TO THE EDITOR: Cutaneous infiltration in multiple myeloma (MM) is an extremely rare condition with poor prognosis that accounts for only approximately 0.6% of patients with MM according to a recent large-scale study [1]. Primary plasma cell leukemia (PCL) constitutes only 2%–5% of all myeloma cases, with a higher proportion of light-chain-only cases presenting as PCL rather than as MM. Typically, the immunophenotype of PCL differs from that of multiple myeloma (MM) in that it lacks aberrant CD56 expression and more frequently shows an abnormal karyotype [2]. Hemophagocytosis in the bone marrow is a characteristic feature of some aggressive disorders, such as hemophagocytic syndrome by histiocytes, but more rarely by myeloblasts, in acute myeloid leukemia. Furthermore, this characteristic is extremely rare among plasma cells, particularly in PCL, and only a few reports on such cases have been published [3,4,5,6,7,8]. Herein, we report a rare case of cutaneous infiltration of malignant plasma cells, which initially presented as bicytopenia, combined with primary lambda-type light-chain PCL characterized by marked phagocytosis of erythrocytes and platelets by neoplastic plasma cells.
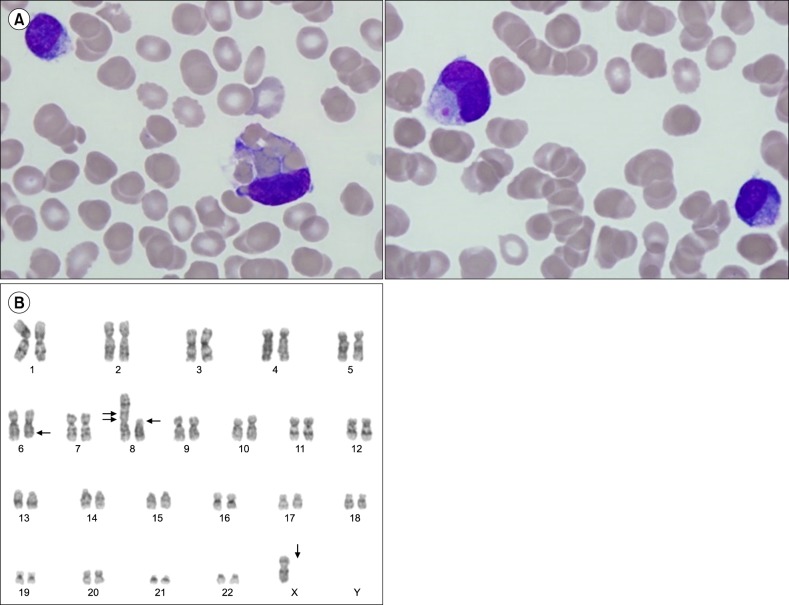
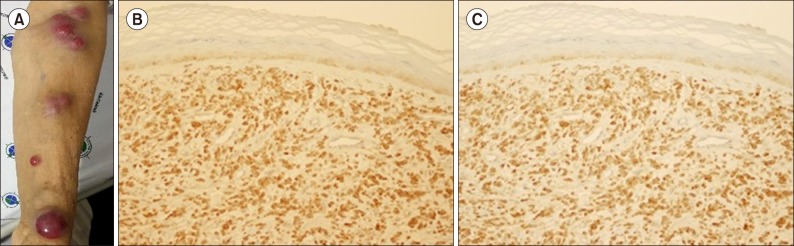
A 77-year-old woman experienced fatigue for several weeks and presented to our hospital with bicytopenia. A complete blood cell count analysis showed normocytic normochromic anemia with thrombocytopenia and leukocytosis (hemoglobin, 9.6 g/dL; platelets, 22.0×109/L; white blood cell count, 16.8×109/L). Laboratory tests revealed normal levels of calcium, blood urea nitrogen, creatinine, and lactate dehydrogenase. However, magnetic resonance imaging exhibited diffuse bone marrow signal change without definite mass or lytic lesion formation. Serum and urine protein electrophoresis displayed a monoclonal band in the beta region, and immunofixation revealed only lambda light-chain monoclonality with markedly increased serum lambda light-chain level (2,952 mg/L; normal range, 5.71–26.30 mg/L). The β-2 microglobulin level was also increased (12.06 mg/L; normal range, 0.0–2.4 mg/L). A peripheral blood smear demonstrated the presence of atypical plasma cells in various sizes with cytoplasmic vacuolations, which constitute up to 52.0%. Bone marrow aspiration showed hypercellular marrow particles with a myeloid–to-erythroid ratio of 8:1 with decreased megakaryocytes. Neoplastic plasma cells accounted for up to 67.2% of all nucleated cells. Numerous binucleated or multinucleated plasma cells were observed, of which 5.7% displayed prominent phagocytosis, primarily of erythrocytes and platelets (Fig. 1A). Flow cytometric analysis revealed that the plasma cells lacked CD56 expression, which is frequently found in PCL, and no other aberrant expression was observed. In addition, immunohistochemical analysis showed that the plasma cells were positive for CD138 and negative for CD20. Karyotypic analysis revealed an abnormal 45,X,−X,+1,dic(1;8)(p13; p23),del(6)(q22q25),del(8)(p21) karyotype (Fig. 1B). The patient was diagnosed with primary PCL, fulfilling the diagnostic criteria of the International Myeloma Working Group, which suggested more than 20% circulating plasma cells and an absolute plasma cell count of greater than 2×109/L [9]. Bortezomib, melphalan, and prednisolone (VMP) therapy was initiated, which reverted her serum lambda light-chain level to normal after one treatment cycle. A second treatment cycle with VMP was planned, but the patient refused further chemotherapy. After 5 months from the termination of the first cycle of VMP therapy, multiple subcutaneous nodules developed on her left upper limb, trunk, back, and lower extremities (Fig. 2A), and her serum lambda light-chain level increased again to 127.65 mg/L. Skin biopsy showed the nodules as plasmacytoma, and immunohistochemistry showed CD138 and lambda light-chain positivity (Fig. 2B, C). The patient was treated with lenalidomide and dexamethasone chemotherapy, but this salvage therapy was effective for preventing nodule dissemination only for 3 months. The cutaneous plasmacytoma aggravated again, and she is now on palliative radiotherapy.
