Abstract
A new genus and species of fossil bat is described from New Zealand’s only pre-Pleistocene Cenozoic terrestrial fauna, the early Miocene St Bathans Fauna of Central Otago, South Island. Bayesian total evidence phylogenetic analysis places this new Southern Hemisphere taxon among the burrowing bats (mystacinids) of New Zealand and Australia, although its lower dentition also resembles Africa’s endemic sucker-footed bats (myzopodids). As the first new bat genus to be added to New Zealand’s fauna in more than 150 years, it provides new insight into the original diversity of chiropterans in Australasia. It also underscores the significant decline in morphological diversity that has taken place in the highly distinctive, semi-terrestrial bat family Mystacinidae since the Miocene. This bat was relatively large, with an estimated body mass of ~40 g, and its dentition suggests it had an omnivorous diet. Its striking dental autapomorphies, including development of a large hypocone, signal a shift of diet compared with other mystacinids, and may provide evidence of an adaptive radiation in feeding strategy in this group of noctilionoid bats.
Introduction
The main islands of New Zealand are the largest emergent part of the continental fragment of Zealandia, other landmasses of which today include New Caledonia, Lord Howe, Chatham and Campbell Islands1,2. Zealandia separated from the Australia-Antarctica part of Gondwana in a split that began 130 Ma (million years ago), with the Tasman Sea opening from south to north in the interval 83–52 Ma3,4 and with ~1600 km of ocean now separating Australia and New Zealand. Australia, Antarctica and South America remained connected until ~40 Ma, as the last vestiges of Gondwana5–7.
Today, New Zealand has a biogeographically highly distinctive fauna that includes many old endemic lineages and recent immigrants, with both vicariance and dispersal implicated in its assembly8,9. Its modern terrestrial mammal fauna comprises three bat species, all other modern mammals having been introduced during the last 800 years10. Chalinolobus tuberculatus, of the cosmopolitan bat family Vespertilionoidae, is closely related to its Australian congeners and probably made a trans-Tasman crossing from Australia less than 2 Ma11. The other two Recent bat species, Mystacina tuberculata and M. robusta, are the only living members of the family Mystacinidae. These are morphologically and ecologically very distinctive chiropterans, also known as burrowing bats, which spend 30% of their foraging time on the forest floor, under leaf litter and on tree branches12. Mystacina tuberculata is considered vulnerable to extinction and M. robusta critically endangered or extinct13,14.
Mystacinidae is one of the six to seven extant families that make up the bat superfamily Noctilionoidea, along with the Neotropical families Phyllostomidae, Noctilionidae, Mormoopidae, Furipteridae and Thyropteridae15. Madagascar’s Myzopodidae is also typically included in Noctilionoidea as sister to the remaining families (e.g.16,17), but some analyses of molecular data suggest it has a sister-group relationship with Vespertilionoidea (e.g.18,19), or that it is sister to Emballonuroidea, or (within Emballonuroidea) Nycteridae20.
With or without Myzopodidae included, Noctilionoidea is the only bat superfamily interpreted to have a Gondwanan origin16. The noctilionoid fossil record is poor, especially for the Paleogene21,22, but biogeographic reconstructions suggest that this morphologically and ecologically diverse superfamily probably originated in Africa (e.g.18,22,23), with subsequent dispersal and radiation producing Australasia’s mystacinids and the five modern Neotropical noctilionoid families. According to molecular data, the divergence of the Australasian and South American noctilionoid clades occurred ~50–37 Ma20,24,25.
Fossils show that mystacinids once occurred in Australia (26–12 Ma; refs26,27) and were present in New Zealand from at least the early Miocene28. In New Zealand, remains of the two modern Mystacina species have been recovered from numerous Pleistocene and Holocene cave deposits29. The Miocene mystacinid Mystacina miocenalis has been described30 and material indicative of two smaller mystacinid species has been reported28 from freshwater lake sediments (16–19 Ma) near St Bathans, Central Otago, South Island. The St Bathans fossil assemblage also includes plants, invertebrates, fish, frogs, lizards, kiwi, moa, New Zealand wrens, parrots, waders and many other water birds, a tuatara, crocodilian and turtle, and fragments of a small non-volant archaic mammal (e.g.9,31–37). As Zealandia’s only known Tertiary terrestrial vertebrate fauna, the St Bathans fossil assemblage offers critical insight into the deep-time history for most of its vertebrate lineages.
Here, we describe a new bat genus and species from St Bathans, and discuss its bearing on hypotheses regarding the radiation of the southern superfamily Noctilionoidea and the family Mystacinidae in the Australian region. This fossil bat indicates that there once was greater ecological diversity in the New Zealand’s bat fauna, and, as only the third bat genus recorded from New Zealand, it signals substantial loss of diversity since the Miocene.
Systematic palaeontology
Order Chiroptera Blumenbach, 1779
Suborder Yangochiroptera Van den Bussche & Hoofer, 2004
Superfamily Noctilionoidea Gray, 1821
Family Mystacinidae Dobson, 1875
Vulcanops jennyworthyae gen. et sp. nov.
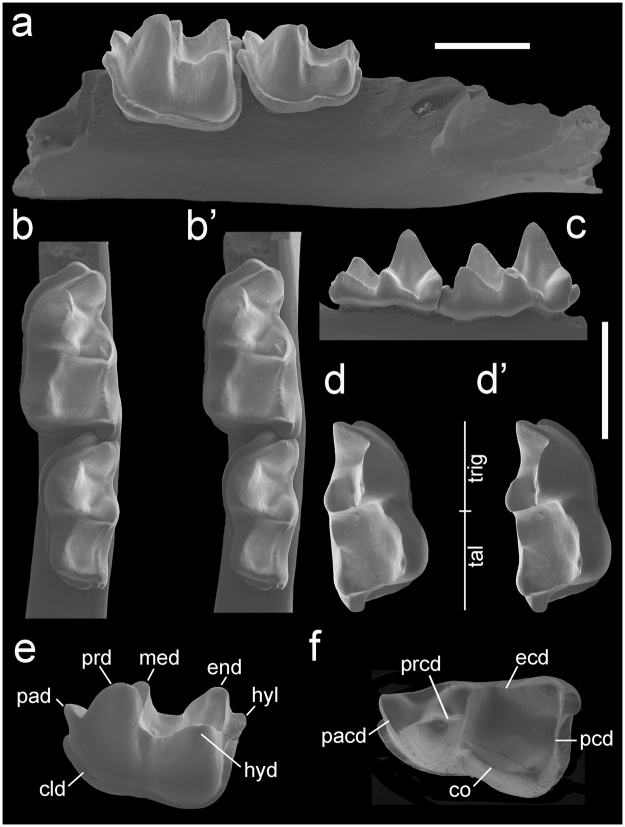
Figure 1.
