Abstract
Tree-level intrinsic water-use efficiency (iWUE) is derived from the tree-ring 13C isotope composition (δ13C) and is an important indicator of the adaptability for trees to climate change. However, there is still uncertainty regarding the relationship between long-term forest ecosystem carbon sequestration capacity and iWUE. To determine whether elevated atmospheric CO2 concentration (Ca) increase iWUE and tree growth (basal area increment, BAI), dendrochronological methods and stable isotope analyses were used to examine annual changes in the tree-ring width and carbon isotope composition (δ13C) of Platycladus orientalis in northern China. The iWUE derived from δ13C has increased significantly (p < 0.01). Long-term iWUE trend was largely and positively driven by the elevated atmospheric CO2 concentration and temperature. We observed a general increase in averaged BAI, which had significant positive correlation with iWUE (R2 = 0.3186, p < 0.01). Increases in iWUE indeed translated into enhanced P. orientalis growth in semi-arid areas of northern China. Elevated atmospheric CO2 concentration significantly (p < 0.01) stimulated P. orientalis biomass accumulation when Ca was less than approximately 320 ppm in the early phase; however, this effect was not pronounced when Ca exceeded 320 ppm.
Introduction
Human activities, including the burning of fossil fuels and changes in land use, have altered the composition of the atmosphere and influenced terrestrial biomes via climate change, particularly in developing countries1,2. During the last 150 years, the CO2 mole fraction in the atmosphere has increased from 280 to 380 µmol mol−1, 3. Long-term forest productivity and carbon sequestration capacity are affected by climate change, and have become a global concern4. The ability to use water and nutrient resources plays a significant role in the adaptation of taxa in terrestrial biomes to variations in the environment5. Tree-level intrinsic water-use efficiency (iWUE) is estimated as the ratio of net photosynthetic CO2 assimilation (A) to stomatal conductance (gs). iWUE is an important component of water-carbon coupling and process management in terrestrial ecosystems6, as well as a way for trees to adapt the changing environment7,8. Stomatal conductance is expected to decline as the concentration of CO2 in the environment increases9,10, which affects the gradient between atmospheric CO2 (Ca) and internal leaf CO2 (Ci), and in turn determines the 13C isotope composition (δ13C) of assimilated carbon5. The “Suess effect” refers to a change in the ratio of the atmospheric concentrations of heavy isotopes of carbon (13C), resulting from the admixture of large amounts of fossil-fuel derived CO2, which is depleted in 13CO2 11. During carbon fixation, fractionation associated with physical and enzymatic processes causes plant matter to become 13C-depleted in comparison with the air. To distinguish variations in the δ13C of source CO2 from the effects of plant metabolic processes, the δ13C signatures of plant organic material are usually translated to carbon isotope discrimination (Δ13C), and Δ13C is related to the ratio of Ci to Ca during photosynthesis in C3 plants12. Thus, any variability in the iWUE is recorded in the carbon isotopes of tree tissues. Because carbon builds up in tissues over time, the δ13C values do not represent the iWUE in a given instant but rather the average iWUE during the formation of tree-tissue organic matter.
Many studies have shown that rising atmospheric CO2 concentrations can speed up tree growth by stimulating photosynthesis in a process known as “CO2 fertilization”; however most of these studies were conducted over short time periods in growth chambers with seedlings or young plants7,13,14. The long-term responses of forests to gradually changing environment are complicated by factors that affect several fluxes at the same time, and by complex interactions among the factors15,16. For example, the direct response of photosynthesis to an increase in atmospheric CO2 concentration will require additional N and other nutrients, and the production response will therefore decline as nutrient limitation progresses. Results derived from short-term experiments may not represent longer-term responses of plants to multiple factors in the natural environment4. Compared with short-term experiments, tree ring information provides a more reliable summary of physiological responses to environmental variations over time and can be used to predict the behavior of mature trees17–21. Studies of tree-ring stable carbon isotopes at a global scale have found variable tree responses to climate change, reflecting the complex relationship between environmental variability and tree growth, and suggest that adaptation strategies vary widely across species and sites22–25. Our knowledge of the long-term relationship between iWUE and tree growth remains incomplete. Tree rings are ideal proxies for assessing changes in physiology and growth over time and under different environmental conditions.
The Yanshan Mountains of northern China, between the Mongolia Plateau and the North China Plain, are forested and dominated by P. orientalis. This area plays a significant role in the global carbon cycle due to its typical monsoon climate. Platycladus orientalis, an evergreen coniferous tree species that is endemic to China, is the main tree species used for afforestation in the semi-arid areas of northern China. Climate change research in China over the last century has focused on the importance of the adaptation strategies and productivity of typical evergreen conifers in the semi-arid region of northern China13. In this study, we examined annual changes in carbon isotope composition and tree-ring width, investigating trends in tree-ring carbon discrimination (Δ13C) and iWUE over the last century in P. orientalis of the Yanshan Mountains. The specific goals of the study were to (1) determine whether tree-level iWUE increased over time (i.e., across subsequent calendar years) and whether that translated into tree growth and (2) assess how iWUE and tree growth (as measured by the basal area increment [BAI]) responded to changes in atmospheric CO2 concentrations, annual temperature, and precipitation.
Materials and Methods
Study sites, tree sampling, and analysis
The study was carried out in the Hongmenchuan watershed (40°26′N, 117°10′E) of northern China, which is an important ecological refuge for Beijing and more than two million people. The Hongmenchuan watershed, located in the transition zone of the North China Plain and the Mongolia Plateau, has a complex geology and belongs to the Yanshan Mountain range (200–1200 m a.s.l.). The region is characterized by a sub-humid, warm and temperate continental monsoon climate. The vegetation in the Hongmenchuan watershed is natural forests with no forest management, dominated by P. orientalis with a density of 739 trees per ha, along with the co-dominant tree species Pinus tabulaeformis, with a density of 116 trees per ha; these data were obtained through sample plot investigation (data not shown).
