Abstract
Background and Objectives
Herpes zoster (HZ) and its most frequent complication, post-herpetic neuralgia (PHN), have been shown to considerably impact quality of life (QoL). This has not yet been demonstrated in Japan.
Methods
QoL in HZ and PHN patients was evaluated using the Zoster Brief Pain Inventory (ZBPI), EuroQoL-5 Dimension (EQ-5D), Short-Form 12 version 2.0, and short-form McGill Pain Questionnaire up to 270 days after rash onset as part of a prospective, observational, cohort study conducted in Kushiro, Hokkaido, Japan.
Results
This study involved 412 adults ≥ 60 years of age diagnosed with HZ, 38 of whom developed PHN. QoL in daily activity performance and emotional and physical functioning was impaired at Day 0 (rash onset) and almost resolved by Day 90. Although the mean ZBPI worst pain score for HZ patients without PHN improved from 4.1 at Day 0 to 0.1 at Day 90, the score for HZ patients with PHN at Day 90 was comparable to that for HZ patients without PHN at Day 0. While the EQ-5D score in HZ without PHN improved, on average, from 0.755 to 0.949, the score for HZ with PHN was dependent on PHN duration and did not improve until PHN disappearance.
Conclusions
HZ impaired QoL in daily activity performance and emotional and physical functioning. The negative impact on QoL was more prevalent in patients with a longer PHN duration compared with HZ without PHN.
ClinicalTrials.gov identifier: NCT01873365.
Key Points
| This is the first study demonstrating that herpes zoster (HZ) and its complication, post-herpetic neuralgia (PHN) impact the overall quality of life (QoL) of patients in Japan. |
| We evaluated the QoL of HZ and PHN in Japanese patients ≥ 60 years of age up to 270 days after rash onset. |
| Results have shown that QoL, measured in daily activity performance, and emotional and physical functioning, was impaired at Day 0 (rash onset) and almost resolved by Day 90; however, some patients developed PHN beyond Day 90 and their impaired QoL did not improve until PHN was resolved. |
Introduction
Herpes zoster (HZ) occurs when varicella-zoster virus (VZV) reactivates due to waning of cell-mediated immunity. More than 90% of the Japanese population is infected with VZV by 20 years of age or older [1] and therefore have a 30% lifetime risk of HZ [2]. HZ-related pain resolves by approximately 90 days for many patients with HZ, but approximately 20% of them will develop post-herpetic neuralgia (PHN) [3], debilitating, long-lasting pain that can continue for months or years and which therefore requires long-term pain management.
Annually, approximately 500,000 individuals in Japan aged 60 years or older develop HZ [3, 4], and this number is expected to increase due to aging of the population, with an expected increase in the number of patients aged ≥ 60 years from 40 million (32%) in 2012 to 45 million (41%) by 2050 [5]. Even with treatment, HZ patients may experience pain for a prolonged period of time [6], and almost half of HZ patients aged over 70 years were shown to experience pain persisting for more than 1 year [7]. Indeed, the risk of HZ recurrence increases with age [8].
A number of studies in different countries reported a significant negative impact of HZ and PHN on patients’ quality of life (QoL) and that pain experienced due to the disease interfered with many aspects of the patient’s daily life [9–11]. In addition, the presence of PHN was associated with a greater impact on most domains of QoL [12–15]. However, to date, data on QoL in HZ patients are very limited in Japan [16]. Cultural differences have been known in patient-reported outcomes across countries [14, 17]. Whether similarity or difference in the outcomes is observed in Japan compared with other countries is not known. The present study assessed the impact of HZ and PHN on the QoL of adults aged 60 years or older in Japan.
Subjects and Methods
Study Design
The QoL data were collected as part of a prospective, observational, multicenter, physician practice-based cohort study of people aged 60 years or older conducted in Kushiro, Japan, between June 2013 and February 2015. The details of the study have been published elsewhere [18].
Study participants were recruited among patients presenting at seven dermatology clinics or hospitals, and HZ was diagnosed by the physician at the initial consultation or at a subsequent visit within 7 days of the initial visit. The maximal duration of the study for each patient was 270 days from the initial visit. At 90 and 180 days after the initial visit, patients with a pain score of 0 were considered as having completed the study. PHN was defined by the presence of HZ-associated pain rated as ≥ 3 in response to item 3 of the Zoster Brief Pain Inventory (ZBPI) questionnaire [19], persisting or appearing more than 90 days after onset of the HZ rash. Only HZ patients who were considered to comply with the protocol requirements (e.g. complete the booklets by themselves) were included in the study.
The study is registered at ClinicalTrials.gov (NCT01873365). It was conducted in accordance with the Ethical Guidelines for Epidemiological Research [20] and approved by a hospital-based Ethics Committee (Kushiro Red Cross Hospital and Kushiro City General Hospital) or a centralized Institutional Review Board (Clinical Research Hospital, Tokyo). Written informed consent was obtained from all participants.