Vulcanops jennyworthyae gen. et sp. nov., Bannockburn Formation, St Bathans, Central Otago, New Zealand. Lower dentition. CM 2013.18.790, holotype, left dentary fragment containing m2-3. (a) Buccal view; (b–b’) stereopair, occlusal view; (c) lingual view m2-3. NMNZ S.52078, paratype, right m1. (d–d’) Stereopair, oblique occlusal view; (e) buccal view; (f) occlusal view. Abbreviations: cld, cingulid; co, cristid obliqua; end, entoconid; ecd, entocristid; hyd, hypoconid; hyl, hypoconulid; med, metaconid; pacd, paracristid; pad, paraconid; pcd, postcristid; prcd, protocristid; prd, protoconid; tal, talonid; trig, trigonid. Scale bars = 2 mm.
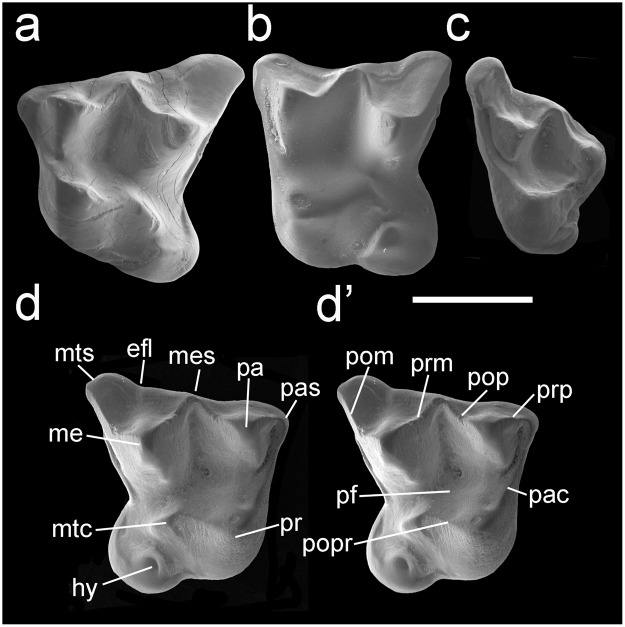
Figure 2.
Vulcanops jennyworthyae gen. et sp. nov. Upper dentition. (a) CM 2013.18.916, left M1, oblique occlusal view. (b) NMNZ S.50383, right M2, oblique occlusal view (reversed). (c) NMNZ S.52400, left M3, oblique occlusal view. (d–d’) NMNZ S.44071, right M1, stereo-pair, oblique occlusal view. Abbreviations: efl, ectoflexus; hy, hypocone; me, metacone; mes, mesostyle; mtc, metaconule; mts, metastyle; pa, paracone; pac, paracingulum; pas, parastyle; pf, protofossa; pr, protocone; prp preparacrista; prm, premetacrista; pom, postmetacrista; pop, postparacrista; popr, postprotocrista. Scale bar = 2 mm.
Generic diagnosis
As for the type and only species.
Stratigraphic and geographic distribution
Lower Miocene of Central Otago, New Zealand.
Etymology
From Vulcan, mythological god of fire and volcanoes (Roman), and ops, a suffix commonly used for bats; in reference to New Zealand’s tectonically active nature, as well as to the historic Vulcan Hotel, centre of the hamlet of St Bathans, from which the fauna takes its name. The species name honours Jennifer P. Worthy in recognition of her pivotal role in revealing the diversity of the St Bathans Fauna.
Holotype
CM 2013.18.790, left dentary fragment with m2-3 (Fig. 1a–c), HH1a, Bannockburn Formation, Manuherikia River, Home Hills Station, Otago, New Zealand (see Locality and age).
Referred specimens
NMNZ S.42876, right m1, HH1a; NMNZ S.52078, right m1 (Fig. 1d–f), HH1a; NMNZ S.52076, left m1/2 (fragment), HH1a; CM 2013.18.916, left M1, HH1a (Fig. 2a); NMNZ S.44071, right M1, HH1a (Fig. 2d); NMNZ S.51461, right M1, HH1b Trench; CM 2013.18.1, left M1, Croc Site Layer 1; NMNZ S.51746, left M2, HH1b Trench; NMNZ S.50383, right M2, HH1a (Fig. 2b); NMNZ S.50778, right M1/2 (posterolingual fragment), HH1a; NMNZ S.52400, left M3, HH1b Trench (Fig. 2c); NMNZ S.52351, right incomplete M3, HH1a; NMNZ S.50384, left M3 incomplete, HH1a. A minimum of four individuals is represented. Measurements of the fossils are given in Table 1.
Table 1.
Measurements (mm) of lower molars (m) and upper molars (m) of Vulcanops jennyworthyae gen. et sp. nov. from the lower Miocene Bannockburn Formation, St Bathans, Central Otago, New Zealand. *Figured, ‡holotype.
| Specimen no. | Position | Length | Width | Trig. width | Tal. width |
|---|---|---|---|---|---|
| NMNZ S.52078 | m1 | 2.90 | 1.55 | 1.70 | |
| NMNZ S.42876* | m1 | 2.90 | 1.45 | 1.80 | |
| CM 2013.18.790*‡ | m2-3 | 5.30 | |||
| m2 | 2.80 | 1.80 | 2.00 | ||
| m3 | 2.55 | 1.55 | 1.30 | ||
| NMNZ S.51461 | M1 | 2.95 | 3.00 | ||
| NMNZ S.44071* | M1 | 2.85 | 2.85 | ||
| CM2013.18.1 | M1 | [3.00] | |||
| CM 2013.18.916* | M1 | 3.05 | 3.10 | ||
| NMNZ S.50383* | M2 | 2.95 | 3.15 | ||
| NMNZ S.52400* | M3 | 2.25 | 2.80 |
Locality and age
Bed HH1a (New Zealand Fossil Record File number H41/f088), a 5–10 cm thick sandy conglomerate, 6.88–7.0 m above base of Bannockburn Formation, Manuherikia River section, Home Hills Station, St Bathans, Otago, New Zealand; 44.907944°S, 169.858222°E. HH1b Trench (H41/f0103), a 10 cm thick sandy conglomerate, 9.5–9.58 m above the base of the Bannockburn Formation, foot of hill 50 m across terrace from river bank, Manuherikia River section, Home Hills Station, Otago; 44.90780°S; 169.85844°E. Croc Site, Layer 1 (H41/f084), c.10 cm thick sand and cobble layer, in 3 m cliff on the north slope of a small hill on the west side of Mata Creek, Dunstanburn Station, St Bathans, Otago; 44.889500°S 169.837833°E. Altonian local stage, lower Miocene, 19–16 Ma35.
