki H. Origin and fate of the central macrophages of erythroblastic islands in the fetal and neonatal mouse liver. Microsc Res Tech. 1997;39:398–405. doi: 10.1002/(SICI)1097-0029(19971201)39:5<398::AID-JEMT2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Martin MA, Bhatia M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005;14:493–504. doi: 10.1089/scd.2005.14.493. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas E, Newsome PN, Harrison DJ, Plevris JN. Hematopoietic stem cell trafficking in liver injury. FASEB J. 2005;19:1225–1231. doi: 10.1096/fj.04-2604rev. [DOI] [PubMed] [Google Scholar]
- 6.Wulf-Goldenberg A, Keil M, Fichtner I, Eckert K. Intrahepatic transplantation of CD34+ cord blood stem cells into newborn and adult NOD/SCID mice induce differential organ engraftment. Tissue Cell. 2012;44:80–86. doi: 10.1016/j.tice.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Navarro-Montero O, Romero-Moya D, Montes R, et al. Intrahepatic transplantation of cord blood CD34+ cells into newborn NOD/SCID-IL2Rγ(null) mice allows efficient multi-organ and multi-lineage hematopoietic engraftment without accessory cells. Clin Immunol. 2012;145:89–91. doi: 10.1016/j.clim.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Choi B, Chun E, Kim M, et al. Human T cell development in the liver of humanized NOD/SCID/IL-2Rγ(null)(NSG) mice generated by intrahepatic injection of CD34(+) human (h) cord blood (CB) cells. Clin Immunol. 2011;139:321–335. doi: 10.1016/j.clim.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Brehm MA, Shultz LD, Luban J, Greiner DL. Overcoming current limitations in humanized mouse research. J Infect Dis. 2013;208(Suppl 2):S125–S130. doi: 10.1093/infdis/jit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie JL, Gan OI, Doedens M, Dick JE. Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood. 2005;106:1259–1261. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
- 12.Lavender KJ, Messer RJ, Race B, Hasenkrug KJ. Production of bone marrow, liver, thymus (BLT) humanized mice on the C57BL/6 Rag2(-/-)γc(-/-)CD47(-/-) background. J Immunol Methods. 2014;407:127–134. doi: 10.1016/j.jim.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Lent AU, Dontje W, Nagasawa M, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–7655. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake AC, Chen Q, Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell Mol Immunol. 2012;9:215–224. doi: 10.1038/cmi.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]