Abstract
Oxygen is thought to be an important light element in Earth’s core but the amount of oxygen in Earth’s core remains elusive. In addition, iron-rich iron oxides are of great interest and significance in the field of geoscience and condensed matter physics. Here, static calculations based on density functional theory demonstrate that I4/mmm-Fe2O is dynamically and mechanically stable and becomes energetically favorable with respect to the assemblage of hcp-Fe and -FeO above 270 GPa, which indicates that I4/mmm-Fe2O can be a strong candidate phase for stable iron-rich iron oxides at high pressure, perhaps even at high temperature. The elasticity and anisotropy of I4/mmm-(FexNi1−x)2O at high pressures are also determined. Based on these results, we have derived the upper limit of oxygen to be 4.3 wt% in Earth’s lower outer core. On the other hand, I4/mmm-(FexNi1−x)2O with high AV S is likely to exist in a super-Earth’s or an ocean planet’s solid core causing the locally seismic heterogeneity. Our results not only give some clues to explore and synthesize novel iron-rich iron oxides but also shed light on the fundamental information of oxygen in the planetary core.
Introduction
Earth’s core is widely accepted to be dominantly composed of iron-nickel alloys. Light elements, which are cosmically abundant and soluble in iron at high pressures such as H, C, O, Si, S and P, are required to explain the 8~10% and 4~5% density deficit of Earth’s outer and inner core from pure iron, respectively1–3. Additionally, the positive density jump from Earth’s liquid outer core to the solid inner core at the inner-outer core boundary (ICB) is too large to be explained by the solidification across the ICB alone4. Therefore, both suggest that there should be more light elements in Earth’s liquid outer core.
Oxygen is thought to be an important candidate for compensating the density deficit of Earth’s core. Previous ab initio calculations and models have shown that oxygen strongly partitions into Earth’s liquid outer core from the solid inner core5,6. Therefore, much attention has been paid to explore oxygen in the outer core. For example, shockwave data in the Fe-S-O system demonstrated that adding oxygen into the liquid iron could not match the density and velocity profiles of the outer core simultaneously and indicated an oxygen-depleted outer core7. In contrast, ab initio molecular dynamic calculations found no oxygen-free iron alloy that fitted the seismological observations well8. This finding leads to the conclusion that oxygen is required as a major light element in Earth’s outer core and the best fitting result is 3.7 wt% O and 1.9 wt% Si without S or C in iron-nickel alloys8. Recent melting experiments have shown that O and Si could not exist at high concentrations simultaneously under outer core conditions and that SiO2 saturation sets limits on O and Si concentrations in Earth’s outer core9. These different results indicate that the amount of oxygen in the outer core remains elusive. On the other hand, investigations into the Fe-FeO system at high pressures give direct clues to determine the temperature at ICB and provide an alternative method to estimate the amount of oxygen in the outer core10–12. Melting experiments showed that the Fe-FeO system remains eutectic to at least 93 GPa10. However, the end member, FeO undergoes a series of transitions under higher pressure and temperature conditions, which will affect the phase relation of the Fe-FeO system13–16. This raises the question as to whether the Fe-FeO system keeps eutectic under core conditions.
In addition to oxygen-bearing iron alloys in Earth’s core, iron oxides have a widespread occurrence in Earth’s crust and mantle, which have a significant influence on the oxidation state and the phase balance of Earth’s interior. Therefore, extensive experiments have been conducted at relevant P-T conditions of Earth’s interior to investigate structural and physical properties of iron oxides13,16–21. In particular, recent studies have successfully synthesized a series of iron oxides with new stoichiometries at high P-T conditions such as Fe4O5, Fe5O6, Fe5O7, Fe7O9 and FeO2 21–26. These findings have revealed a complex phase diagram of the Fe-O system at extreme conditions and indicated different scenarios in Earth’s interior. For example, Fe4O5 and Fe5O6 as well as those well-known iron oxides are stable and some of them are likely to coexist from 10 GPa to 20 GPa resulting in a complicated oxygen buffer in Earth’s transition zone23,27,28. However, it is obvious that these newly synthesized compounds are all oxygen-rich iron oxides with Fe/O < 1. Heretofore, investigations into iron-rich iron oxides with Fe/O > 1 are rare. Although some theoretical studies have been performed to explore iron-rich iron oxides under high pressure29–32, their structural and physical properties as well as thermodynamic stability with respect to other components in the Fe-O system are poorly understood.
Here we investigate the stability, elastic and seismic properties of (FexNi1−x)2O under high pressure by first-principle calculations based on density functional theory (DFT). According to present results and previous data, we further discuss the existence of (FexNi1−x)2O and its potential effect on geochemical and geophysical processes in Earth’s and a super-Earth’s interior.