Species diagnosis
A bat with: m1-2 myotodont, with talonid longer and conspicuously wider than trigonid (particularly on m1), and rounded talonid basin; m1-2 paraconid buccally displaced, not aligned with metaconid and entoconid; m1-2 entoconid very tall, with pre-entocristid interrupted such that talonid opens lingually; m1-3 cristid obliqua curved, with inflection close to trigonid, contacting trigonid conspicuously buccal to midpoint between protoconid and metaconid; m1-3 with complete anterior, buccal and posterior cingulid; m1-3 with relatively shallow hypoflexid; m3 reduced in length and width, talonid narrower than trigonid, myotodont with small hypoconulid; M1-2 as wide as long, three rooted with anteroposteriorly extended lingual root, paracone reduced in volume but subequal in height with protocone, metacone taller, parastyle conical, non-cuspidate mesostyle on buccal margin of crown, postmetacrista elongated, large posterolingually directed heel (hypocone shelf) bearing tall, bulbous hypocone, metaconule in postprotocrista with short posterolingual crest not reaching hypocone, protofossa long, deep and open posteriorly, narrow paracingulum present, posterior cingulum indistinct, lacking paraloph, metaloph and anterolingual cingulum; M3 large, metacone with complete premetacrista but no postmetacrista, heel (hypocone shelf) with small hypocone. An expanded description is given in the Supplementary Information online.
Differential diagnosis
Differs from other mystacinids (species of Mystacina and Icarops) in exhibiting the following traits: m1-2 paraconid buccally displaced, not aligned with metaconid and entoconid, with talonid conspicuously wider than trigonid; m1-3 cristid obliqua curved rather than straight, with inflection near trigonid, contacting trigonid buccal rather than at midpoint between protoconid and metaconid; m1-3 with only shallow hypoflexid; m3 more reduced in length and width; M1-2 with hypocone present and conical parastyle; M3 long with broad angles between ectoloph cristae and with hypocone shelf and hypocone present. Differs additionally from Mystacina spp. in M1-2 having long, wide heel (hypocone shelf).
Differs from myzopodids in having: m1-3 cristid obliqua curving lingually rather than buccally; all trigonids with equally wide trigonid angle; m3 with small hypoconulid present. Differs additionally from Myzopoda spp. in having: M1-3 with hypocone shelf and hypocone; M1-2 as wide as long, with broader angles between ectoloph cristae, preparacrista shorter than postparacrista and postmetacrista elongated, ectocingulum variably present but indistinct.
Differs from thyropterids and furipterids in having: m1-2 paraconid buccally displaced, not aligned with metaconid and entoconid; cristid obliqua contacting trigonid conspicuously buccal to midpoint between protoconid and metaconid; m1-3 with only shallow hypoflexid; m3 reduced in length and width; M1-3 lacking paraloph and metaloph; M1-2 with hypocone shelf and hypocone; M1-2 long with broader angles between ectoloph cristae; postmetacrista elongated; presence of ectocingulum; lacking buccally extruded mesostyles (deep ectoflexi?); M1-2 without continuous lingual cingulum. Additionally differs from furipterids in its M1-2 lacking metacingulum; m1-3 myotodont rather than nyctalodont, without conical entoconid and lacking postmetacristid. Additionally differs from thyropterids in its M3 smaller (e.g. narrower) than M1.
Differs from noctilionids in its: m1-3 without tall, long/continuous entocristid closing talonid; with only shallow hypoflexid; m1-3 trigonids not anteroposteriorly compressed, with protocristid and cristid obliqua curved, the latter lingually with inflection near trigonid, contacting trigonid conspicuously buccal to midpoint between protoconid and metaconid (rather than extending to lingual margin of crown); M1-2 with postparacrista and premetacrista meeting on buccal margin such that centrocrista continuous; M1-2 with rounded (rather than sharply/pointed) posterolingually directed and unbasined heel; lacking strong paraloph and metaloph that close the protofossa anteriorly and posteriorly; M3 with hypocone shelf and small hypocone, lacking paraloph.
Differs from mormoopids in its: m1-3 cristid obliqua meeting trigonid buccal of centre; talonid basin rounded rather than triangular (with lingually curved rather than straight cristid oblique); M1-2 with shorter, posterolingually (rather than posteriorly) directed heel; angles between ectostyle cristae wider; without hooked parastyle; incomplete/absent lingual cingulum; M3 with hypocone.
Differs from desmodontine, stenodermatine, carolliine and rhinophylline phyllostomids in having dilambdodont molars. Differs from most phyllostomines, macrotines, micronycterines, lonchophyllines, lonchorhynines and glossophagines in its myotodont rather than nyctalodont lower molars. Differs additionally from phyllostomines in M3 being large and with hypocone.
Body mass
Using the equations of Gunnell et al.38 and the proxies of upper first molar (M1) area, lower first molar (m1) area, and diameter of mid-shaft humerus, the body mass of eight of the ten known extinct and extant mystacinid taxa are given in Table 2. For the previously known mystacinids, these values range from ~8.5 g (Icarops paradox) to 39.3 g (Mystacina miocenalis). For Vulcanops jennyworthyae, the estimates are 42.6 g (based on M1 area) and 39.8 g (m1 area). This indicates a relatively large bat, compared with the median value of 13.8 g for 905 extant bat species (refs38,39; see Discussion).
Table 2.