Fig. 1. Hemophagocytic plasma cells and abnormal karyotyping result. (A) Microscopic examination of bone marrow (BM) showing large, bizarre plasma cells with characteristic hemophagocytic features mostly with mature erythrocytes and platelets (Wright-Giemsa staining, ×1,000). (B) Conventional BM chromosome analysis result showing 45,X,−X,+1,dic(1;8)(p13;p23),del(6)(q22q25), del(8)(p21) karyotype.
Fig. 2. Multiple skin nodules and their biopsy showing infiltration of plasma cells. Multiple skin nodules in the patient's extremities (A). Biopsy of the skin-infiltrating plasma cells showing positive immunohistochemical staining for CD138 (B) and lambda light-chain (C) (×200).
Cutaneous plasmacytoma is an extremely rare condition with poor prognosis, and the median overall survival is only 8.5 months [10,11]. This condition is not associated with a specific myeloma immunoglobulin type, although a more aggressive course is observed in light-chain-only subtypes [10]. Requena et al. [11] analyzed 8 cases of cutaneous plasmacytoma and revealed that malignant plasmacytes are homogeneous in their immunophenotype with strong expression of CD79a, CD138, and epithelial membrane antigen. In addition, RB1 gene deletion in skin-infiltrated plasmacytes was reported to be associated with poor prognosis [11]. A recent retrospective study of 53 cutaneous plasmacytoma cases showed no correlation between CD56 negativity or cytogenetic abnormality with skin infiltration of malignant plasma cells and that the plasmablastic morphology in the skin lesion indicated a worse overall survival [10]. In the present case, the malignant plasma cells were positive for CD138 and negative for CD56, CD19, CD20, and CD22, which correlated to the immunophenotypic characterization of malignant plasma cells. The RB1 gene deletion could not be analyzed, and no significant plasmablastic appearance of plasma cell infiltration was observed in the patient's skin lesion.
Although hemophagocytosis by neoplastic plasma cells has rarely been described in the literature, this rare condition does not appear to be associated with a specific immunophenotype, immunoglobulin or light-chain subtype, or karyotype [6]. One hypothesis is that hemophagocytic plasma cell formation may be attributed to the expansion of rare B-cell clones with innate phagocytic potential, although this proposition remains to be confirmed [5,7]. Similar to our case, the hemophagocytic feature of plasma cells is more frequently found in female patients, and it appears to be dominant in mature erythrocytes and platelets [4,7]. Some reports also suggest that phagocytosis by neoplastic plasma cells resulted in peripheral blood cytopenia; however, whether this complication is a direct consequence of hemophagocytosis by plasma cells remains to be determined [2,4].
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Deng S, Xu Y, An G, et al. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single-center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15:286–291. doi: 10.1016/j.clml.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 3.Invernizzi R, Pecci A. A case of phagocytic multiple myeloma. Haematologica. 2000;85:318. [PubMed] [Google Scholar]
- 4.Kanoh T, Saigo K. Phagocytic myeloma cells in asymptomatic multiple myeloma. Tohoku J Exp Med. 1987;153:207–210. doi: 10.1620/tjem.153.207. [DOI] [PubMed] [Google Scholar]
- 5.Kucukkaya RD, Hacihanefioglu A, Yenerel MN, et al. CD15-expressing phagocytic plasma cells in a patient with multiple myeloma. Blood. 2001;97:581–583. doi: 10.1182/blood.v97.2.581. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig H, Pavelka M. Phagocytic plasma cells in a patient with multiple myeloma. Blood. 1980;56:173–176. [PubMed] [Google Scholar]
- 7.Ramos J, Lorsbach R. Hemophagocytosis by neoplastic plasma cells in multiple myeloma. Blood. 2014;123:1634. doi: 10.1182/blood-2013-11-536771. [DOI] [PubMed] [Google Scholar]
- 8.Savage DG, Zipin D, Bhagat G, Alobeid B. Hemophagocytic, non-secretory multiple myeloma. Leuk Lymphoma. 2004;45:1061–1064. doi: 10.1080/10428190310001623856. [DOI] [PubMed] [Google Scholar]
- 9.Fernández de, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurczyszyn A, Olszewska-Szopa M, Hungria V, et al. Cutaneous involvement in multiple myeloma: a multi-institutional retrospective study of 53 patients. Leuk Lymphoma. 2016;57:2071–2076. doi: 10.3109/10428194.2015.1128542. [DOI] [PubMed] [Google Scholar]
- 11.Requena L, Kutzner H, Palmedo G, et al. Cutaneous involvement in multiple myeloma: a clinicopathologic, immunohistochemical, and cytogenetic study of 8 cases. Arch Dermatol. 2003;139:475–486. doi: 10.1001/archderm.139.4.475. [DOI] [PubMed] [Google Scholar]