Results
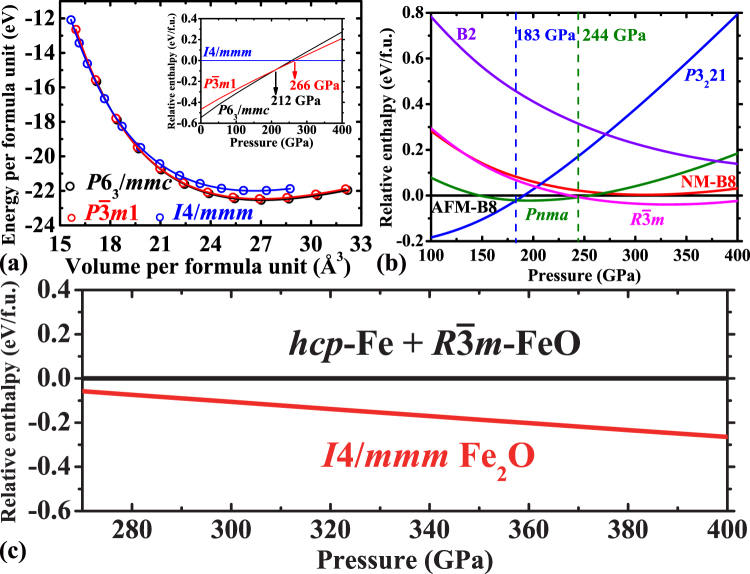
The static calculation results of Fe2O are displayed in Fig. 1(a) and the corresponding Equation of State (EoS) parameters are listed in Table S1. This figure illustrates that Fe2O undergoes structural transitions from P63/mmc to at 212 GPa and further to I4/mmm at 266 GPa, consistent with previous simulations32. The later transition is accompanied by an increase of the coordination number of oxygen from six to eight. To investigate the dynamic stability of the I4/mmm-type Fe2O, ab initio lattice-dynamics calculations are performed [see Supplementary Fig. S2]. The phonon spectrum without any imaginary frequency implies that the I4/mmm structure is dynamically stable at 320 GPa.
Figure 1.
(a) The total energy vs. volume for Fe2O with P63/mmc (black circles), (red circles) and I4/mmm (blue circles). The inset refers to calculated enthalpy differences as a function of pressure among the aforementioned structures. (b) Calculated enthalpy differences as a function of pressure for FeO with antiferromagnetic-B8 (black line), nonmagnetic-B8 (red line), P3221 (blue line), Pnma (olive line), (pink line) and B2 (purple line). (c) Calculated relative enthalpy of I4/mmm-Fe2O as a function of pressure compared with the assemblage of hcp-Fe and -FeO.
In order to evaluate the relative stability of Fe2O versus FeO at pressures corresponding to Earth’s core, the following chemical reaction is considered in the present study:
| 1 |
For FeO five candidate structures (P63/mmc (B8), (B2), P3221, Pnma and ) are considered above 100 GPa in our simulations [see Supplementary Table S1]. Our simulations predict phase transitions form P3221 to Pnma and further to at 183 GPa and 244 GPa, respectively [Fig. 1(b)]. These results are inconsistent with previously experimental observations, where B8 is the most stable structure above 100 GPa at moderate temperatures and will transform into B2 above 240 GPa at high temperatures13–15,33,34. However, our results are in good agreement with previous theoretical calculations32. The present calculations are performed at 0 K (static calculations) while previous experiments were conducted at high temperatures. In addition, the ideal chemical stoichiometric compound FeO is considered in our simulations but the non-stoichiometric Fe1−xO was often used in experiments. For Fe the hcp structure is predicted to be stable at high pressures corresponding to Earth’s core compared with the fcc structure [see Supplementary Table S1]. Based on these parameters, we have calculated the relative enthalpy of I4/mmm-Fe2O as a function of pressure compared with the assemblage of hcp-Fe and -FeO [Fig. 1(c)]. Figure 1(c) demonstrates that I4/mmm-Fe2O becomes energetically favorable above ~270 GPa and is likely to be stable at least up to 400 GPa. Additionally, the enthalpy difference between I4/mmm-Fe2O and the assemblage of hcp-Fe and FeO increases with increasing pressure indicating that I4/mmm-Fe2O becomes more stable upon compression.
The compression of volumes as a function of pressure displayed in Fig. S3 illustrates a marginal volume change across the chemical reaction (1). The volume reduction of reaction (1) is about 1.45% at 260 GPa and increases with increasing pressure. Previous experiments have detected B2-FeO under ultra-high pressure and temperature conditions15 and therefore calculated results of the assemblage of hcp-Fe and B2-FeO are also included for comparison [see Supplementary Fig. S3]. Similarly, I4/mmm-Fe2O has a smaller volume compared with that of hcp-Fe + B2-FeO. Though the volume reduction of reaction (1) is marginal, generally smaller than 1.60% up to 400 GPa, it may have important contributions to stabilize the I4/mmm-type Fe2O at high pressures.