Body mass estimates (g) of extinct and extant mystacinids from New Zealand (NZ) and Australia (Aus) based on equations in Gunnell et al.22 and using the proxies of upper first molar (M1) area, lower first molar (m1) area, and humerus mid-shaft diameter.
| Taxon | Location | Age | Body mass estimate | Live weight | ||
|---|---|---|---|---|---|---|
| M1 | m1 | humerus | ||||
| †Vulcanops jennyworthyae 1 | NZ | E Miocene | 42.6 (1) | 39.8 (1) | — | — |
| †Mystacina miocenalis 2 | NZ | E Miocene | 39.3 (1) | — | — | — |
| †Mystacinid indet. 13 | NZ | E Miocene | — | — | 114.33 (1) | — |
| Mystacina tuberculata 4 | NZ | Holocene | 12.77 (20) | 14.28 (20) | 112.23 (5) | 13.6 (300) |
| Mystacina robusta 5 | NZ | Holocene | 22.90 (12) | 22.19 (12) | 117.70 (1) | — |
| †Icarops paradox 6 | Aus | E Miocene | 8.43 (2) | 10.31 (1) | — | — |
| †Icarops aenae 7 | Aus | L Oligo-E Mio | 17.74 (2) | 21.52 (1) | 115.07 (2) | — |
| †Mystacinid indet.8 | Aus | L Oligocene | 11.34 (1) | — | — | — |
† Indicates extinct taxon; E, Early; L, Late; (#), number of specimens. Humerus mid-shaft measured in this work. Dental and weight data from: 1, this paper (Table 1); 2,30; 3,28; 4,87 (Codfish Is),73; 5, 30,87 (Stewart Is); 6 & 7,27; 8,45. No estimates available for Icarops breviceps (known from m2-3;26) but tooth size similar to I. aenae 27, nor Mystacinid indet. 2 but distal humerus is smaller than in Mystacinid indet. 128.
Phylogeny
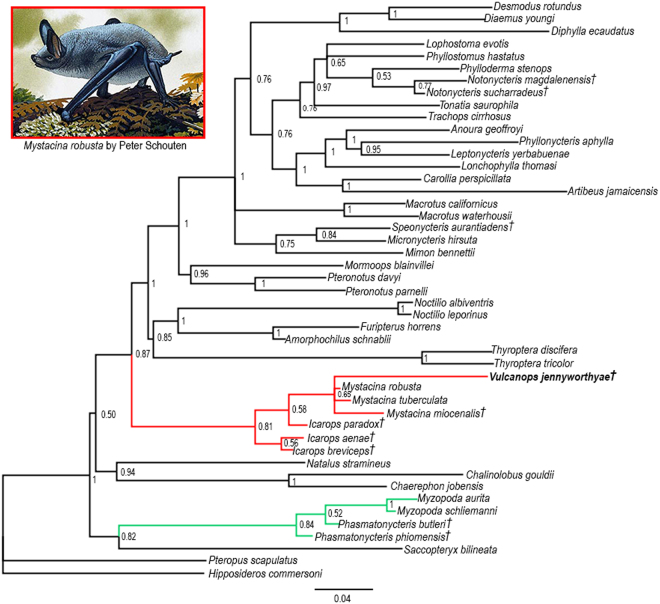
The 50% majority rule consensus of post-burn-in trees from our Bayesian total evidence analysis is given in Fig. 3. Mystacinidae, Furipteridae + Noctilionidae, Thyropteridae, and Mormoopidae + Phyllostomidae formed clades, all with relatively high support (posterior probabilities shown in Fig. 3). Yangochiroptera had 100% support; Noctilionoidea and Vespertilionoidea were sister groups but with low support (50%). Myzopodidae and the emballonurid Saccopteryx bilineata grouped with relatively high support of 82%. Vulcanops fell within Mystacinidae, with a relatively high posterior probability of 81% but with relationships within the family less strongly supported (posterior probabilities 55–65%). Of the fossil taxa, Speonycteris aurantiadens grouped with phyllostomids rather than mormoopids40,41, but the others grouped in agreement with the results of previous studies, namely Phasmatonycteris spp. with Myzopoda spp. in Myzopodidae22, Australian Icarops spp. with New Zealand Mystacina spp.27,30 and Notonycteris spp. with phyllostomine phyllostomids42,43.
Figure 3.
50% majority rule consensus tree of post-burn-in trees from Bayesian total evidence analysis of 292 dental characters plus 11.1 kb of mitochondrial and nuclear DNA sequence data. Values at nodes represent Bayesian posterior probabilities values > 0.5. † Indicates extinct taxon; green, Myzopodidae; red, Mystacinidae + Vulcanops. Illustration of Mystacina robusta by Peter Schouten.
The Bayesian analysis identified eight unequivocal synapomorphies uniting Mystacinidae (i.e. Icarops + Mystacina + Vulcanops), of which four can be scored in Vulcanops: ectocingulum present but weak (character 71:1), secondary cusp present on postprotocrista of M1 and M2 (character 92:1), m1 hypoflexid shallow (character 252:1), and m3 cristid obliqua contacts trigonid (character 271:0). A full list of synapomorphies for all nodes in the topologies shown in Fig. 3, under both Accelerated Transformation (ACCTRAN) and Delayed Transformation (DELTRAN), is given in Supplementary Information.
Discussion
Bayesian total evidence analysis (mitochondrial and nuclear genes plus dental characters) places the New Zealand Miocene bat Vulcanops jennyworthyae among Australasia’s living and fossil mystacinids. The overall results of our phylogenetic analysis are broadly congruent with recent large-scale molecular studies of bats17,19,20,24,25. Like some of these studies (e.g.20), our analysis raises questions about the inclusion of Africa’s Myzopodidae within Southern Hemisphere Noctilionoidea, suggesting instead that myzopodids may be more closely related to cosmopolitan emballonurids.
Our analysis finds a sister-group relationship between Madagascar’s extant Myzopoda species and North Africa’s Phasmatonycteris species, supporting referral of those fossil taxa to the family Myzopodidae22. These fossil taxa were described by Gunnell et al.22 from the Eocene Birket Qarun (~37 Ma) and Oligocene Upper Jebel Qatrani (~30 Ma) Formations of the Fayum in Egypt and referred to Myzopodidae on the basis of their lower dentitions (upper teeth are unknown). Although there are similarities between Vulcanops and myzopodids in the morphology of the lower dentition (e.g. m1-2 paraconid buccally displaced, not aligned with metaconid and entoconid, with talonid conspicuously wider than trigonid; m1-3 cristid obliqua curved rather than straight, with inflection near trigonid, and contacting trigonid buccal to rather than at midpoint between protoconid and metaconid; m1-3 with only shallow hypoflexid; m3 reduced in length and width with respect to m1-2; see Differential diagnosis), our phylogenetic analysis indicates these similarities are likely homoplastic.