Quality-of-Life Questionnaires
QoL data were collected through several questionnaires used previously in other studies in HZ patients [10–15]. The first questionnaire survey on QoL of patients with HZ was conducted at the study site, while the remaining surveys were performed through self-administered questionnaires at 15, 30, 60, 90, 120, 150, 180, 210, 240, and 270 days after the initial visit. Data from the ZBPI and the EuroQoL-5 dimension (EQ-5D) questionnaire were collected at all time points specified above, while data for the Short-Form 12 version 2.0 (SF-12) were collected at 0 and 90 days after the initial visit, and data for the short-form McGill Pain Questionnaire (SF-MPQ) were collected at the initial visit.
The ZBPI questionnaire was used to quantify HZ pain and its related interference with seven functional statuses: general activity, mood, walking ability, work, relations with others, sleep, and enjoyment of life [19]. Pain was rated between 0 (no pain) and 10 (pain as bad as you can image), while interference was also rated between 0 (does not interfere) and 10 (completely interferes).
The EQ-5D 3-level scale [21] was used to assess the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with each dimension having three levels: no problems, some problems, and extreme problems. The level for each dimension was combined to generate a health profile and then converted to a continuous single utility score between 0.000 (worst imaginable health state) and 1.000 (best imaginable health state). In the present study, the Japanese Time-Trade-Off EQ-5D value sets were used to generate the utility score [22].
The SF-12 was used to assess eight QoL domains: physical functioning (PF), role-physical (RP, role limitations due to physical problems), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE, role limitations due to emotional problems), and mental health (MH) [23]. From these domains, the physical health component score (PCS) and mental health component score (MCS) were computed. A standardized Statistical Analysis System (SAS) macro was used to perform the scoring [24], and the score for each domain was expressed between 0 (worst health state) and 100 (best health state). The component summary score was represented by a norm-based scoring having means of 50 and standard deviation (SD) of 10 in the general Japanese population.
The SF-MPQ measures diverse dimensions of pain using a set of descriptors of both the sensory and affective dimensions of pain [25].
Statistical Analyses
The first day of onset of HZ rash was defined as Day 0, consistent with the analysis of Coplan et al. [19]. To be included in the analysis as Day 0, the first questionnaire must have been filled in within 7 days after onset of HZ rash. Questionnaires at other time points were considered evaluable if they were filled out within the time windows detailed in Table 1.
Table 1.
Classification time windows at each questionnaire time point
| Time window | Time point |
|---|---|
| Day 0–Day 7 | Day 0 |
| Day 8–Day 22 | Day 15 |
| Day 23–Day 45 | Day 30 |
| Day 46–Day 75 | Day 60 |
| Day 76–Day 105 | Day 90 |
| Day 106–Day 135 | Day 120 |
| Day 136–Day 165 | Day 150 |
| Day 166–Day 195 | Day 180 |
| Day 196–Day 225 | Day 210 |
| Day 226–Day 255 | Day 240 |
| ≥Day 256 | Day 270 |
Compliance with questionnaire completion was evaluated at each time point as the percentage of patients with at least one evaluable questionnaire out of the number of patients who were still in the study at the respective time point. Patients could be included in the analysis with only one questionnaire in total at any time point. Additionally, the missing questionnaires were assumed to be missing at random.
In a post hoc analysis not planned in the study protocol, patients developing PHN were separated from the group of all HZ patients, and mean ZBPI and EQ-5D scores over the first 3 months were estimated separately for the group of HZ patients not developing PHN and those with PHN.
Descriptive statistics (e.g. mean and SD) were presented for the multi-item SF-12, EQ-5D, and ZBPI scales, as well as the SF-MPQ, for four age groups (60–64, 65–69, 70–79, and ≥ 80 years) and overall over time using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). The Kaplan–Meier estimates were used to measure the duration of PHN.
Results
Overall, 412 HZ patients, including 38 (9.8%) PHN patients, were included in this study. Patients had a mean age of 71.7 years and approximately 60% were female (Table 2).
Table 2.
Patient demographics (N = 412)
| Characteristic | Value (N = 412) |
|---|---|
| Age group, years [n (%)] | |
| 60–64 | 92 (22.3) |
| 65–69 | 85 (20.6) |
| 70–79 | 163 (39.6) |
| ≥ 80 | 72 (17.5) |
| Age, years [mean (SD)] | 71.67 (7.46) |
| Gender [n (%)] | |
| Female | 248 (60.2) |
| Male | 164 (39.8) |
| HZ status [n (%)] | |
| Without PHN | 374 (90.8) |
| With PHN by Day 180 | 23 (5.6) |
| With PHN by Day 270 | 15 (3.6) |
SD standard deviation, HZ herpes zoster, PHN post-herpetic neuralgia, N total number of patients, n number of patients (among the total)
Completion rates were higher than 80% for all time points for all questionnaires, except for Day 240 for ZBPI and EQ-5D, where the completion rate was slightly lower (73.3%). The mean delay between initial HZ rash onset and first clinical visit was 4.4 days (range 0–26). Approximately 90% of patients visited the doctor ≤ 7 days after HZ rash onset.