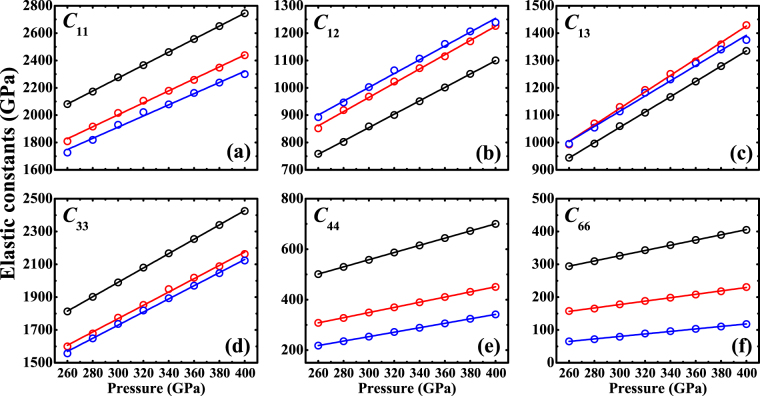
In order to evaluate the effect of Ni on elastic and seismic properties of Fe2O at high pressures, the components Ni2O and (Fe0.5Ni0.5)2O are also included in present simulations [see Supplementary Fig. S1(b) and Table S1]. (It is worth mentioning that different arrangements of Ni in (Fe0.5Ni0.5)2O were considered but they were unstable. When the atomic positions and unit-cell parameters were allowed to relax at each given volume, the I4/mmm structure would destroy.) The zero-pressure bulk modulus (K 0) of (FexNi1−x)2O decreases with the Ni content [see Supplementary Fig. S4]. The elastic constants of (FexNi1−x)2O increase monotonically with pressure [Fig. 2 and see Supplementary Table S2]. These three components all present mechanically stable in the calculated pressure range supported by the Born-Huang criterion35. The substitution of Ni for Fe in (FexNi1−x)2O tends to significantly decrease C 11, C 33, C 44 and C 66 and increase C 12 and C 13. Figure 2 also reveals a moderate anisotropy in the axial incompressibility along a axis and c axis. The c axis is more compressible than a axis with C 11 > C 33 for (FexNi1−x)2O.
Figure 2.
Calculated single-crystal elastic constants of I4/mmm-(FexNi1−x)2O as a function of pressure. The black, red and blue open circles represent Fe2O, (Fe0.5Ni0.5)2O and Ni2O, respectively. The black, red and blue lines are obtained by linear fitting.
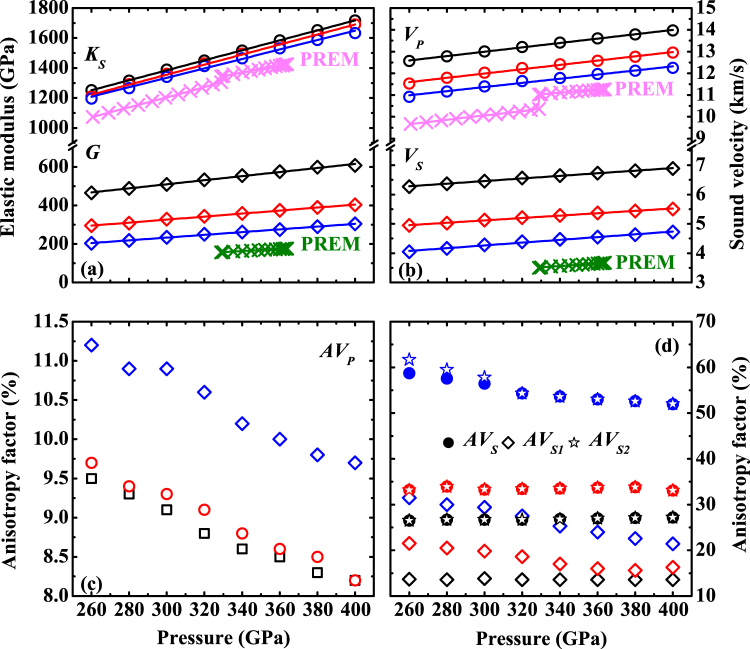
Using the aforementioned C ij of (FexNi1−x)2O, the adiabatic bulk and shear moduli (K S and G) at high pressures are calculated according to the Voigt-Reuss-Hill averages36 [Fig. 3(a) and see Supplementary Table S2]. The Ks and G of I4/mmm-(FexNi1−x)2O increase monotonically with pressure and are all greater than PREM37. The substitution of Ni in (FexNi1−x)2O slightly affects the K S but significantly reduces the G. Furthermore, the aggregate V P and V S of (FexNi1−x)2O are obtained from their moduli and densities [Fig. 3(b) and see Supplementary Table S2]. In contrast to the moduli of (FexNi1−x)2O, the substitution of Ni in (FexNi1−x)2O largely reduces V P and V S because both of them are partially controlled by the G.
Figure 3.
(a) Bulk and shear moduli (K S and G) and (b) aggregate velocities (V P and V S) of I4/mmm-(FexNi1−x)2O as a function of pressure. (c) Variation of P wave anisotropies (AV P) and (d) shear wave splitting factors (AV S) and anisotropy factors of two polarized S waves (AV S1 and AV S2) for I4/mmm-(FexNi1−x)2O at high pressures. The black, red and blue marks represent Fe2O, (Fe0.5Ni0.5)2O and Ni2O, respectively. The lines in (a) and (b) are obtained by linear fitting. The elastic and seismic profiles of PREM (pink and olive crosses) (ref 37) are plotted for comparison.