Unequivocal noctilionoid families, from the Americas and Australasia, first appear in the fossil record slightly later: mormoopids 32–30 Ma in Florida44, mystacinids 26 Ma in South Australia27,45, phyllostomids 21 Ma in Panama46, and noctilionids and thyropterids 13–12 Ma in Colombia47,48. Furipterids are first recorded from the Pleistocene of Brazil, French Guiana, and Peru49. Older dates for the divergence of these lineages are estimated from recent molecular clock analyses (which use fossils as calibrations): Mystacinidae at 50.3 to 37.3 Ma20,24,25, and the base of the neotropical noctilionoid radiation (Thyropteridae + Furipteridae + Noctilionidae + Mormopteridae + Phyllostomidae) at 47.0 to 37.3 Ma17,25.
With respect to Southern Hemisphere palaeogeography, these divergence times long postdate estimated dates for the separation of India-Madagascar and Africa from Gondwana (>100 Ma), with Madagascar isolated in the Indian Ocean for more than 80 Ma50. The divergence dates, however, span those estimated for the breakup of the Australia-Antarctica-South America landmass, with Australia and Antarctica separating ~45 Ma and South America and Antarctica ~41 Ma5,6. New Zealand has been isolated in the South Pacific from ~52 Ma3,4, possibly before the divergence of the mystacinid lineage from other noctilionoids.
Based on phylogenetic inference and tectonic events, a number of biogeographic hypotheses have been proposed to explain the modern distribution of noctilionoids in the Southern Hemisphere. These include: a trans-Atlantic dispersal of stem noctilionoids from Africa to North or South America in the Eocene (e.g.18,23); a North American origin (or transit) of stem noctilionoids, with dispersal to South America via an Eocene proto-Caribbean archipelago (e.g.21); or an American origin or transit with subsequent dispersal of ancestral mystacinids to Australasia (e.g.51). Gunnell et al.22 proposed that noctilionoids originated and initially diversified in Africa (giving rise there to myzopodids) with a subsequent dispersal to Australia (producing mystacinids) and then to South America via Antarctica (this lineage leading to the five neotropical noctilionoid families).
Even if myzopodids are not noctilionoids, as suggested by some recent molecular data and by our total evidence analysis, one of these scenarios may still be valid. The modern bat crown-clade is thought to have originated in either Africa52–54 or Eurasia55, with estimates for the age of the base of the extant bat radiation ranging from 62.6 Ma20 to 50.3 Ma25. Potential living sister-groups of Noctilionoidea (sensu 20, i.e. excluding Myzopodidae) are vespertilionoids and emballonuroids. These two speciose groups have cosmopolitan distributions, occurring on all continents except Antarctica today, but molecular data suggest their roots were in Africa (stem and crown) and their oldest fossils are from North Africa54. These data, and an estimated divergence time of ~50 Ma to 37 Ma for Noctilionoidea20,24,25, are not inconsistent with the many previous biogeographical hypotheses for the distribution of superfamily Noctilionoidea outlined above.
The data are also potentially consistent with a vicariant origin of Mystacinidae (e.g.56). In the early Paleogene, global temperatures were up to 12 °C higher than today, mainland Antarctica supported a frost-free, paratropical flora until 50 Ma and Nothofagus forests until at least 15 Ma, and intercontinental distances in the Southern Hemisphere were generally less than now57,58. The Paleogene remnants of Gondwana may have supported a broadly distributed noctilionoid fauna. If so, final fragmentation of the supercontinent may have led to the extinction of noctilionoids in Neogene Antarctica as ice-sheets grew59, with Mystacinidae vicariantly isolated in the Australian region. However, fossil bats have yet to be found in Antarctica, and a divergence date for mystacinids from other noctilionoids of ~50 to 37 Ma, after isolation of New Zealand in the Pacific ~52 Ma3,4, suggests that their presence in at least New Zealand probably reflects one or more post-Gondwanan dispersals.
Other bats were present in the Australian region in the early Paleogene, as demonstrated by the archaic Australonycteris clarkae from the 55 Ma Tingamarra fauna of southeastern Queensland, Australia60,61. The likely route taken by the first bats to reach Australia is unknown (the relationship of Australonycteris to other early chiropterans from Northern and Southern Hemispheres is unclear62;). Between 55 and 26 Ma, there is long gap in the Australian mammal record63,64 but when it resumes in the late Oligocene mystacinids were widespread, occurring in deposits in both central and northern Australia27. In New Zealand’s oldest terrestrial mammal-bearing deposit, in 19–16 Ma sediments of the lower Bannockburn Formation near St Bathans, mystacinids are present and there is evidence that long-term ecological associations between Mystacina and its arthropod prey and roost trees and food plants were already established30.
If Vulcanops is a mystacinid, as we suggest here, it brings the number of representatives of this bat family in the Miocene St Bathans fauna to four28,30. In Australia, at least another four mystacinid species, all in the genus Icarops, are recorded from Oligocene to Miocene deposits in South Australia, Queensland and the Northern Territory, with two species co-occurring in some Queensland deposits27. In our total evidence analysis (Fig. 3), Vulcanops forms a clade with Mystacina species, with Icarops species paraphyletic relative to Vulcanops + Mystacina; this arrangement is congruent with a single origin of New Zealand mystacinids from an Australian source, but the topology receives only weak support.
A striking feature distinguishing the dentition of Vulcanops from previously known mystacinids (Mystacina spp. and Icarops spp.) is the presence of a large hypocone on its upper molars (Fig. 2). This structure is similar to that found in neotropical noctilionoids (phyllostomids and mormoopids). In that speciose group, it appears to have evolved multiple times43, but it is otherwise uncommon (and particularly rare on M3) in bats with a dilambdodont dentition. Outside Noctilionoidea, a large bulbous hypocone also occurs in the late Eocene Egyptian bat Aegyptonycteris knightae Simmons, Seiffert & Gunnell, 201665. The latter is known only from its dilambdodont M2-3 and is the only member of its family whose relationships to other bats are unknown65. This large fossil bat differs significantly from Vulcanops in that its M2-3 also have a large conule at the base of the metacone and an ectostyle on the buccal margin, two features unknown in other bat families, living or extinct65. Among mammals, a hypocone increases occlusal area, effectively doubling the tooth surface devoted to processing food66. It is strongly correlated with a less strictly carnivorous diet, often involving an increase in plant consumption66. In Vulcanops, a long, broad, deep talonid on m1-2, low curved postcristid (=posthypocristid), cristid obliqua lacking carnassial notches, and long broad protocone on M1-2 are also horizontal shearing adaptations associated with a relatively more herbivorous diet. At the same time, elongation of the molar crests as also seen in Vulcanops (postmetacrista on M1-2 twice length of preparacrista, shallow ectoloph, open angle of m1-3 trigonids, cristid obliqua meeting trigonid buccal to centre of crown) are adaptations for vertical shearing, possibly indicating relatively more flesh eating.