The detailed information for each plot is shown in Table 1 26. P. orientalis is mainly distributed in areas with an altitude of 200 m–600 m, on shady and sunny slopes27. Therefore, we selected 10 typical plots with altitudes ranging from 332 m to 562 m located on each slope aspect. Within each plot, four P. orientalis trees that were at least 90 years of age were selected for sampling. Two or three cores were taken from each tree at breast height (1.3 m) from different sections of the stem using a 5-mm increment borer (a total of 92 cores were collected). To prevent contamination from other carbon sources, the collected samples were stored in glass tubes.
Table 1.
Geographical, topographical, and structural characteristics of the sampled sites. The numbers 1 to 10 represent the site codes. DBH (cm) is diameter at breast height. Values shown are the means ± SE.
| Site code | Latitude (N) | Longitude (E) | Aspect | Elevation (m) | Slope (°) | DBH (cm) | Height (m) |
|---|---|---|---|---|---|---|---|
| 1 | 40°28′ | 117°8′ | NE | 562 | 30 | 21.4 ± 3.7 | 18.5 ± 1.5 |
| 2 | 40°28′ | 117°7′ | NE | 537 | 16 | 20.9 ± 4.3 | 18 ± 1 |
| 3 | 40°27′ | 117°9′ | NW | 496 | 19 | 22.3 ± 4.6 | 20.5 ± 1.5 |
| 4 | 40°26′ | 117°10′ | N | 435 | 26 | 20.7 ± 2.9 | 17 ± 2 |
| 5 | 40°25′ | 117°8′ | SE | 508 | 27 | 21.6 ± 4.4 | 19.5 ± 1 |
| 6 | 40°26′ | 117°7′ | SW | 522 | 23 | 22.5 ± 3.4 | 20.5 ± 1 |
| 7 | 40°25′ | 117°7′ | SE | 369 | 25 | 23.4 ± 3 | 22.5 ± 1.5 |
| 8 | 40°24′ | 117°5′ | SW | 332 | 21 | 22.3 ± 4.5 | 21.5 ± 2 |
| 9 | 40°24′ | 117°6′ | S | 414 | 22 | 23.9 ± 3.7 | 22 ± 1.5 |
| 10 | 40°25′ | 117°4′ | SE | 420 | 20 | 21.2 ± 2.8 | 20.5 ± 1 |
Ring-width, BAI, and stable carbon isotope analysis
The core samples were sanded with grain paper that varied from 100 to 800 mesh to make the tree-ring more clearly visible for cross-dating. Then, COFECHA was used to check dating, find errors in the individual series, and improve the cross-dating accuracy7,28. Eight of the cores had rotted or had been damaged by woodworms, and 11 of the cores were missing rings, which would cause inaccuracies in the test data. Cores with poor quality were excluded from analysis. Thus, only 71 cores were selected for analysis, and these were divided into two sections: 35 cores were used for width measurements, and the remaining 36 cores were used to evaluate stable carbon isotopes. After natural drying, fixation, and grinding, tree ring widths were measured at a resolution of 0.01 mm from each of the cores using LINTAB 6 measurement equipment (Frank Rinn, Heidelberg, Germany) and the data were analyzed using Time Series Analysis and Presentation (TSAP) software package (Frank Rinn, Heidelberg, Germany). Cross-dating of the tree-ring data was verified using COFECHA, which assesses the quality of cross-dating and the measurement accuracy of tree-ring series using a segmented time-series correlation technique28.
Tree-ring width decreases with age in mature trees due to the diameter growth of the stem. Therefore, BAI is more appropriate than DBHI (diameter at breast height increment) for modeling tree growth and forest productivity. BAI was estimated according to the following formula:
| 1 |
where R is the radius at breast height and n is the year of tree-ring formation. We measured tree-ring width of 35 cores included 12 tree-ring cores more than 150 years older. We calculated BAI with the beginning width in 1855 calendar year, then we gave up the first 25 years data of each core considering the less number of sample cores and “juvenile age effects” might rise the errors of tree-ring widths (similar consideration applied to the analysis of tree-ring δ13C)13. Then we calculated the BAI between 1880 to 2014 of each tree.
Prior to the isotope analysis, the first 25 years of each core were removed to avoid “juvenile age effects” on the tree-ring isotopes13. In this study, pooling was necessary because P. orientalis is a slow-growing conifer with very narrow rings, making it difficult to obtain enough material to measure the individual isotopic series. In addition, the small ring size would have created an unacceptably high risk of measurement error. Several recent studies have tested and proved the representativeness of pooled isotopic series for tree-ring δ13C compared to individual isotopic series29–34. The cores were divided into individual rings using a scalpel to cut along ring lines under a stereomicroscope (40× magnification) and rings within the same year were pooled. Earlywood and latewood were not separated for the isotopic analyses, as recent studies have shown that there is no difference between the two wood types at an isotopic level35,36. Each pooled sample was ground into a powder using a ball mill, followed by sieving through an 80-mesh sieve. Cellulose was extracted from all samples to avoid isotope variations during the iWUE calculation, so that they were based purely on the changes in the relative abundance of individual wood constituents that typically have different isotope signatures37. The method for cellulose extraction was modified from Loader38. Cellulose samples and whole wood samples were used for δ13C analysis using a high temperature reactor (HT-O, HEKAtech GMBH, Wegberg, Germany) coupled to a DELTAplus XP Mass Spectrometer (ThermoFinnigan, Thermo Fisher Scientific Inc., Waltham, MA, USA). Each sample was analyzed three times, and the average values were calculated.
The results from the isotope ratio deviations are presented using the common δ notation:
| 2 |
where R refers to the ratio of the 13C to 12C isotopes in the sample (‘sa’) and the reference [‘re’, compared to the PDB (Pee Dee Belemnite) standard].
Therefore, cellulose Δ13C was calculated as follows:
| 3 |
where δ13Ca and δ13Cp are the ratios in atmospheric CO2 and tree-ring cellulose, respectively.
The relative rates of carbon fixation and stomatal conductance are the primary factors that determine Δ. According to the model proposed by Farquhar12, Δ13C and δ13C have different trends.
| 4 |
where a is the discrimination against 13CO2 during CO2 diffusion through the stomata (a = 4·4‰), b is the discrimination associated with carboxylation (b = 27‰), and Ci and Ca are the intercellular and ambient CO2 concentrations, respectively.