To understand the evolution of velocity anisotropies of the I4/mmm-type (FexNi1−x)2O as a function of pressure, the percentage of velocity anisotropies and their distributions are calculated based on C ij and density at each given pressure38,39 [Fig. 3(c), (d) and see Supplementary Fig. S5, Table S2]. The AV P of Fe2O is 9.5% at 260 GPa and decreases to 8.2% at 400 GPa [Fig. 3(c) and see Supplementary Table S3]. The AV P of (Fe0.5Ni0.5)2O is similar to that of Fe2O while the AV P of Ni2O is about 1.7% larger than that of Fe2O at each pressure point. These results indicate that the small substitution of Ni for Fe will not change the AV P of (FexNi1−x)2O significantly in the calculated pressure range. At 320 GPa, the fastest P wave (13.82 km/s) propagates along a axis direction and the slowest P wave (12.65 km/s) distributes in the (110) plane in Fe2O. The fastest and slowest P waves both reduce with the increasing amount of Ni in (FexNi1−x)2O. It is worth mentioning that (FexNi1−x)2O has a high AV S, i.e., the AV S are 26.5%, 33.2% and 58.7% for Fe2O, (Fe0.5Ni0.5)2O and Ni2O at 260 GPa, respectively [Fig. 3(d)]. The high AV S mainly results from the high anisotropy of V S2, though AV S1 is, in fact not small. The layered I4/mmm structure consists of OX8 blocks (X: Fe or Ni) along c axis contributing to a high AV S, which is similar to that of post-perovskite40.
Discussion and Implications
Previous static simulations calculated the stability of -Fe3O and -Fe4O compared with the assemblage of hcp-Fe and B8-FeO under Earth’s core conditions29. The results have shown that the chemical combination of Fe and FeO into Fe3O or Fe4O is energetically unfavorable under Earth’s core pressures. Additionally, the enthalpy difference increases with pressure indicating the large instability of Fe3O or Fe4O even at higher pressures. A BiI3-like triclinic Fe3O was later found to possess much lower enthalpy than that of Fe3O with previously predicted structures30. But the enthalpy of this new phase was still over 1 eV higher than that of the assemblage of hcp-Fe and B8-FeO. The present results demonstrate that the I4/mmm-type Fe2O is energetically favorable than the hcp-Fe + -FeO assemblage. And the -FeO is more stable than B8-FeO based on our simulations. Thus, the I4/mmm-type Fe2O will be much more stable than the triclinic Fe3O at 0 K. Recent static calculations investigated the stability of -Fe3O and P4/nmm-Fe3O at 350 GPa and 500 GPa, respectively by ab initio random structure searching32. These structures are still unstable with respect to the dissociation, though they are very close to the convex hull. Furthermore, the structure of Fe3O is found to be a mixture of phases consisting of Fe and Fe2O and the P4/nmm structure is found to consist of Fe, Fe2O and FeO. Therefore, combining previous data with our results, we propose that it is unlikely to form Fe3O or Fe4O by the chemical combination of Fe and Fe2O under Earth’s core pressures. Our static calculations indicate that the I4/mmm-type Fe2O is a stable phase in iron-rich iron oxides at 0 K.
The present static calculations have shown that the I4/mmm-type Fe2O is dynamically stable and has the smaller enthalpy and volume than those of the assemblage of Fe and FeO at high pressures, which all favor the formation of Fe2O by the combination of Fe and FeO. However, our high-pressure simulations are limited at 0 K in contrast to the high P-T conditions of Earth’s core. As shown in Fig. 1(c), the enthalpy difference between the I4/mmm-type Fe2O and the Fe + FeO assemblage at high pressure of the ICB is of the order of 0.15 eV corresponding to ~1750 K. In terms of B2-FeO, which has been experimentally confirmed at high P-T conditions, the enthalpy difference is of the order of 0.35 eV (~4100 K). Thus it is likely that the I4/mmm-type Fe2O can be stable at high P-T conditions. On the other hand, the entropy is going to play an important role at high temperature but its effect on the relative stability of the target system is not considered in the present study. Previous ab initio simulations have found that when the entropic effect is included in the calculation, the Gibbs free energy of the reaction, 62Fe + FeO → Fe63O, can be substantially reduced by ~3 eV31. As for the present target system, the introduction of entropic effect may correspondingly favor the combination of Fe and FeO at high P-T conditions. But, on the contrary, it can have an opposite effect causing the reaction energetically unfavorable. That means it might change our conclusion significantly and invalidate our further interpretations for the deep Earth at ultra-high temperature. Further investigations on the present target system by either ab initio molecular dynamics methods or high P-T experiments are definitely required to verify the reaction, Fe + FeO → Fe2O. It is to be noted that Earth’s core contains diverse light elements. The present of one light element can affect the relative stability and the amount of the other one in Earth’s core8,9. Thus, the relative stability and physical properties of the iron-rich iron oxide or oxygen-bearing iron alloys should be investigated as a function of not only pressure and temperature but also compositions in order to comprehensively elucidate the nature of oxygen in Earth’s core. But they are beyond the scope of the present study and we will work on them in the future.