As body size increases in bats, species with dilambdodont molars often include small vertebrates in their diets65–70. The presence of a well-developed hypocone in the ~40 g Vulcanops, however, argues against a strictly carnivorous diet. A tall, rounded hypocone is absent in flesh-eating bats (e.g. nycterids, megadermatids and phyllostomines Vampyrum and Trachops;71), although a crestiform hypocone is present in fish-eating noctilionids (Noctilio spp.) and is similar to the condition seen in some specimens of Vulcanops (e.g. CM 2013.18.916; Fig. 2a). Other aspects of Noctilio teeth that are possibly adaptations for piscivory (e.g. the discontinuous centrocrista of M1-2, in which the central blades reach the buccal margin of the crown, and the cristid obliqua of m1-2, which extends to the lingual margin of the crown) are very different from those of Vulcanops. The latter’s dentition, and diet, was perhaps most similar to some phyllostomines that consume invertebrates, nectar, fruit, flowers, as well as small vertebrates (e.g. the large-bodied omnivorous Phyllostomus hastatus;65).
New Zealand’s Recent Mystacina species also have very broad, omnivorous diets consisting of nectar, pollen, fruit, flowers, and flying and terrestrial arthropods, but are not known to hunt small vertebrates72,73. However, Vulcanops exhibits several dental apomorphies, such as a large, blunt hypocone and long, broad, deep talonid, that are lacking in Mystacina species (as well as in Australia’s extinct Icarops species) and suggest additional feeding capabilities in this extinct bat. No other extant or extinct bat known from the Australasian region has similar dental features. If a large blunt hypocone is indicative of increased herbivory in bats, as argued above (see also65), this may provide evidence for the wider adoption, both geographically and taxonomically, of phytophagy in noctilionoid bats by the early Miocene74. It may also have relevance to phylogenetic reconstructions of the ancestral diet in Noctilionoidea and its constituent families17,43,74–77.
There is some evidence from dental remains that Australia’s extinct Icarops species were more insectivorous than New Zealand’s omnivorous extant and extinct Mystacina species27,30. The derived features present in the dentition of Vulcanops that are absent in other mystacinids signal a further shift in diet. This could reflect exploitation of new, abundant and/or underutilized food resources in New Zealand compared with Australia where omnivorous peramelemorphian (bandicoot) and phalangeridan (possum and kangaroo) marsupials were morphologically diverse, speciose and abundant in forest ecosystems shared with mystacinids27,64. Baker et al.76 have argued that the adaptive radiation of feeding strategies seen in phyllostomid noctilionoids – the most radical derived from a common ancestor for any monophyletic group of mammals – was triggered by the dietary inclusion of plant material in addition to insects, in concert with new environmental opportunities in Oligo-Miocene South America.
The large body size (~40 g) estimated for the early Miocene New Zealand mystacinids Vulcanops jennyworthyae and Mystacina miocenalis Hand, Lee, Worthy & Archer, 201530 is notable compared with other extant and extinct mystacinids (Table 2), and especially given that the ancestral body mass for noctilionoids and the Mystacina lineage has been estimated at ~10–14 g78. The evolution of relatively large size in certain bat lineages has been associated with ecological release from the biophysical constraints imposed by flight and echolocation during aerial insectivory, and occurs in lineages exhibiting divergent dietary and behavioural specializations such as frugivory (e.g. pteropodids) or gleaning and perch-hunting behaviour in extreme insectivory and animalivory (e.g. megadermatids, noctilionids)78. That some mystacinids have reached notably large sizes may be another example of this evolutionary trend. Mystacinids are renowned for their peculiar walking habits which enable them to exploit an exceptionally broad range of plant and animal resources72, including ground-flowering plants and large invertebrate prey that they can pursue on foot.
In early Miocene New Zealand, V. jennyworthyae was part of a diverse faunal community living in semitropical to warm-temperate Gondwanan rainforest on the shores of the vast 5000 sq km Manuherikia palaeolake30,79,80. A number of distinctive vertebrate taxa present in the early Miocene St Bathans assemblage, like Vulcanops, disappeared sometime before the late Pleistocene. These include crocodilians, terrestrial turtles, flamingo-like palaelodids, swiftlets, several pigeon, parrot and shorebird lineages and non-volant mammals (e.g.8,9,31–33,36,37). Most of these were probably warm-adapted species8,9,81. After the middle Miocene, global climate change59 brought colder and drier conditions to New Zealand, with significant changes to vegetation and palaeoenvironments80,82. It is possible that this general cooling and drying trend also drove extinction of the Vulcanops lineage, and overall loss in mystacinid diversity over time. In Australia, the Icarops lineage also went extinct, sometime after the late middle Miocene, with Mystacinidae being the only one of eight crown bat families known to have become extinct on that continent62. The reasons for this remain unclear, in part because the later Miocene and Pliocene Australian mammal record is too poor to pinpoint the time of their disappearance62,64.
Methods
Stratigraphic nomenclature for the St Bathans region follows Schwarzhans et al.35. Dental terminology follows Hand et al.27 and Dávalos et al. (ref.43: MorphoBank P891). Case denotes upper (e.g. M1) and lower (e.g. m1) teeth. The prefix CM refers to specimens held in the fossil collections of the Canterbury Museum, Christchurch, New Zealand; NMNZ to the Museum of New Zealand Te Papa Tongarewa, Wellington. Species examined in this study are listed in Supplementary Information online (see also MorphoBank P2737).