Fick’s law is as follows:
| 5 |
The ratio of leaf conductance to water vapor is 1.6 g CO2, and the change in 13C can be related to the ratio as follows:
| 6 |
iWUE was calculated using the following equation:
| 7 |
Climate data, CO2 records, and δ13Ca
All weather data were obtained from the Miyun National Forest Farm Meteorological Station (117°07′E, 40°22′N) located at 352 m a.s.l., 5 km from the study area. The data were checked for quality and consistency, and included the annual minimum, mean, and maximum temperatures, as well as annual precipitation, from 1951 to 2014. As Fig. 1 shows, mean annual temperatures in the study area ranged from 10.45 °C to 14.02 °C, with an average temperature of 12.2 °C, and there was an increasing trend of 0.039 °C per year during the study period. A similar trend was seen for the minimum annual temperatures and no obvious change was observed in maximum temperature. Annual precipitation exhibited a slight decrease over the study period (−3.4625 mm per year), and differed significantly among calendar years, ranging from 261.4 mm to 1404.6 mm. Annual atmospheric CO2 concentration records have been monitored at the Shangdianzi Atmospheric Background Station since 1981. Data prior to 1981 were obtained from the National Oceanic and Atmospheric Administration. We used δ13Ca data that were recorded at the Shangdianzi National Atmospheric Background Station (117°07′01″E, 40°39′00″N), located at 112 m.a.s.l., 31.8 km from the study area.
Figure 1.
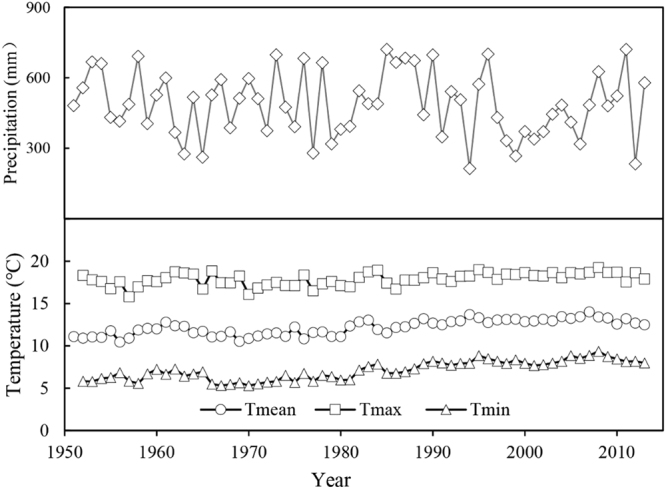
Annual observations of meteorological conditions during the study period: precipitation and mean (Tmean), minimum (Tmin), and maximum (Tmax) temperature between 1951 and 2014.
Statistical analysis
To model tree growth, we measured all cores ring widths and calculated BAI of each tree and then averaged them. One-way Analysis of Variance (ANOVA) was used to examine the BAI variation among trees. We used pooled wood samples, mixing material of the same cross-dated ring for measuring annual δ13C and then calculated iWUE. Regression analyses and t-test were used to identify significant trends in δ13C, Δ13C, iWUE, and BAI from 1880 to 2014, and to test the relationships between iWUE and BAI, between iWUE and environmental factors (include atmospheric CO2 concentration, annual temperature and precipitation). R2 and slope p-values were also estimated. All statistical analyses were carried out using the SPSS 11.0 statistical software package (SPSS, Chicago, IL, USA).
Results
Patterns of δ13C, Δ13C, iWUE, and BAI
Figure 2 shows the whole wood δ13Cw and cellulose δ13Cc trends under decreased stable atmospheric carbon isotopes (δ13Ca) (“Suess effect”) from 1880 to 2014. The t-test revealed that a significant difference between δ13Cw and δ13Cc, implying that δ13Cc cannot be replaced by δ13Cw. We detected a decreased trend in both δ13Cw and δ13Cc curves. The δ13Cc pattern could be divided into approximately three periods, i.e., two plateau periods from 1880 to 1917 and from 1975 to 2014 and one decreasing period from 1918 to 1974. The δ13Cc declined from 1918 to 1974 and exhibited considerable fluctuation during that time, and both the maximum (−21.57‰, 1918) and minimum (−23.97‰, 1962) values were detected in that period. By comparison, there was no clear trend in the other two periods, during which time the δ13Cc curve was relatively stable.
Figure 2.
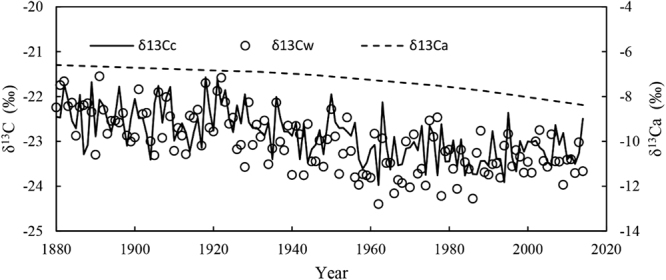
Annual variations in: the tree ring cellulose stable carbon isotope (δ13Cc, polyline) record for P. orientalis, the tree ring whole wood stable carbon isotope (δ13Cw, circles) record for P. orientalis, and the atmospheric stable carbon isotope (δ13Ca, dotted line) between 1880 and 2014. We connected the annual δ13Cc points to a polyline, for convenient comparison with δ13Cw.
iWUE increased significantly, particularly since 1974, with changes in the Ci and CO2 concentrations (Fig. 3), while the discrimination (Δ13C) trends exhibited the opposite pattern (a decrease). There was a significant increase in iWUE, at a rate of 0.49 μmol mol−1 per year, throughout the last century (p < 0.05). iWUE ranged from 82.35 μmol mol−1 (1920) to 138.12 μmol mol−1 (2014) during the study period, and a significant increase was detected from 1974 to 2014, at a rate of 1.1 μmol mol−1 per year. Higher Ci values were associated with lower discrimination (Δ13C) and higher iWUE.
Figure 3.