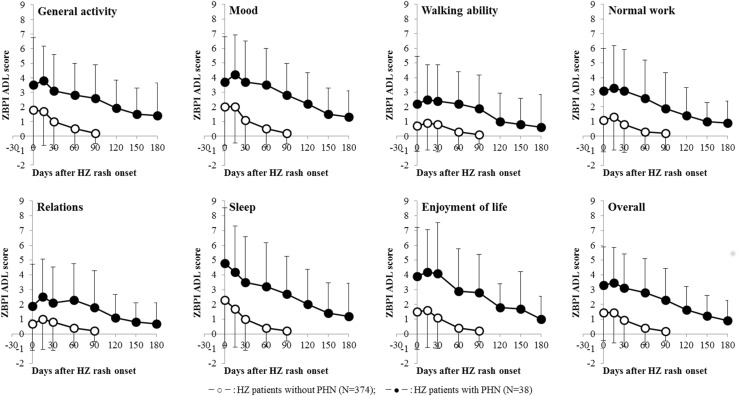
The mean ZBPI worst pain score at Day 0 was higher in the 38 patients who developed PHN (5.8) than for the 374 HZ patients who did not develop PHN (4.1) (Fig. 1). HZ-associated pain interfered with all ZBPI activities of daily living (ADL) dimensions, especially sleep, mood, general activity, and enjoyment of life (Fig. 2). Although both ZBPI pain and ADL scores for HZ patients without PHN had almost improved by Day 90, the scores for HZ patients with PHN at Day 90 remained high and were the same as or higher than the scores for HZ patients without PHN at Day 0. All the mean ZBPI worst pain scores in this study are presented by age and study period (see Table 3). The median duration of PHN was 185 days (interquartile range 134–274). Overall, 34% (95% confidence interval 0.21–0.54) of PHN patients still had PHN at Day 270.
Fig. 1.
Changes in ZBPI worst pain score over time. The symbol and vertical bar represent the mean and standard deviation. The number of data collected at each point is included in Table 3. HZ herpes zoster, PHN post-herpetic neuralgia, ZBPI Zoster Brief Pain Inventory
Fig. 2.
Interference of HZ-associated pain with ZBPI ADL scores over time. The symbol and vertical bar represent the mean and standard deviation. HZ herpes zoster, PHN post-herpetic neuralgia, ZBPI Zoster Brief Pain Inventory, ADL activities of daily living
Table 3.
ZBPI worst scores by age group over time
| 60–64 years | 65–69 years | 70–79 years | ≥ 80 years | Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Mean ± SD | N | n | Mean ± SD | N | n | Mean ± SD | N | n | Mean ± SD | N | n | Mean ± SD | |
| HZ patients without PHN | |||||||||||||||
| Day 0 | 87 | 77 | 4.4 ± 2.84 | 78 | 70 | 3.8 ± 2.77 | 143 | 132 | 4.2 ± 2.75 | 66 | 57 | 4.1 ± 2.89 | 374 | 336 | 4.1 ± 2.79 |
| Day 15 | 76 | 2.9 ± 2.71 | 66 | 2.6 ± 2.47 | 118 | 3.2 ± 2.83 | 49 | 2.8 ± 2.93 | 309 | 3.0 ± 2.74 | |||||
| Day 30 | 71 | 1.1 ± 1.97 | 64 | 1.6 ± 2.52 | 108 | 1.4 ± 2.03 | 48 | 1.6 ± 2.66 | 291 | 1.4 ± 2.24 | |||||
| Day 60 | 70 | 0.3 ± 1.35 | 63 | 0.5 ± 1.92 | 110 | 0.6 ± 1.48 | 45 | 0.6 ± 1.66 | 288 | 0.5 ± 1.58 | |||||