In terms of phase relations of the Fe-FeO system, Sherman 29 indicated there should be no solid solution between Fe and FeO under Earth’s core conditions due to the aforementioned reasons. In contrast, later simulations implied that oxygen dissolved in iron might be stabilized at concentrations up to a few mol% under core conditions because of the significant entropic effect in the dilute solution31. Recent thermodynamic simulations suggested the feasibility of the ideal solution model to calculate the Fe-FeO liquid property under outer core conditions and yielded the eutectic compositions of Fe-7.2~9.1 wt% O12. Based on their thermodynamic simulations, they concluded that an overall oxygen-rich bulk outer core model should be excluded. In the present study, it is proposed that Fe2O can be a strong candidate phase for stable iron-rich iron oxides at high pressure, perhaps even at high temperature, which can potentially change phase relations of the Fe-FeO system at pressures corresponding to Earth’s lower outer core.
The accurate measurement of the sound velocity of liquid iron alloys at high pressure is very challenging. Previous studies have discussed the sound velocity of the liquid phase can be addressed from the bulk sound velocity of the solid phase with identical compositions7. In addition, theoretical calculations have shown that the bulk sound velocity (V φ) of the solid phase is comparable to V P of the liquid phase (the difference is about 2.5%) and both are marginally dependent of temperature at a given high pressure41,42. Indeed, recent experiments have presumed V φ of the solid FeS2 is equivalent to V P of the liquid counterpart and thus estimated the sulfur content in Earth’s outer core43. Furthermore, in the I4/mmm-type Fe2O, the coordination number of oxygen is eight and each iron is surrounded by four oxygen and nine iron, which is similar to the recent report of structural properties of the liquid oxygen-bearing iron alloys by first-principles molecular dynamics44. Thus, it is reasonable that the target system can be used to roughly derive the amount of oxygen in the lower outer core. We obtain V φ of (FexNi1−x)2O as a function of pressure [Fig. 4 and see Supplementary Table S2] and estimate the volume fraction of Fe2O by the following relation:
| 2 |
where t is the volume fraction of Fe2O. We find a volume fraction, t ~ 35% [Fig. 4]. Then, if oxygen is the only light element in Earth’s lower outer core, the maximum possible oxygen content is ~4.3 wt%. However, thermoelastic parameters of (FexNi1−x)2O at realistic outer core conditions are not well constrained, which certainly warrants further explorations.
Figure 4.
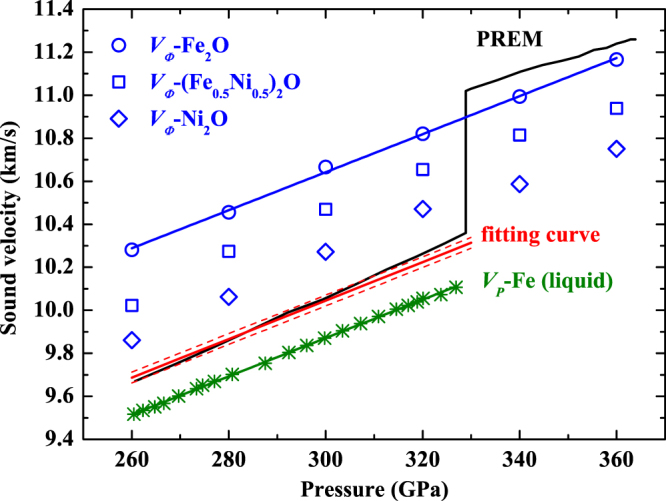
Bulk sound velocity of I4/mmm-(FexNi1−x)2O (blue marks) as a function of pressure. The seismic profile of PREM (black line) (ref 37) and V P profile of liquid iron (olivine stars) (ref 42) are plotted for comparison. The red fitting curve is calculated from 35 vol% I4/mmm-Fe2O + 65 vol% iron.
On the other hand, for a terrestrial super-Earth, unlike Earth, the metal core is mainly composed of solid iron alloys and only a small portion of the core is liquid because of its high mass. Therefore, there will be more oxygen in a super-Earth’s solid core than that in Earth’s inner core assuming that they have similar cosmochemical compositions. There also exist a type of so-called ocean planets, where a thick layer of ice covers the rocky materials and the center temperature can be much lower than that of super-Earths’45,46. Based on current results at 0 K, above 270 GPa, it is energetically favorable for the formation of the I4/mmm-type Fe2O. The enthalpy difference becomes larger upon further compression, which can stabilize the I4/mmm-type Fe2O even at high temperature. Thus, it is likely that I4/mmm-(FexNi1−x)2O could exist in the solid core of a super-Earth or an ocean planet. In addition, Fig. 3(d) demonstrates that I4/mmm-(FexNi1−x)2O has a high AV S in the calculated pressure range due to its layered structure. If (FexNi1−x)2O can accumulate in the solid core locally, it will cause the locally seismic anisotropy in the solid core, which might be even observed by advanced instruments.