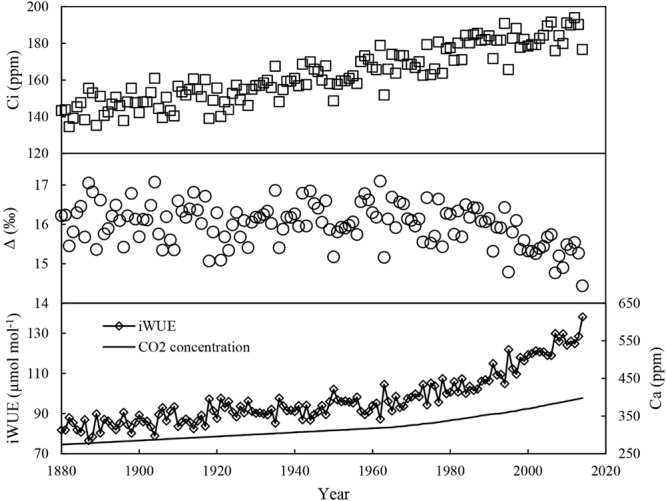
Annual variations in the tree ring indices for P. orientalis: (a) intercellular CO2 concentrations (Ci), (b) tree ring cellulose carbon isotope discrimination (Δ13C), (c) intrinsic water use efficiency (iWUE) derived from tree ring cellulose carbon isotopes (δ13Cc) and atmospheric CO2 concentrations between 1880 and 2014.
Tree-ring width tended to fluctuate during the last century, ranging from 0.029 cm to 0.193 cm, with an average of 0.1 cm (Fig. 4). Tree-ring width increased initially (from 1880 to 1961) and then decreased from 1962 to 2001, as shown in Fig. 4. Since 2002, there has been a slight increase in tree-ring width. The averaged BAI showed a significant quadratic curve increase from 1880 to 2014 (R2 = 0.6353, p < 0.01), especially during the early years of growth, after which the increasing trend weakened since 1960. The increase in BAI in the earlier decades ranged from 2.01 cm2 to 19.7 cm2, which accounted for approximately 82.7% of the total increase, indicating that the trees grew rapidly during that period. The rapid increasing phase was followed by a decrease in BAI, which has fluctuated since the 1960s.
Figure 4.
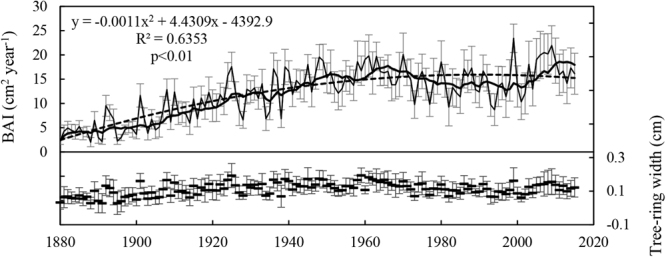
Annual variations in tree-ring width and averaged basal area increment (BAI, cm2) for P. orientalis. The solid line represent the averaged BAI and the bold curve represents the smoothed results using a 10-year fast Fourier transform (FFT) filter to emphasize long-term variations. The dotted line is the trend line. Error bars indicate the standard error of the means.
Relationships between iWUE and temperature, precipitation, and atmospheric CO2
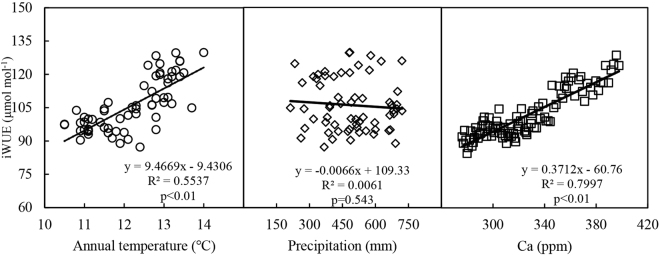
Correlations between iWUE and annual temperature were significant and positive (R2 = 0.5537, p < 0.01, Fig. 5). The slope of the regression was 9.4669, indicating that every 0.1 °C increase in annual temperature was accompanied by an by a 1.21 μmol mol−1 increase in iWUE per year. Annual precipitation fluctuated greatly, ranging from 250 mm to 800 mm, and decreased in recent years. However, no significant relationship between iWUE and precipitation was observed. The results of the correlation analysis indicated that iWUE was positively correlated with elevated Ca (r 2 = 0.7997, p < 0.01).
Figure 5.
Relationships between iWUE and annual temperature, precipitation, and atmospheric CO2 concentration (Ca). Lines indicate the change trends. The regression equations (iWUE and annual temperature, iWUE and annual precipitation, iWUE and annual atmospheric CO2 concentration), coefficients, and levels of significance are also shown.
Relationships between BAI and temperature, precipitation, Ca, and iWUE
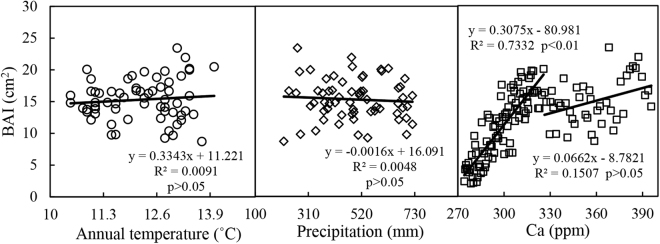
No significant relationship was observed between BAI and annual temperature (R2 = 0.0091, p > 0.05, Fig. 6), indicating that the influence of annual temperature on BAI was not as strong as that on iWUE. In addition, the relationship between BAI and precipitation were weak and not significant (R2 = 0.0048, p > 0.05). There was a nonlinear relationship between BAI and Ca. Elevated atmospheric CO2 concentration significantly (p < 0.01) stimulated P. orientalis biomass accumulation when Ca was less than approximately 320 ppm in the early phase; however, this effect was not pronounced when Ca exceeded 320 ppm. The correlation between BAI and iWUE was positive and significant (p < 0.01, Fig. 7).
Figure 6.
Relationships between averaged BAI and annual temperature (1951–2014), precipitation (1951–2014), and atmospheric CO2 concentration (Ca) (1880–2014). Lines represent the change trends. Regression equations (averaged BAI and annual temperature, annual atmospheric CO2 concentration), coefficients, and levels of significance are also shown.