| Day 90 | 71 | 0.1 ± 0.69 | 67 | 0.1 ± 0.98 | 116 | 0.1 ± 0.99 | 47 | 0.2 ± 0.84 | 301 | 0.1 ± 0.90 | |||||
| HZ patients with PHNa | |||||||||||||||
| Day 0 | 5 | 4 | 4.8 ± 3.20 | 7 | 5 | 7.0 ± 2.12 | 20 | 15 | 5.9 ± 3.20 | 6 | 6 | 5.2 ± 2.40 | 38 | 30 | 5.8 ± 2.85 |
| Day 15 | 4 | 4.8 ± 1.26 | 6 | 6.0 ± 3.10 | 15 | 6.3 ± 2.19 | 4 | 5.8 ± 2.63 | 29 | 5.9 ± 2.30 | |||||
| Day 30 | 5 | 3.4 ± 2.07 | 5 | 4.4 ± 1.95 | 18 | 5.3 ± 2.91 | 6 | 6.0 ± 2.68 | 34 | 5.0 ± 2.67 | |||||
| Day 60 | 4 | 2.3 ± 2.63 | 5 | 3.2 ± 1.92 | 19 | 4.4 ± 2.43 | 4 | 5.8 ± 2.63 | 32 | 4.1 ± 2.49 | |||||
| Day 90 | 5 | 4.4 ± 3.21 | 7 | 4.1 ± 2.73 | 20 | 4.1 ± 2.46 | 6 | 5.5 ± 1.87 | 38 | 4.3 ± 2.49 | |||||
| Day 120 | 4 | 3.0 ± 2.45 | 7 | 3.7 ± 2.75 | 19 | 2.6 ± 2.48 | 5 | 5.4 ± 3.29 | 35 | 3.3 ± 2.71 | |||||
| Day 150 | 5 | 2.6 ± 2.61 | 7 | 1.9 ± 2.34 | 17 | 1.0 ± 1.62 | 4 | 4.0 ± 3.74 | 33 | 1.8 ± 2.36 | |||||
| Day 180 | 5 | 3.2 ± 2.95 | 4 | 2.3 ± 2.87 | 18 | 0.7 ± 1.64 | 4 | 3.8 ± 3.77 | 31 | 1.7 ± 2.56 | |||||
| Day 210 | 3 | 3 | 3.3 ± 3.51 | 2 | 1 | 3.0 | 7 | 6 | 2.0 ± 1.55 | 3 | 3 | 3.7 ± 4.04 | 15 | 13 | 2.8 ± 2.52 |
| Day 240 | 2 | 2.5 ± 3.54 | 1 | 5.0 | 5 | 2.0 ± 1.87 | 3 | 5.7 ± 2.52 | 11 | 3.4 ± 2.62 | |||||
| Day 270 | 3 | 2.0 ± 3.46 | – | – | 5 | 2.6 ± 2.61 | 3 | 4.3 ± 2.31 | 11 | 2.9 ± 2.66 | |||||
SD standard deviation, HZ herpes zoster, PHN post-herpetic neuralgia, ZBPI Zoster Brief Pain Inventory, N total number of patients, n number of patients (among the total)
aBeyond Day 180, 15 patients among 38 PHN patients continued the study
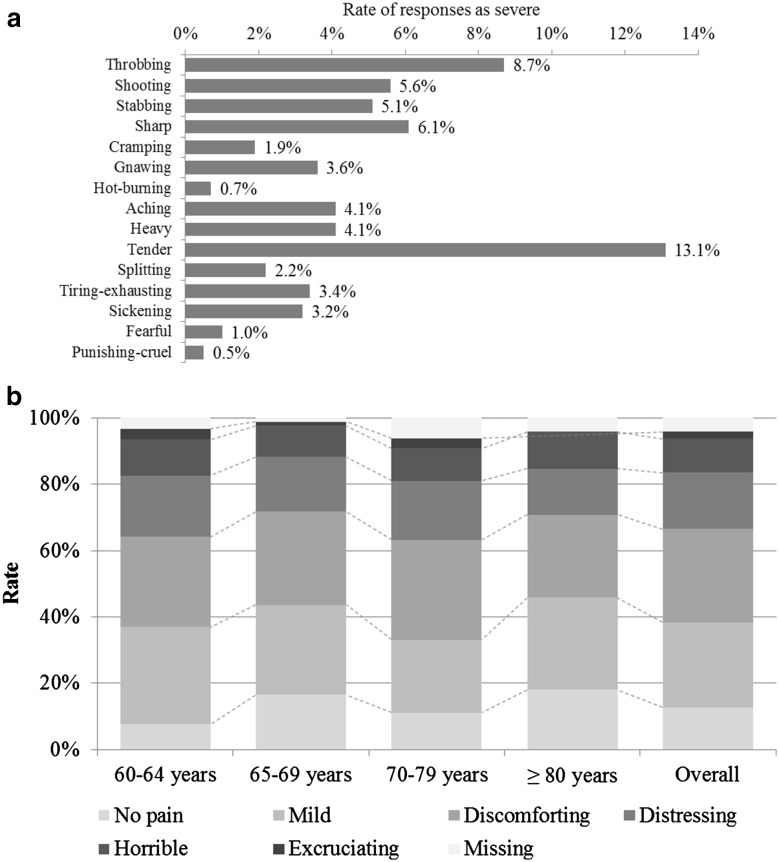
At onset of rash, 13.1% of all patients reported tender pain classified as severe, 8.7% reported severe throbbing pain, 6.1% reported severe sharp pain, 5.6% reported severe shooting pain, and 5.1% reported severe stabbing pain (Fig. 3a). When patients described their present pain intensity at the onset of rash, 12.6% reported no pain, 25.7% reported mild pain, 28.2% reported discomforting pain, 17% reported distressing pain, 10.2% reported horrible pain, and 2.2% reported excruciating pain (Fig. 3b).