Conclusions
In conclusion, the high-pressure behavior of (FexNi1−x)2O has been studied based on first-principle density functional calculations. The end member Fe2O, is predicted to undergo structural transitions from P63/mmc to at 212 GPa and further to I4/mmm at 266 GPa, consistent with previous simulations. The dynamically and mechanically stable I4/mmm-Fe2O becomes energetically favorable with respect to the assemblage of hcp-Fe and -FeO above 270 GPa. These results indicate that I4/mmm-Fe2O can be a strong candidate phase for stable iron-rich iron oxides at high pressure, perhaps even at high temperature, which requires further investigations by ab initio molecular dynamics methods or high P-T experiments. The elastic and seismic properties of I4/mmm-(FexNi1−x)2O at high pressures have been discussed in detail. Combining previous data with present results, we roughly estimate that if oxygen is the only light element in Earth’s lower outer core, less than 4.3 wt% oxygen content is required to match the seismic observations. On the other hand, I4/mmm-(FexNi1−x)2O may exist in a super-Earth’s or an ocean planet’s solid core. If (FexNi1−x)2O can accumulate in its solid core locally, it is likely to cause the locally seismic heterogeneity because of its high AV S.
Methods
Three candidate structures (P63/mmc, and I4/mmm [see Supplementary Fig. S1(a)]) for Fe2O were considered in the present study and structural details could be found in Table S1. First-principle calculations were performed based on density functional theory with the projected augmented wave method (PAW) implemented in Vienna ab-initio simulation package (VASP)47–49. The Perdew-Burke-Ernzerhof (PBE) version of the generalized gradient approximations (GGA) was selected to treat the exchange correlation potential50. The kinetic energy cut-off was set to 1000 eV. The energy convergence criterion for the electronic self-consistent calculation was 10−6 eV. The total energy difference was converged to 1×10−5 eV/formula unit (f.u.) with respect to the energy cutoff and k-points, respectively. The force difference was converged to 1×10−3 eV/Å (less than 0.1 GPa). The spin-polarization of iron of Fe2O with various structures was not included because the existence of magnetic moments under Earth’s core conditions could be safely ruled out. For each crystalline phase, the atomic positions and unit-cell parameters were allowed to relax at each given volume to obtain the minimum total energy. Once the minimum total energies of each phase were obtained at different volumes, they were fitted to the third-order Birch-Murnaghan EoS51,52. Additionally, the enthalpy (H = E + PV) of each phase was compared with each other to identify the most stable structure at the given pressure.
The phonon dispersions were calculated using the Phonopy Code by the supercell method53 and a 2×2×2 supercell was constructed for the I4/mmm-type Fe2O. Single crystal elastic constants of I4/mmm-(FexNi1−x)2O were computed from the stress-strain relations (σ ij = c ijkl ε kl, where σ ij stands for the stresses, c ijkl for the elastic moduli and ε kl for the strains)54. For the I4/mmm symmetry, we derived six independent elastic constants (C 11, C 12, C 13, C 33, C 44 and C 66). We applied positive and negative strains of magnitude of 0.5% in order to accurately determine the stresses in the appropriate limit of zero strain.
Electronic supplementary material
Acknowledgements
X. W. and S. Q. acknowledge financial support from the National Science Foundation of China (41473056 and 41472037).
Author Contributions
X.W. and S.H. conceived the research. S.H. performed the theoretical calculations. S.H., X.W. and S.Q. analyzed the data. S.H., X.W. and S.Q. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18678-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Birch F. Density and composition of mantle and core. J. Geophys. Res. 1964;69:4377–4388. doi: 10.1029/JZ069i020p04377. [DOI] [Google Scholar]
- 2.Poirier JP. Light elements in the Earth’s outer core: Acritical review. Phys. Earth Planet. Inter. 1994;85:319–337. doi: 10.1016/0031-9201(94)90120-1. [DOI] [Google Scholar]
- 3.Anderson OL, Isaak DG. Another look at the core density deficit of Earth’s outer core. Phys. Earth Planet. Inter. 2002;131:19–27. doi: 10.1016/S0031-9201(02)00017-1. [DOI] [Google Scholar]
- 4.Shearer P, Masters G. The density and shear velocity contrast at the inner core boundary. Geophys. J. Int. 1990;102:491–498. doi: 10.1111/j.1365-246X.1990.tb04481.x. [DOI] [Google Scholar]
- 5.Alfè D, Gillan MJ, Price GD. Composition and temperature of the Earth’s core constrained by combining ab initio calculations and seismic data. Earth Planet. Sci. Lett. 2002;195:91–98. doi: 10.1016/S0012-821X(01)00568-4. [DOI] [Google Scholar]
- 6.Badro J, et al. Effect of light elements on the sound velocities in solid iron: Implications for the composition of Earth’s core. Earth Planet. Sci. Lett. 2007;254:233–238. doi: 10.1016/j.epsl.2006.11.025. [DOI] [Google Scholar]
- 7.Huang H, et al. Evidence for an oxygen-depleted liquid outer core of the Earth. Nature. 2011;479:513–516. doi: 10.1038/nature10621. [DOI] [PubMed] [Google Scholar]
- 8.Badro J, Côté AS, Brodholt JP. A seismologically consistent compositional model of Earth’s core. Proc. Natl. Acad. Sci. USA. 2014;111:7542–7545. doi: 10.1073/pnas.1316708111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirose K, et al. Crystallization of silicon dioxide and compositional evolution of the Earth’s core. Nature. 2017;543:99–102. doi: 10.1038/nature21367. [DOI] [PubMed] [Google Scholar]