Figure 7.
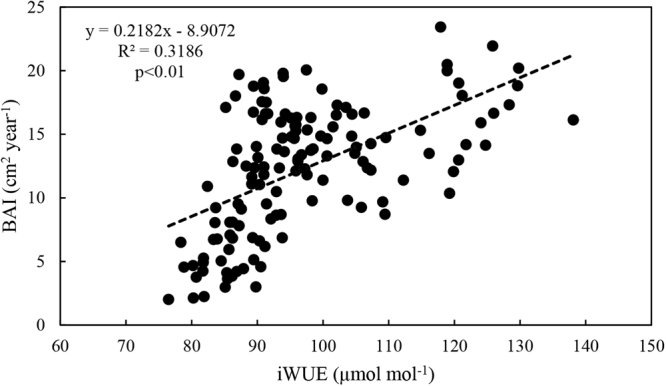
Relationship between averaged BAI and iWUE. Lines represent the change trends. Regression equations (averaged BAI and iWUE), coefficients, and levels of significance are also shown.
Discussion
We found that in the first half of the last century, the error in the P. orientalis tree-ring δ13C was relatively high, but it decreased gradually over time. It seems that saplings exhibited varying responses to environmental change; this, can be described as the “juvenile effect”. One reason for this age-related trend in δ13C could be the recycling of respired air that has already been depleted of 13C by young trees growing close to the forest floor. Plant tissue δ13C is significantly lower than that found in the atmosphere because atmospheric CO2 contains 98.9% of light-stable carbon isotope and 1.1% heavy-stable carbon isotope, and the light-stable carbon isotope is preferentially absorbed during photosynthesis, resulting in carbon isotope fractionation, which is known as the “carbon isotope effect”39. This process may be influenced by biotic factors, such as tree age and height, as well as abiotic factors such as meteorological conditions (radiation regime, temperature, precipitation, atmospheric pressure, etc.), CO2 concentration, site conditions (water availability, nutritional constraints, elevation and slope exposure, etc.), and forest management and anthropogenic perturbations (fires, climate change, etc.)31,39–41. Chen et al.42 suggested that altitudinal variation in δ13C may be negative or unchanged for plants growing in arid and/or semiarid regions, and atmospheric pressure has a negative effect on plant δ13C. Di Matteo et al.43 conducted a replicated field trial to characterize the interacting effects of aspect and intensifying drought on foliar δ13C and to quantify temporal variation in stomatal conductance. They found a significant reduction in stomatal conductance (p < 0.01) as the drought increased along a sunny slope, and foliar δ13C increased significantly (p < 0.01). In this study, we selected 10 typical plots, for which the altitude ranged from 332 m to 562 m and located on two slope aspects, to carry out our study with the original intention of understanding the average conditions of iWUE and tree growth of P. orientalis in northern China. Therefore, we did not take into account the effect of differences in exposure and elevation on tree-ring δ13C.
During the study period, the P. orientalis iWUE was significantly and positively correlated with annual temperature. This result agrees with research conducted on other tree species around the world7,37,44. This phenomenon can be explained by plant ecophysiology. First, when plants are subjected to high temperatures their stomata partially close, and then gs decreases to prevent an excessive loss of water, thereby reducing the internal CO2 concentration of the leaves. The absorption and utilization rate of internal leaf CO2 increase to maintain high photosynthetic rate (An). At the same time, an increase in temperature causes an increase in the vapor-pressure deficit and in the transpiration rate (Tr); accordingly, iWUE and temperature were positively related in this study. The number of days for which the daily maximum temperature was higher than 30 °C increased substantially in the study area each year, together with the frequency of extreme temperature values, leading to reduced stomatal conductance and altering the environmental and physiological functions of P. orientalis.
Following the second industrial revolution, atmospheric CO2 concentrations have gradually increased, rising from 276.1 ppm to 397.6 ppm in northern China according to the China Global Atmosphere Watch Baseline Observatory. We found that the iWUE increased significantly under elevated atmospheric CO2 concentrations. Our findings are consistent with other studies conducted on the effects of elevated CO2 on leaf physiological activity, which demonstrating that increased CO2 promotes WUE (determined by traditional measurement methods: weighing, gas exchange, etc.)45,46 Jackson et al.47 found that increased CO2 led to 50% decreases in stomatal conductance and transpiration, while the photosynthetic rate increased by 70% and WUE nearly doubled with sufficient illumination. Curtis et al.48 found that leaf photosynthetic products increased by approximately 50% under elevated CO2 concentrations after combining 38 groups of experimental data. Gagen et al.49 noted that northern boreal Pinus sylvestris may have reached a threshold with respect to its ability to increase WUE under rising atmospheric CO2 concentrations.
In this study, we used the averaged BAI to characterize trees growth. Then we did the ANOVA between averaged BAI and each tree’s BAI, a total of 35 pairs of data series, found that the 77.1% pairs had no significant difference. Thus the variation among trees could be ignored and averaged BAI could represent most trees growth in this region. The averaged BAI showed a significant increasing tendency (p < 0.01); however, the coefficient of determination between BAI and iWUE was relatively low (R2 = 0.3299), and there was still some difference values between before and after the 1970s. The BAI increased significantly before the 1970s and then fluctuated but did not increase significantly. It seems that there is a limitation beyond which the increase of BAI would abate. Some scholars found that when atmospheric CO2 concentration exceeds approximately 350 ppm, the BAI decreased with increasing atmospheric CO2 concentration4. In this study, we found that the tipping point of the concentration for the BAI response curve was approximately 320 ppm. Thus, there is a contrast between the results of short-term experiments and long-term studies. A notable difference is nitrogen availability, which may positively stimulate leaf area, photosynthesis, and plant growth50. However, the effect of nitrogen on plants may be less pronounced or may even become negative over time due to altered soil N transformation processes and progressive nutrient limitations in ecosystems approaching and surpassing N saturation4,51. It is likely that these effects caused the difference in the results between short-term experiments and long-term studies.