Fig. 3.
Pain descriptions at rash onset. a Rate of responses as severe for Pain Rating Index; and b Present Pain Intensity
The mean utility score at Day 0 was lower (representing worse QOL) in HZ patients with PHN (0.679) than in HZ patients without PHN (0.755) (Fig. 4). While the mean score in HZ patients without PHN improved to 0.949 at Day 90, the mean score at Day 90 in HZ patients with PHN was comparable to scores for HZ patients without PHN at Day 0. EQ-5D scores were similar to ZBPI pain scores as they did not appear to improve over time in PHN patients. All the utility scores in this study are presented by age and study period (see Table 4).
Fig. 4.

Changes in EQ-5D utility score over time. The symbol and vertical bar represent the mean and standard deviation. The number of data collected at each point is included in Table 4. HZ herpes zoster, PHN post-herpetic neuralgia, EQ-5D EuroQoL-5 Dimension
Table 4.
Estimated EQ-5D utility scores by age group over time
| 60–64 years | 65–69 years | 70–79 years | ≥ 80 years | Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Mean ± SD | N | n | Mean ± SD | N | n | Mean ± SD | N | n | Mean ± SD | N | n | Mean ± SD | |
| HZ patients without PHN | |||||||||||||||
| Day 0 | 87 | 75 | 0.761 ± 0.129 | 78 | 70 | 0.805 ± 0.134 | 143 | 132 | 0.737 ± 0.136 | 66 | 56 | 0.729 ± 0.162 | 374 | 333 | 0.755 ± 0.141 |
| Day 15 | 73 | 0.822 ± 0.126 | 67 | 0.845 ± 0.153 | 119 | 0.809 ± 0.173 | 48 | 0.750 ± 0.228 | 307 | 0.811 ± 0.171 | |||||
| Day 30 | 71 | 0.912 ± 0.124 | 65 | 0.898 ± 0.173 | 106 | 0.852 ± 0.156 | 48 | 0.782 ± 0.219 | 290 | 0.865 ± 0.170 | |||||
| Day 60 | 71 | 0.949 ± 0.099 | 63 | 0.968 ± 0.084 | 112 | 0.896 ± 0.189 | 48 | 0.859 ± 0.187 | 294 | 0.918 ± 0.157 | |||||
| Day 90 | 74 | 0.962 ± 0.145 | 68 | 0.990 ± 0.048 | 118 | 0.942 ± 0.144 | 44 | 0.883 ± 0.180 | 304 | 0.949 ± 0.139 | |||||
| HZ patients with PHNa | |||||||||||||||
| Day 0 | 5 | 4 | 0.665 ± 0.167 | 7 | 6 | 0.688 ± 0.194 | 20 | 16 | 0.676 ± 0.135 | 6 | 6 | 0.687 ± 0.102 | 38 | 32 | 0.679 ± 0.139 |
| Day 15 | 4 | 0.768 ± 0.000 | 6 | 0.704 ± 0.179 | 15 | 0.629 ± 0.130 | 4 | 0.594 ± 0.082 | 29 | 0.659 ± 0.134 | |||||
| Day 30 | 5 | 0.796 ± 0.124 | 4 | 0.757 ± 0.022 | 19 | 0.671 ± 0.139 | 6 | 0.554 ± 0.130 | 34 | 0.679 ± 0.144 | |||||
| Day 60 | 4 | 0.857 ± 0.171 | 6 | 0.759 ± 0.129 | 19 | 0.722 ± 0.156 | 4 | 0.584 ± 0.144 | 33 | 0.728 ± 0.161 | |||||
| Day 90 | 5 | 0.793 ± 0.125 | 7 | 0.795 ± 0.092 | 20 | 0.735 ± 0.142 | 4 | 0.654 ± 0.132 | 36 | 0.746 ± 0.132 | |||||
| Day 120 | 4 | 0.752 ± 0.032 | 7 | 0.857 ± 0.137 | 18 | 0.759 ± 0.155 | 5 | 0.725 ± 0.184 | 34 | 0.773 ± 0.148 | |||||
| Day 150 | 5 | 0.802 ± 0.114 | 6 | 0.884 ± 0.127 | 15 | 0.797 ± 0.146 | 4 | 0.698 ± 0.235 | 30 | 0.802 ± 0.153 | |||||
| Day 180 | 5 | 0.848 ± 0.141 | 5 | 0.888 ± 0.158 | 16 | 0.844 ± 0.128 | 4 | 0.519 ± 0.464 | 30 | 0.809 ± 0.225 | |||||
| Day 210 | 3 | 3 | 0.732 ± 0.062 | 2 | 1 | 0.768 | 7 | 4 | 0.772 ± 0.009 | 3 | 3 | 0.792 ± 0.198 | 15 | 11 | 0.766 ± 0.096 |
| Day 240 | 2 | 0.715 ± 0.076 | 1 | 0.768 | 4 | 0.698 ± 0.116 | 3 | 0.595 ± 0.106 | 10 | 0.677 ± 0.106 | |||||
| Day 270 | 3 | 0.810 ± 0.173 | – | – | 6 | 0.724 ± 0.168 | 3 | 0.690 ± 0.025 | 12 | 0.737 ± 0.143 | |||||
SD standard deviation, HZ herpes zoster, PHN post-herpetic neuralgia, EQ-5D EuroQoL-5 Dimension, N total number of patients, n number of patients (among the total)
aBeyond Day 180, 15 patients among 38 PHN patients continued the study
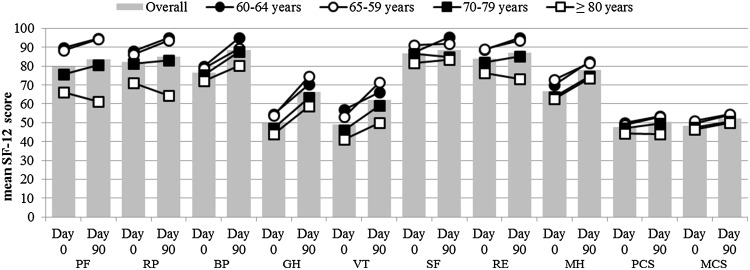