To assess its likely phylogenetic affinities, Vulcanops was added to a large morphological character matrix (MorphoBank Project 2737; http://morphobank.org/permalink/?P2737) comprising 292 dental characters scored for 45 yangochiropterans (35 extant and 10 fossil species) plus 2 yinpterochiropteran outgroup taxa. Vulcanops could be scored for 143 of 292 characters, rendering it 49% complete. 112 characters representing plausible morphoclines were specified as ordered. We also created a total evidence matrix by combining the morphological dataset with the molecular dataset of Amador et al. 20. This comprises DNA sequence data from five nuclear genes [dentin matrix protein 1 (DMP1), recombination activating protein 1 (RAG1), recombination activating protein 2 (RAG2), exon 11 of the breast cancer susceptibility protein 1 (BRCA1) and exon 28 of the von Willebrand factor (VWF)] and four mitochondrial genes [cytochrome b (MT-CYB), NADH:ubiquinone oxidoreductase core subunit 1 (MT-ND1), and 12 S (MT-RNR1) and 16 S (MT-RNR2) rRNAs]. We maintained Amador et al.’s20 alignment, but deleted the third codon position of MT-CYB, because this partition showed the greatest evidence of compositional heterogeneity (calculated using BaCoCa; Kück & Struck83), leaving 11.1 kb of sequence data. We then pruned this modified alignment down to the extant taxa present in our morphological matrix.
The total evidence matrix was analysed using an undated Bayesian approach in MrBayes 3.2.684. First, PartitionFinder 2.1.185 was used to select an appropriate partitioning scheme and set of models for the molecular data, assuming linked branch lengths, and using the “greedy” algorithm and AICc for model selection; only models implemented by MrBayes were tested. The morphological data was assigned the Mk model of Lewis86, assuming that variable characters had been scored, and with a gamma distribution with four rate categories to model rate heterogeneity among the morphological characters. The MrBayes analysis comprised four runs of four chains (three “heated,” one “cold”), sampling trees every 5000 generations. The analysis was run for 5 × 106 generations, with the first 25% of sampled trees discarded as burn-in; the post-burn-in trees were summarised using 50% majority rule consensus, with Bayesian posterior probabilities as support values.
To estimate body mass in extinct bats, Gunnell et al.38 developed a set of algorithms based on dental, skeletal and weight measurements in 1,160 extant bats from eight families. We used these equations, and the proxies of upper first molar (M1) area, lower first molar (m1) area, and diameter of mid-shaft humerus, to estimate the body mass of eight of the ten known extinct and extant mystacinid taxa (Table 2).
The morphological datasets generated or analysed during this study are included in this published article’s tables or are available in the MorphoBank repository as Project 2737 (http://morphobank.org/permalink/?P2737).
Nomenclatural Act
This published work and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the International Code of Zoological Nomenclature. The ZooBank life science identifiers can be resolved and the associated information viewed by appending the life science identifiers to the prefix http://zoobank.org/. The life science identifier for this publication is 13BDAB9F-4BC3-4711-A331-4E883DE52DC2, for Vulcanops is 498FA8AA-7DAF-4703-94F3-02931CE7F85F, and for V. jennyworthyae is 3A625804-F490-46F6-BDA0-C93A6523EE6D.
Electronic supplementary material
Acknowledgements
This fossil research was supported by grants from the Australian Research Council (DP0770660, DP120100486, DE120100957, DP130100197), Canterbury Museum and Museum of New Zealand Te Papa Tongarewa, R.S. Allan Memorial Fund of Canterbury Museum, and the Marsden Fund Council from Government funding, managed by the Royal Society Te Apārangi. We thank: landowners Euan and Ann Johnstone (Home Hills Station) and Tony Enright (Dunstanburn; Southern Lakes Holdings Ltd) for generously allowing us access to the St Bathans fossil deposits; many colleagues for helping in the St Bathans excavations over several years; and Euan Johnstone for hours spent on a mechanical digger to facilitate our quarrying operations. The following people kindly arranged access to comparative specimens in their institutions: M. Samaan, General Director and A.H. Sileem, Curator of Vertebrates, Egyptian Geological Museum, Cairo; J. Gauthier and C. Norris, Yale Peabody Museum, New Haven; E. Westwig and N. Duncan, American Museum of Natural History, New York; H. Kafka, National Museum of Natural History, Smithsonian Institution, Washington D.C.; B. Patterson, L. Heaney and the late W. Stanley, Field Museum, Chicago; D. Reed, University of Florida, Gainesville; K. Krohmann, Naturmuseum Senckenberg, Frankfurt; J.-M. Pons, Muséum National d’Histoire Naturelle, Paris; R. Coory and G. Stone, Museum of New Zealand Te Papa Tongarewa, Wellington; S. Ingleby and T. Ennis, Australian Museum, Sydney; H. Janetzki, Queensland Museum, Brisbane; K. Travouillon, Western Australian Museum, Perth. We thank our editor and two reviewers for constructive comments on our submission.
Author Contributions
S.J.H. & R.M.D.B. drafted the paper; T.H.W., A.J.D.T., R.P.S., M.A., S.W.S., V.D.P., R.M.D.B. carried out fieldwork; N.B.S., G.F.G., S.J.H. coded taxa for phylogenetic analysis; R.M.D.B. performed phylogenetic analyses; S.J.H. produced the figures; all authors wrote parts of the paper, and read and approved the final version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18403-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campbell, H. & Hutching, G. In search of ancient New Zealand. Penguin and GNS Sciences, Wellington, New Zealand, 240 pp. (2007).
- 2.Goldberg J, Trewick SA, Paterson AM. Evolution of New Zealand’s terrestrial fauna: a review of molecular evidence. Phil. Trans. R. Soc. B. 2008;363:3319–3334. doi: 10.1098/rstb.2008.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaina C, et al. The tectonic history of the Tasman Sea: a puzzle with 13 pieces. J. Geophys. Res. 1998;103:12413–12433. doi: 10.1029/98JB00386. [DOI] [Google Scholar]
- 4.Schellert W, Lister G, Toy V. A late Cretaceous and Cenozoic reconstruction of the Southwest Pacific region: Tectonics controlled by subduction and slab rollback processes. Earth-Sci. Rev. 2006;76:191–233. doi: 10.1016/j.earscirev.2006.01.002. [DOI] [Google Scholar]
- 5.Scher H, Martin E. Timing and climatic consequences of the opening of Drake Passage. Science. 2006;312:428–430. doi: 10.1126/science.1120044. [DOI] [PubMed] [Google Scholar]
- 6.White LT, Gibson GM, Lister GS. A reassessment of paleogeographic reconstructions of eastern Gondwana: Bringing geology back into the equation. Gondwana Res. 2013;24:984–998. doi: 10.1016/j.gr.2013.06.009. [DOI] [Google Scholar]
- 7.Eagles G, Jokat W. Tectonic reconstructions for paleobathymetry in Drake Passage. Tectonophysics. 2014;611:28–50. doi: 10.1016/j.tecto.2013.11.021. [DOI] [Google Scholar]
- 8.Tennyson AJD. The origin and history of New Zealand’s terrestrial vertebrates. N. Z. J. Ecol. 2010;34:6–27. [Google Scholar]
- 9.Worthy TH, De Pietri VL, Scofield RP. Recent advances in avian palaeobiology in New Zealand with implications for understanding New Zealand’s geological, climatic and evolutionary histories. N. Z. J. Zool. 2017;44:177–211. doi: 10.1080/03014223.2017.1307235. [DOI] [Google Scholar]
- 10.Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc. Natl. Acad. Sci. USA. 2008;105:7676–7680. doi: 10.1073/pnas.0801507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell, C. F. J. New Zealand long-tailed bat in The handbook of New Zealand mammals. 2nd edition (ed. King, C. M.) 98–109 (Oxford University Press, 2005).