In addition, there was no clear response of the iWUE to changes in precipitation. In the rocky mountainous area of northern China, precipitation mainly causes increases in runoff with some percolation through the soil. Changes in precipitation typically affect plant growth conditions by altering the soil moisture content52. There were no significant changes in precipitation during the study period and only a slight decrease was observed after the year 2000. In any case, the inter-annual variability of rainfall is very high, and we did not found any relationship between annual precipitation and iWUE or BAI. It seems that P. orientalis is well adapted to dry conditions and can cope withthe limitation posed by dry conditions by increasing iWUE. This finding agrees with that of Niinemets et al.53 for evergreens that exhibited greater responsiveness to increased CO2.
Many studies have focused on the relationship between tree growth and water use efficiency, but it is unclear whether increased water use efficiency translates into greater tree growth54,55. Sleen56 found that while increasing atmospheric CO2 concentrations generally result in more efficient water use in the monsoon forests of Thailand, tree growth still tends to decline. This means that changes in other limiting climatic factors exert stronger opposing effects on tree growth. Wang et al.57 found that since 1800, tree radial growth was not always associated with increased iWUE in the Xinglong Mountains of northwestern China; in fact, tree growth rates declined during periods of drought and particularly in the past decade. These results suggested that increases in iWUE are unable to compensate for the negative effects of severe limitations on tree radial growth.
In contrast with other studies, we found evidence for long-term increases in BAI that were associated with increases in iWUE. P. orientalis growth increased as iWUE increased in the mountainous area of northern China due to drought tolerance and high temperature resistance. In addition, the long-term water use efficiency of the P. orientalis forest ecosystem increased under elevated CO2 concentrations and rising temperatures, suggesting that trees overcame the unfavorable factors and made full use of favorable growth conditions. This is mainly because of the special leaf structure, i.e., photosynthetic limitation by mesophyll diffusion varies strongly with the leaf structure58. This limitation caused by internal diffusion conductance is expected to be particularly large in sclerophyllous evergreens such as P. orientalis, which have a structure that enables them to adapt to water limitations59. Furthermore, P. orientalis, supports numerous leaf age classes, leading to high leaf-area indices as 8–13 m2 m−2 on a projected leaf area basis60. With a more robust leaf structure, typical reductions in stomatal conductance observed in response to elevated CO2 are likely to alter photosynthesis reduction, and generate a stronger response of iWUE53,61. In the context of climate change, the eco-physiological functions of the P. orientalis forest ecosystem are not inhibited, and maintains a high potential for carbon sequestration.
Conclusions
Global climate change has had significant impacts on forest ecosystem function worldwide, and these effects vary across regions. Understanding the responses of iWUE and tree growth to climate change has great importance for predicting how plants may respond to climate variations in the future and its implications for forest management and conservation. In our study, we detected a significantly increasing trend in iWUE curves (p < 0.01) and a significant positive correlation between iWUE and BAI (p < 0.01). Long-term iWUE trend could be largely and positively driven by the elevated CO2 concentration and temperature. Elevated atmospheric CO2 concentration stimulated P. orientalis BAI accumulation significantly (p < 0.01) when Ca was below about 320 ppm in the early phase. Then the promotion effect were not pronounced when Ca was above 320 ppm. There was not a decrease in the growth of drought-tolerant conifers in northern China, suggesting that growth of P. orientalis in semi-arid areas benefited from the elevated atmospheric CO2 concentrations. The study outcomes may be used to improve forest management and conservation in semi-arid mountainous areas.
Acknowledgements
The research project on which this paper is based was funded by the National Natural Science Foundation of China (No. 41430747), the Graduate Training and Development Program of the Beijing Municipal Commission of Education (BLCXY201624), and the Beijing Municipal Education Commission (CEFF-PXM2017_014207_000043). All experiments were in compliance with the current laws of the country in which they were performed.
Author Contributions
Weiwei Lu and Hanzhi Li designed and performed the experiment. Weiwei Lu analyzed the data and wrote the manuscript. Guodong Jia and Ziqiang Liu contributed significantly to data analysis, manuscript preparation and practice of experiment. Xinxiao Yu revised the paper and finished the submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Climate change 2013: The Physical Science Basis., Cambridge, UK: Cambridge University Press. http://www.ipcc.ch/report/ar5/wg1/#.Uq_tD7KBRR1 (2013).
- 2.Silva LC, Horwath WR. Explaining global increases in water use efficiency: why have we overestimated responses to rising atmospheric CO(2) in natural forest ecosystems? PLoS One. 2013;8:e53089. doi: 10.1371/journal.pone.0053089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibt U, Rajabi A, Griffiths H, Berry JA. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia. 2008;155:441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- 4.Sun FF, et al. Long-term tree growth rate, water use efficiency, and tree ring nitrogen isotope composition of Pinus massoniana L. in response to global climate change and local nitrogen deposition in Southern China. Journal of Soils and Sediments. 2010;10:1453–1465. doi: 10.1007/s11368-010-0249-8. [DOI] [Google Scholar]
- 5.Battipaglia G, et al. Elevated CO2 increases tree‐level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytologist. 2013;197:544–554. doi: 10.1111/nph.12044. [DOI] [PubMed] [Google Scholar]
- 6.Osmond, C. B., Björkman, O. & Anderson, D. J. Physiological processes in plant ecology: toward a synthesis with Atriplex. Vol. 36 (Springer Science & Business Media, 2012).
- 7.Nock CA, et al. Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Global Change Biology. 2011;17:1049–1063. doi: 10.1111/j.1365-2486.2010.02222.x. [DOI] [Google Scholar]
- 8.Farquhar, G., Hubick, K., Condon, A. & Richards, R. in Stable isotopes in ecological research 21–40 (Springer, 1989).