The SF-12 scores in all eight domains and two summary components were higher at Day 90 than at Day 0, representing an improvement in QoL (Fig. 5). However, in the ≥ 80 years age group, decreases in PF (from 66.1 to 61.1), RP (from 71.2 to 64.4), RM (from 76.4 to 73.1), and PCS (from 44.4 to 43.9) were observed from Day 0 to Day 90, representing a worsening of symptoms. In general, SF-12 scores were lower in older patients.
Fig. 5.
SF-12 scores by age group at Day 0 and Day 90. SF-12 Short-Form 12 version 2.0, PF physical functioning, RP role-physical, BP bodily pain, GH general health, VT vitality, SF social functioning, RE role-emotional, MH mental health, PCS physical health component score, MCS mental health component score
Discussion
To our knowledge, this is the first study assessing the impact of HZ and PHN on QoL in Japan. Our study demonstrated that HZ impaired QoL, impacting sleep, mood, general activity, and emotional and physical functioning at rash onset. Pain score and impaired QoL were almost resolved for HZ patients who did not develop PHN by Day 90; however, some patients developed PHN beyond Day 90 and their impaired QoL did not improve until PHN was resolved, suggesting that the negative impact on QoL was greater in HZ patients with PHN than in HZ patients without PHN.
Several studies have reported the negative impact of HZ and PHN on QoL using the ZBPI [10–15, 26], as well as generic QoL measures such as the EQ-5D [10–13] and SF-12 [10, 26]. Increased levels of pain have known to be associated with increased levels of interference in ADL, such as sleep, mood, and general activity [10, 27, 28]. In a Japanese study, the QoL of HZ patients was evaluated using the Dermatology Life Quality Index and the SF-36 at rash onset, week 1, and week 2. The results of that study suggested that valaciclovir hydrochloride therapy for HZ not only improved skin symptoms and pain but also patients’ QoL [32].
A prospective study in Taiwan by Tsai et al., which assessed the severity and duration of HZ-associated pain and QoL, found that among patients aged 50 years or older, the mean ZBPI worst pain score improved from 5.95 at rash onset to 1.04 after 90 days [11]. The reported pain scores in our study were slightly milder than those in that study, which might be caused by differences in patient characteristics such as hospitalization rate (3.4% in our study vs. 20.7% in the Taiwanese study) [11, 18].
The ZBPI pain scores remained higher for HZ patients who developed PHN for longer periods than for HZ patients who did not. This agrees with previously reported data for HZ and PHN patients aged 50 years or older that were conducted in European countries and Canada [14, 15, 29].
The presence of pain has consistently been shown to significantly interfere with patients’ health-related quality of life [10, 30, 31]. Among Asian populations, a prospective study in South Korean adults with HZ who were older than 50 years of age showed that all ZBPI-assessed ADLs were impacted by the pain experienced, and that the interference increased with the intensity of the reported pain [32]. Again, comparing our results with the prospective study by Tsai et al., larger values for mean interferences in HZ patients for all ADLs were observed at rash onset [11], in parallel with the pain intensity. In addition, a review of studies assessing the humanistic burden of HZ in the EU summarized mean levels of pain interferences by ZBPI ADL scores ranging from 1.7 to 4.9 in HZ patients, and from 1.7 to 6.5 in PHN patients [9]. These differences warrant further investigation, but patients with different cultural backgrounds have been suggested to report discrepant levels of pain or ADL in HZ patients [14].