- 10.Seagle CT, Heinz DL, Campbell AJ, Prakapenka VB, Wanless ST. Melting and thermal expansion in the Fe-FeO system at high pressure. Earth Planet. Sci. Lett. 2008;265:655–665. doi: 10.1016/j.epsl.2007.11.004. [DOI] [Google Scholar]
- 11.Sata N, et al. Compression of FeSi, Fe3C, Fe0.95O, and FeS under the core pressures and implication for light element in the Earth’s core. J. Geophys. Res. 2010;115:B09204. doi: 10.1029/2009JB006975. [DOI] [Google Scholar]
- 12.Komabayashi T. Thermodynamics of melting relations in the system Fe-FeO at high pressure: Implications for oxygen in the Earth’s core. J. Geophys. Res. Solid Earth. 2014;119:4164–4177. doi: 10.1002/2014JB010980. [DOI] [Google Scholar]
- 13.Fei Y, Mao HK. In situ determination of the NiAs phase of FeO at high pressure and temperature. Science. 1994;266:1678–1680. doi: 10.1126/science.266.5191.1678. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa H, Hirose K, Tateno S, Sata N, Ohishi Y. Phase transition boundary between B1 and B8 structures of FeO up to 210 GPa. Phys. Earth Planet. Inter. 2010;179:157–163. doi: 10.1016/j.pepi.2009.11.005. [DOI] [Google Scholar]
- 15.Ozawa H, Takahashi F, Hirose K, Ohishi Y, Hirao N. Phase transition of FeO and stratification in Earth’s outer core. Science. 2011;334:792–794. doi: 10.1126/science.1208265. [DOI] [PubMed] [Google Scholar]
- 16.Fischer RA, et al. Equation of state and phase diagram of FeO. Earth Planet. Sci. Lett. 2011;304:496–502. doi: 10.1016/j.epsl.2011.02.025. [DOI] [Google Scholar]
- 17.Lazor P, Shebanova ON, Annersten H. High-pressure study of stability of magnetite by thermodynamic analysis and synchrotron X-ray diffraction. J. Geophys. Res. Solid Earth. 2004;109:B05201. doi: 10.1029/2003JB002600. [DOI] [Google Scholar]
- 18.Ding Y, et al. Novel pressure-induced magnetic transition in magnetite (Fe3O4) Phys. Rev. Lett. 2008;100:045508. doi: 10.1103/PhysRevLett.100.045508. [DOI] [PubMed] [Google Scholar]
- 19.Shim SH, et al. Electronic and magnetic structures of the postperovskite-type Fe2O3 and implications for planetary magnetic records and deep interiors. Proc. Natl. Acad. Sci. USA. 2009;106:5508–5512. doi: 10.1073/pnas.0808549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JF, et al. Abnormal elastic and vibrational behaviors of magnetite at high pressures. Sci. Rep. 2014;4:6282. doi: 10.1038/srep06282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bykova E, et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 2016;7:10661. doi: 10.1038/ncomms10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavina B, et al. Discovery of the recoverable high-pressure iron oxide Fe4O5. Proc. Natl. Acad. Sci. USA. 2011;108:17281–17285. doi: 10.1073/pnas.1107573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavina B, Meng Y. Unraveling the complexity of iron oxides at high pressure and temperature: Synthesis of Fe5O6. Sci. Adv. 2015;1:e1400260. doi: 10.1126/sciadv.1400260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Q, et al. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen-hydrogen cycles. Nature. 2016;534:241–244. doi: 10.1038/nature18018. [DOI] [PubMed] [Google Scholar]
- 25.Hu Q, et al. Dehydrogenation of goethite in Earth’s deep lower mantle. Proc. Natl. Acad. Sci. USA. 2017;114:1498–1501. doi: 10.1073/pnas.1620644114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinmyo R, et al. Discovery of Fe7O9: a new iron oxide with a complex monoclinic structure. Sci. Rep. 2016;6:32852. doi: 10.1038/srep32852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodland AB, Frost DJ, Trots DM, Klimm K, Mezouar M. In situ observation of the breakdown of magnetite (Fe3O4) to Fe4O5 and hematite at high pressures and temperatures. Am. Mineral. 2012;97:1808–1811. doi: 10.2138/am.2012.4270. [DOI] [Google Scholar]
- 28.Woodland AB, et al. Fe4O5 and its solid solutions in several simple systems. Contrib. Mineral. Petrol. 2013;166:1677–1686. doi: 10.1007/s00410-013-0948-4. [DOI] [Google Scholar]
- 29.Sherman DM. Stability of possible Fe-FeS and Fe-FeO alloy phases at high pressure and the composition of the Earth’s core. Earth Planet. Sci. Lett. 1995;132:87–98. doi: 10.1016/0012-821X(95)00057-J. [DOI] [Google Scholar]
- 30.Alfè D, Price GD, Gillan MJ. Oxygen in the Earth’s core: a first-principles study. Phys. Earth Planet. Inter. 1999;110:191–210. doi: 10.1016/S0031-9201(98)00134-4. [DOI] [Google Scholar]
- 31.Alfè D, Price GD, Gillan MJ. Thermodynamic stability of Fe/O solid solution at inner-core conditions. Geophys. Res. Lett. 2000;27:2417–2420. doi: 10.1029/2000GL011567. [DOI] [Google Scholar]
- 32.Weerasinghe GL, Pickard CJ, Needs RJ. Computational searches for iron oxides at high pressures. J. Phys. Condens. Matter. 2015;27:455501. doi: 10.1088/0953-8984/27/45/455501. [DOI] [PubMed] [Google Scholar]
- 33.Ohta K, Hirose K, Shimizu K, Ohishi Y. High-pressure experimental evidence for metal FeO with normal NiAs-type structure. Phys. Rev. B. 2010;82:174120. doi: 10.1103/PhysRevB.82.174120. [DOI] [Google Scholar]
- 34.Ozawa H, et al. Spin crossover, structural change, and metallization in NiAs-type FeO at high pressure. Phys. Rev. B. 2011;84:134417. doi: 10.1103/PhysRevB.84.134417. [DOI] [Google Scholar]
- 35.Born, M. & Huang K. Dynamical Theory of Crystal Lattices (Oxford University Press, 1954).