- 9.Field CB, Jackson RB, Mooney HA. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant, Cell & Environment. 1995;18:1214–1225. doi: 10.1111/j.1365-3040.1995.tb00630.x. [DOI] [Google Scholar]
- 10.Ainsworth EA, Long SP. What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 11.Tans P, De Jong A, Mook W. Natural atmospheric 14C variation and the Suess effect. Nature. 1979;280:826–828. doi: 10.1038/280826a0. [DOI] [Google Scholar]
- 12.Farquhar GD, O’Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Functional Plant Biology. 1982;9:121–137. [Google Scholar]
- 13.Liu X, et al. Tree growth and intrinsic water-use efficiency of inland riparian forests in northwestern China: evaluation via delta13C and delta18O analysis of tree rings. Tree Physiol. 2014;34:966–980. doi: 10.1093/treephys/tpu067. [DOI] [PubMed] [Google Scholar]
- 14.Peñuelas J, Canadell JG, Ogaya R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Global Ecology and Biogeography. 2011;20:597–608. doi: 10.1111/j.1466-8238.2010.00608.x. [DOI] [Google Scholar]
- 15.Hietz P, Wanek W, Dünisch O. Long-term trends in cellulose δ13 C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree physiology. 2005;25:745–752. doi: 10.1093/treephys/25.6.745. [DOI] [PubMed] [Google Scholar]
- 16.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451:293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 17.Karnosky D, et al. Tropospheric O3 moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project. Functional Ecology. 2003;17:289–304. doi: 10.1046/j.1365-2435.2003.00733.x. [DOI] [Google Scholar]
- 18.Norby RJ. Introduction to a virtual special issue: probing the carbon cycle with 13C. New Phytologist. 2009;184:1–3. doi: 10.1111/j.1469-8137.2009.03020.x. [DOI] [PubMed] [Google Scholar]
- 19.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 20.Domec JC, et al. Acclimation of leaf hydraulic conductance and stomatal conductance of Pinus taeda (loblolly pine) to long-term growth in elevated CO2 (free-air CO2 enrichment) and N-fertilization. Plant Cell and Environment. 2009;32:1500–1512. doi: 10.1111/j.1365-3040.2009.02014.x. [DOI] [PubMed] [Google Scholar]
- 21.Maseyk K, Hemming D, Angert A, Leavitt SW, Yakir D. Increase in water-use efficiency and underlying processes in pine forests across a precipitation gradient in the dry Mediterranean region over the past 30 years. Oecologia. 2011;167:573–585. doi: 10.1007/s00442-011-2010-4. [DOI] [PubMed] [Google Scholar]
- 22.Martínez‐Vilalta J, López B, Adell N, Badiella L, Ninyerola M. Twentieth century increase of Scots pine radial growth in NE Spain shows strong climate interactions. Global Change Biology. 2008;14:2868–2881. doi: 10.1111/j.1365-2486.2008.01685.x. [DOI] [Google Scholar]
- 23.Liu, X. et al. Response and dendroclimatic implications of δ13C in tree rings to increasing drought on the northeastern Tibetan Plateau. Journal of Geophysical Research: Biogeosciences113 (2008).
- 24.Silva LCR, Anand M, Oliveira JM, Pillar VD. Past century changes in Araucaria angustifolia (Bertol.) Kuntze water use efficiency and growth in forest and grassland ecosystems of southern Brazil: implications for forest expansion. Global Change Biology. 2009;15:2387–2396. doi: 10.1111/j.1365-2486.2009.01859.x. [DOI] [Google Scholar]
- 25.Dorman M, Perevolotsky A, Sarris D, Svoray T. The effect of rainfall and competition intensity on forest response to drought: lessons learned from a dry extreme. Oecologia. 2015;177:1025–1038. doi: 10.1007/s00442-015-3229-2. [DOI] [PubMed] [Google Scholar]
- 26.West, P. W. Tree and forest measurement. Vol. 20 (Springer, 2009).
- 27.Wang X, Ma L, Jia ZK, Xu C, Zhang Y. Primary Study on Distribution Pattern Change of Pinus tabulaeformis and Platycladus orientalis Plantations in Mountainous Area of Beijing. Journal of Southwest Forestry College. 2005;22:23–26. [Google Scholar]
- 28.Holmes, R. L. Computer-assisted quality control in tree-ring dating and measurement. Tree-ring bulletin (1983).
- 29.Leavitt SW. Tree-ring isotopic pooling without regard to mass: No difference from averaging delta C-13 values of each tree. Chemical Geology. 2008;252:52–55. doi: 10.1016/j.chemgeo.2008.01.014. [DOI] [Google Scholar]
- 30.Linan ID, et al. Pooled versus separate measurements of tree-ring stable isotopes. Science of the Total Environment. 2011;409:2244–2251. doi: 10.1016/j.scitotenv.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, et al. Individual and pooled tree-ring stable-carbon isotope series in Chinese pine from the Nan Wutai region, China: Common signal and climate relationships. Chemical Geology. 2012;330:17–26. doi: 10.1016/j.chemgeo.2012.08.008. [DOI] [Google Scholar]
- 32.Szymczak S, Joachimski M, Bräuning A, Hetzer T, Kuhlemann J. Are pooled tree ring δ 13 C and δ18 O series reliable climate archives?—a case study of Pinus nigra spp. laricio (Corsica/France) Chemical Geology. 2012;308:40–49. doi: 10.1016/j.chemgeo.2012.03.013. [DOI] [Google Scholar]
- 33.Woodley EJ, et al. Estimating uncertainty in pooled stable isotope time-series from tree-rings. Chemical Geology. 2012;294:243–248. doi: 10.1016/j.chemgeo.2011.12.008. [DOI] [Google Scholar]
- 34.Wu G, et al. Elevation-dependent variations of tree growth and intrinsic water-use efficiency in Schrenk spruce (Picea schrenkiana) in the western Tianshan Mountains, China. Front Plant Sci. 2015;6:309. doi: 10.3389/fpls.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borella S, Leuenberger M, Saurer M, Siegwolf R. Reducing uncertainties in δ13C analysis of tree rings: pooling, milling, and cellulose extraction. Journal of Geophysical Research: Atmospheres. 1998;103:19519–19526. doi: 10.1029/98JD01169. [DOI] [Google Scholar]