Similar to a post hoc analysis of the ARIZONA study [33], physical functioning at Day 0, evaluated using the SF-12, appeared to decrease with age without any age-related changes in pain levels, possibly related to comorbidities associated with aging rather than HZ infection per se. This should be carefully interpreted.
The strength of this study is its prospective study design with PHN case definition based on the validated ZBPI questionnaire, which accounted for various degrees of pain intensity [19]. One of the limitations of our study was that patients were limited to those who agreed to provide written informed consent and responded to the questionnaire survey. Thus, non-respondents and selection biases may have affected the overall results. In addition, there were variations in the observation period, and some patients may have had pain scores ≥ 3 at a later time point beyond their observation period, resulting in these scores not being captured in this study. This may have led to an underestimated duration and proportion of PHN.
Acknowledgements
The authors thank Drs. Kazuhiro Asano, Akihiro Watanabe, Riri Adachi, Mariko Kiuchi, Keiju Kobayashi, Kenji Iida, and Hiroyuki Nakamura (Investigator Dermatologist) for the study data collection, and would also like to thank Business & Decision Life Sciences [on behalf of GSK] for editorial assistance and manuscript coordination. Gregory Collet coordinated manuscript development and editorial support, and John Bean (Bean Medical Writing) provided medical writing services.
Author Contribution
AM, KS, KH, TK, and DC participated in the conception and design of the study; KA and TM participated in the collection and generation of the study data; DC and SM were involved in the study analysis; and AM, KS, KH, and DC participated in the interpretation of data.
Compliance with Ethical Standards
Conflict of interest
Koichi Adachi received a study grant from the GSK group of companies, personal fees for medical advice during the conduct of the study, and personal fees for lectures from the GSK group of companies outside the submitted work. Katsiaryna Holl, Desmond Curran, Keiko Sato and Toshihiko Kaise are employees of the GSK group of companies and hold shares in the GSK group of companies as part of their employee remuneration. Taizo Matsuki and Akiko Mizukami are employees of the GSK group of companies. Sean Matthews is a freelance consultant working on behalf of GSK. KS, TK, TM, and AM received funding from Japan Vaccine Co. Ltd (a 50/50 joint venture of GSK/Daiichi Sankyo Company, Ltd) for the conduct of the study.
Funding
GlaxoSmithKline Biologicals SA funded the study and was involved in all study activities and overall data management (collection, analysis and interpretation). They also funded all costs associated with developing and publishing this manuscript. All authors had full access to the data, and the corresponding author was responsible for submission of the publication (ClinicalTrials.gov identifier: NCT01873365).
Footnotes
Akiko Mizukami and Keiko Sato are co-first authors on this work.
References
- 1.National Institute of Infectious Diseases. Infectious Agents Surveillance Report (in Japanese). 2016;37:116–8. Available at: https://www.niid.go.jp/niid/ja/varicella-m/varicella-iasrd/6331-436d05.html. Accessed 1 Sept 2017.
- 2.Centers for Disease Control and Prevention. Shingles (Herpes Zoster). Clinical Overview. Available at: http://www.cdc.gov/shingles/hcp/clinical-overview.html. Accessed 6 July 2016.
- 3.Takao Y, Miyazaki Y, Okeda M, Onishi F, Yano S, Gomi Y, et al. Incidences of Herpes Zoster and Postherpetic Neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ study. J. Epidemiol. 2015;25:617–25. Available at: https://www.jstage.jst.go.jp/article/jea/25/10/25_JE20140210/_article. Accessed 5 July 2016. [DOI] [PMC free article] [PubMed]
- 4.Portal site of Official Statistics of Japan. Total population, October 1, 2014. Available at: http://www.e-stat.go.jp. Accessed 5 July 2016.
- 5.United Nations. Population Ageing and Development 2012. Available at: http://www.un.org/esa/population/publications/2012PopAgeingDev_Chart/2012PopAgeingandDev_WallChart.pdf. Accessed 5 July 2016.