- 36.Hill R. The elastic behavior of a crystalline aggregate. Proc. Phys. Soc. Sect. A. 1952;65:349. doi: 10.1088/0370-1298/65/5/307. [DOI] [Google Scholar]
- 37.Dziewonski AM, Anderson DL. Preliminary reference Earth model. Phys. Earth Planet. Inter. 1981;25:297–356. doi: 10.1016/0031-9201(81)90046-7. [DOI] [Google Scholar]
- 38.Mainprice D. A FORTRAN program to calculate seismic anisotropy from the lattice preferred orientation of minerals. Comput. Geosci. 1990;16:385–393. doi: 10.1016/0098-3004(90)90072-2. [DOI] [Google Scholar]
- 39.Mainprice, D., Barruol, G. & Ismaïl, W. B. The seismic anisotropy of the Earth’s mantle: From single crystal to polycrystal, in Earth’s Deep Interior: Mineral Physics and Tomography From the Atomic to the Global Scale (ed. Karato, S. –I. et al.) 237-264 (AGU, 2000).
- 40.Tsuchiya T, Tsuchiya J, Umemoto K, Wentzcovitch RM. Elasticity of post-perovskite MgSiO3. Geophys. Res. Lett. 2004;31:L14603. doi: 10.1029/2004GL020278. [DOI] [Google Scholar]
- 41.Vočadlo L, Dobson DP, Wood IG. Ab initio calculations of the elasticity of hcp-Fe as a function of temperature at inner-core pressure. Earth Planet. Sci. Lett. 2009;288:534–538. doi: 10.1016/j.epsl.2009.10.015. [DOI] [Google Scholar]
- 42.Ichikawa H, Tsuchiya T, Tange Y. The P-V-T equation of state and thermodynamic properties of liquid iron. J. Geophys. Res. Solid Earth. 2014;119:240–252. doi: 10.1002/2013JB010732. [DOI] [Google Scholar]
- 43.Thompson EC, et al. Equation of state of pyrite to 80 GPa and 2400 K. Am. Mineral. 2016;101:1046–1051. doi: 10.2138/am-2016-5527. [DOI] [Google Scholar]
- 44.Posner ES, Steinle-Neumann G, Vlček V, Rubie DC. Structural changes and anomalous self-diffusion of oxygen in liquid iron at high pressure. Geophys. Res. Lett. 2017;44:3526–3534. doi: 10.1002/2017GL072926. [DOI] [Google Scholar]
- 45.Anderson JD, Lau EL, Sjogren WJ, Schubert G, Moore WB. Gravitational constraints on the internal structure of Ganymede. Nature. 1996;384:541–543. doi: 10.1038/384541a0. [DOI] [Google Scholar]
- 46.Sotin C, Grasset O, Mocquet A. Mass-radius curve for extrasolar Earth-like planets and ocean planets. Icarus. 2007;191:337–351. doi: 10.1016/j.icarus.2007.04.006. [DOI] [Google Scholar]
- 47.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 48.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 49.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 50.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 51.Murnaghan FD. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA. 1944;30:244–247. doi: 10.1073/pnas.30.9.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birch F. Finite Elastic strain of cubic crystals. Phys. Rev. 1947;71:809–824. doi: 10.1103/PhysRev.71.809. [DOI] [Google Scholar]
- 53.Togo A, Oba F, Tanaka I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B. 2008;78:134106. doi: 10.1103/PhysRevB.78.134106. [DOI] [Google Scholar]
- 54.Karki BB, Stixrude L, Wentzcovitch RM. High-pressure elastic properties of major materials of Earth’s mantle from first principles. Rev. Geophys. 2001;39:507–534. doi: 10.1029/2000RG000088. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.