- 36.Matteo G, Angelis P, Brugnoli E, Cherubini P, Scarascia-Mugnozza G. Tree-ring Δ13C reveals the impact of past forest management on water-use efficiency in a Mediterranean oak coppice in Tuscany (Italy) Annals of Forest Science. 2010;67:510–510. doi: 10.1051/forest/2010012. [DOI] [Google Scholar]
- 37.Tognetti R, Lombardi F, Lasserre B, Cherubini P, Marchetti M. Tree-ring stable isotopes reveal twentieth-century increases in water-use efficiency of Fagus sylvatica and Nothofagus spp. in Italian and Chilean mountains. PLoS One. 2014;9:e113136. doi: 10.1371/journal.pone.0113136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loader N, Robertson I, Barker A, Switsur V, Waterhouse J. An improved technique for the batch processing of small wholewood samples to α-cellulose. Chemical Geology. 1997;136:313–317. doi: 10.1016/S0009-2541(96)00133-7. [DOI] [Google Scholar]
- 39.PeÑUelas J, Hunt JM, Ogaya R, Jump AS. Twentieth century changes of tree-ring δ13C at the southern range-edge of Fagus sylvatica: increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Global Change Biology. 2008;14:1076–1088. doi: 10.1111/j.1365-2486.2008.01563.x. [DOI] [Google Scholar]
- 40.Warren JM, et al. Timing and magnitude of C partitioning through a young loblolly pine (Pinus taeda L.) stand using 13C labeling and shade treatments. Tree physiology. 2011;32:799–813. doi: 10.1093/treephys/tpr129. [DOI] [PubMed] [Google Scholar]
- 41.Brienen, R. et al. Tree height strongly affects estimates of water-use efficiency responses to climate and CO2 using isotopes. Nature Communications8 (2017). [DOI] [PMC free article] [PubMed]
- 42.Chen, Z., Wang, G. & Jia, Y. Foliar δ13C showed no altitudinal trend in an arid region and atmospheric pressure exerted a negative effect on plant δ13C. Frontiers in plant science8 (2017). [DOI] [PMC free article] [PubMed]
- 43.Di Matteo G, et al. Changes in foliar carbon isotope composition and seasonal stomatal conductance reveal adaptive traits in Mediterranean coppices affected by drought. Journal of forestry research. 2014;25:839–845. doi: 10.1007/s11676-014-0532-4. [DOI] [Google Scholar]
- 44.Kwak J-H, et al. Temperature and air pollution affected tree ring δ 13 C and water-use efficiency of pine and oak trees under rising CO2 in a humid temperate forest. Chemical Geology. 2016;420:127–138. doi: 10.1016/j.chemgeo.2015.11.015. [DOI] [Google Scholar]
- 45.Linares JC, Camarero JJ. From pattern to process: linking intrinsic water-use efficiency to drought-induced forest decline. Global Change Biology. 2012;18:1000–1015. doi: 10.1111/j.1365-2486.2011.02566.x. [DOI] [Google Scholar]
- 46.Barnard HR, Brooks JR, Bond BJ. Applying the dual-isotope conceptual model to interpret physiological trends under uncontrolled conditions. Tree Physiol. 2012;32:1183–1198. doi: 10.1093/treephys/tps078. [DOI] [PubMed] [Google Scholar]
- 47.Jackson R, Sala O, Field C, Mooney H. CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia. 1994;98:257–262. doi: 10.1007/BF00324212. [DOI] [PubMed] [Google Scholar]
- 48.Curtis PS, Wang X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 49.Gagen M, et al. Evidence of changing intrinsic water-use efficiency under rising atmospheric CO2 concentrations in Boreal Fennoscandia from subfossil leaves and tree ring delta 13C ratios. Global Change Biology. 2011;17:1064–1072. doi: 10.1111/j.1365-2486.2010.02273.x. [DOI] [Google Scholar]
- 50.Guerrieri MR, et al. Impact of different nitrogen emission sources on tree physiology as assessed by a triple stable isotope approach. Atmospheric Environment. 2009;43:410–418. doi: 10.1016/j.atmosenv.2008.08.042. [DOI] [Google Scholar]
- 51.Hyvönen R, et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytologist. 2007;173:463–480. doi: 10.1111/j.1469-8137.2007.01967.x. [DOI] [PubMed] [Google Scholar]
- 52.Eilmann B, Rigling A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012;32:178–187. doi: 10.1093/treephys/tps004. [DOI] [PubMed] [Google Scholar]
- 53.Niinemets U, Flexas J, Penuelas J. Evergreens favored by higher responsiveness to increased CO(2) Trends Ecol Evol. 2011;26:136–142. doi: 10.1016/j.tree.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Cullen LE, Adams MA, Anderson MJ, Grierson PF. Analyses of δ13C and δ18O in tree rings of Callitris columellaris provide evidence of a change in stomatal control of photosynthesis in response to regional changes in climate. Tree Physiology. 2008;28:1525–1533. doi: 10.1093/treephys/28.10.1525. [DOI] [PubMed] [Google Scholar]
- 55.Roden JS, Farquhar GD. A controlled test of the dual-isotope approach for the interpretation of stable carbon and oxygen isotope ratio variation in tree rings. Tree Physiol. 2012;32:490–503. doi: 10.1093/treephys/tps019. [DOI] [PubMed] [Google Scholar]
- 56.Van Der Sleen P, et al. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nature geoscience. 2015;8:24–28. doi: 10.1038/ngeo2313. [DOI] [Google Scholar]
- 57.Wang WZ, Liu XH, An WL, Xu GB, Zeng XM. Increased intrinsic water-use efficiency during a period with persistent decreased tree radial growth in northwestern China: Causes and implications. Forest Ecology and Management. 2012;275:14–22. doi: 10.1016/j.foreco.2012.02.027. [DOI] [Google Scholar]
- 58.Niinemets U, Diaz-Espejo A, Flexas J, Galmes J, Warren CR. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J Exp Bot. 2009;60:2249–2270. doi: 10.1093/jxb/erp036. [DOI] [PubMed] [Google Scholar]
- 59.Galmés J, Medrano H, Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist. 2007;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- 60.Niinemets Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecological Research. 2010;25:693–714. doi: 10.1007/s11284-010-0712-4. [DOI] [Google Scholar]
- 61.McMurtrie RE, et al. Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimisation hypothesis. Functional Plant Biology. 2008;35:521–534. doi: 10.1071/FP08128. [DOI] [PubMed] [Google Scholar]