- 6.Douglas MW, Johnson RW, Cunningham AL. Tolerability of treatments for postherpetic neuralgia. Drug Saf. 2004;27:1217–1233. doi: 10.2165/00002018-200427150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Wood AJJ, Kost RG, Straus SE. Postherpetic Neuralgia—pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 8.Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gater A, Uhart M, McCool R, Préaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15:193. doi: 10.1186/s12889-015-1514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 11.Tsai T-F, Yao C-A, Yu H-S, Lan C-C, Chao S-C, Yang J-H, et al. Herpes zoster-associated severity and duration of pain, health-related quality of life, and healthcare utilization in Taiwan: a prospective observational study. Int J Dermatol. 2015;54:529–536. doi: 10.1111/ijd.12484. [DOI] [PubMed] [Google Scholar]
- 12.Serpell M, Gater A, Carroll S, Abetz-Webb L, Mannan A, Johnson R. Burden of post-herpetic neuralgia in a sample of UK residents aged 50 years or older: findings from the Zoster Quality of Life (ZQOL) study. Health Qual. Life Outcomes. BioMed Central; 2014;12:92. [DOI] [PMC free article] [PubMed]
- 13.Gater A, Abetz-Webb L, Carroll S, Mannan A, Serpell M, Johnson R. Burden of herpes zoster in the UK: findings from the zoster quality of life (ZQOL) study. BMC Infect. Dis. 2014;14:402. Available at: http://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-14-402. Accessed 24 July 2017. [DOI] [PMC free article] [PubMed]
- 14.Lukas K, Edte A, Bertrand I. The impact of herpes zoster and post-herpetic neuralgia on quality of life: patient-reported outcomes in six European countries. Z Gesundh Wiss. 2012;20:441–451. doi: 10.1007/s10389-011-0481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinke T, Edte A, Schmitt S, Lukas K. Impact of herpes zoster and post-herpetic neuralgia on patients’ quality of life: a patient-reported outcomes survey. J Public Health. 2010;18:367–74. Available at: http://link.springer.com/10.1007/s10389-010-0323-0. Accessed 24 July 2017. [DOI] [PMC free article] [PubMed]
- 16.Kubota Y, Moriue J, Moriue T, Nakai K, Yokoi I, Fujita N, et al. Quality of life assessment for patients with herpes zoster and the effect of an oral anti-herpetic on their quality of life. Med Consult New Remedies. 2009;46:922–927. [Google Scholar]
- 17.König H-H, Bernert S, Angermeyer MC, Matschinger H, Martinez M, Vilagut G, et al. Comparison of population health status in six european countries: results of a representative survey using the EQ-5D questionnaire. Med Care. 2009;47:255–261. doi: 10.1097/MLR.0b013e318184759e. [DOI] [PubMed] [Google Scholar]
- 18.Sato K, Adachi K, Nakamura H, Asano K, Watanabe A, Adachi R, et al. Burden of herpes zoster and post-herpetic neuralgia in Japanese adults 60 years of age or older: results from an observational, prospective, physician practice-based cohort study. J Dermatol. 2016;44:414–422. doi: 10.1111/1346-8138.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Japanese Ministry of Health Labour and Welfare. Ethical Guidelines for Epidemiological Research. http://www.mhlw.go.jp/seisakunitsuite/bunya/hokabunya/kenkyujigyou/i-kenkyu/dl/02-02.pdf. Accessed 25 July 2017.
- 21.EuroQol Group EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11:341–353. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hays R. Ron hays: programs and utilities, scoring the SF-12. Available at: http://gim.med.ucla.edu/FacultyPages/Hays/utils/. Accessed 25 July 2017.
- 25.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 26.Bricout H, Perinetti E, Marchettini P, Ragni P, Zotti CM, Gabutti G, et al. Burden of herpes zoster-associated chronic pain in Italian patients aged 50 years and over (2009–2010): a GP-based prospective cohort study. BMC Infect Dis. 2014;14:637. doi: 10.1186/s12879-014-0637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Seventer R, Sadosky A, Lucero M, Dukes E. A cross-sectional survey of health state impairment and treatment patterns in patients with postherpetic neuralgia. Age Ageing. 2006;35:132–137. doi: 10.1093/ageing/afj048. [DOI] [PubMed] [Google Scholar]
- 28.Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract. 2011;11:397–402. doi: 10.1111/j.1533-2500.2010.00432.x. [DOI] [PubMed] [Google Scholar]
- 29.Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182:1731–1736. doi: 10.1503/cmaj.091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342–348. doi: 10.1086/421942. [DOI] [PubMed] [Google Scholar]
- 31.Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6:356–363. doi: 10.1016/j.jpain.2005.01.359. [DOI] [PubMed] [Google Scholar]
- 32.Song H, Lee J, Lee M, Choi WS, Choi JH, Lee MS, et al. Burden of illness, quality of life, and healthcare utilization among patients with herpes zoster in South Korea: a prospective clinical-epidemiological study. Int J Infect Dis. 2014;20:23–30. doi: 10.1016/j.ijid.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Duracinsky M, Paccalin M, Gavazzi G, El Kebir S, Gaillat J, Strady C, et al. ARIZONA study: is the risk of post-herpetic neuralgia and its burden increased in the most elderly patients? BMC Infect Dis. 2014;14:529. doi: 10.1186/1471-2334-14-529. [DOI] [PMC free article] [PubMed] [Google Scholar]