- 12.Daniel MJ. The New Zealand short-tailed bat, Mystacina tuberculata; a review of present knowledge. N. Z. J. Zool. 1979;6:357–370. doi: 10.1080/03014223.1979.10428375. [DOI] [Google Scholar]
- 13.O’Donnell CFJ. Mystacina tuberculata. The IUCN Red List of Threatened Species. 2008;2008:e.T14261A4427784. [Google Scholar]
- 14.O’Donnell CFJ. Mystacina robusta. The IUCN Red List of Threatened Species. 2008;2008:e.T14260A4427606. [Google Scholar]
- 15.Simmons, N. B. Order Chiroptera in Mammal species of the world: a taxonomic and geographic reference (ed. Wilson, D. E. & Reeder, D. M.) 312–529 (Smithsonian Institution Press, 2005).
- 16.Teeling EC, et al. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307:580–584. doi: 10.1126/science.1105113. [DOI] [PubMed] [Google Scholar]
- 17.Rojas D, Warsi OM, Dávalos LM. Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant Neotropical diversity. Syst. Biol. 2016;65:432–448. doi: 10.1093/sysbio/syw011. [DOI] [PubMed] [Google Scholar]
- 18.Eick GN, Jacobs DS, Matthee CA. A nuclear DNA phylogenetic perspective on the evolution of echolocation and historical biogeography of extant bats (Chiroptera) Mol. Biol. Evol. 2005;22:1869–1886. doi: 10.1093/molbev/msi180. [DOI] [PubMed] [Google Scholar]
- 19.Meredith RW, et al. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science. 2011;334:521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- 20.Amador, L. I., Moyers Arévalo, R. L., Almeida, F. C., Catalano, S. A. & Giannini, N. P. Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. J. Mammal. Evol. 10.1007/s10914-016-9363-8 (2016).
- 21.Morgan, G. S. & Czaplewski, N. J. Evolutionary history of the Neotropical Chiroptera: the fossil record in Evolutionary history of bats: fossils, molecules and morphology (ed. Gunnell, G. F. & Simmons, N. B.) 105–161 (Cambridge University Press, 2012).
- 22.Gunnell GF, Simmons NB, Seiffert ER. New Myzopodidae (Chiroptera) from the Late Paleogene of Egypt: Emended family diagnosis and biogeographic origins of Noctilionoidea. PLoS ONE. 2014;9(2):e86712. doi: 10.1371/journal.pone.0086712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim BK. Review of the origins and biogeography of bats in South America. Chiropt. Neotrop. 2009;15:391–410. [Google Scholar]
- 24.Shi JJ, Rabosky DL. Speciation dynamics during the global radiation of extant bats. Evolution. 2015;69:1528–1545. doi: 10.1111/evo.12681. [DOI] [PubMed] [Google Scholar]
- 25.Phillips MJ. Geomolecular dating and the origin of placental mammals. Syst. Biol. 2016;65:546–557. doi: 10.1093/sysbio/syv115. [DOI] [PubMed] [Google Scholar]
- 26.Hand SJ, Murray PF, Megirian D, Archer M, Godthelp H. Mystacinid bats (Microchiroptera) from the Australian Tertiary. J. Paleontol. 1998;72:538–545. doi: 10.1017/S0022336000024318. [DOI] [Google Scholar]
- 27.Hand SJ, Archer M, Godthelp H. Australian Oligo-Miocene mystacinids (Microchiroptera): upper dentition, new taxa and divergence of New Zealand species. Geobios. 2005;38:339–352. doi: 10.1016/j.geobios.2003.11.005. [DOI] [Google Scholar]
- 28.Hand SJ, et al. Miocene mystacinids (Chiroptera: Noctilionoidea) indicate a long history for endemic bats in New Zealand. J. Vertebr. Paleontol. 2013;33:1442–1448. doi: 10.1080/02724634.2013.775950. [DOI] [Google Scholar]
- 29.Worthy, T. H. & Holdaway, R. N. The lost world of the moa: prehistoric life of New Zealand. Indiana University Press, Bloomington, Indiana, xxxiii + 718 pp. (2002).
- 30.Hand SJ, et al. Miocene fossils reveal ancient roots for New Zealand’s endemic Mystacina (Chiroptera) and its rainforest habitat. PLoS ONE. 2015;10(6):e0128871. doi: 10.1371/journal.pone.0128871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worthy TH, et al. Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proc. Natl. Acad. Sci. USA. 2006;103:19419–19423. doi: 10.1073/pnas.0605684103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worthy TH, et al. Biogeographical and phylogenetic implications of an Early Miocene wren (Aves, Passeriformes, Acanthisittidae) from New Zealand. J. Vertebr. Paleontol. 2010;30:479–498. doi: 10.1080/02724631003618033. [DOI] [Google Scholar]
- 33.Worthy TH, Tennyson AJD, Hand SJ, Godthelp H, Scofield RP. Terrestrial turtle fossils from New Zealand refloat Moa’s Ark. Copeia. 2011;2011(1):72–76. doi: 10.1643/CH-10-113. [DOI] [Google Scholar]
- 34.Worthy TH, Tennyson AJD, Scofield RP, Hand SJ. Early Miocene fossil frogs (Anura: Leiopelmatidae) from New Zealand. J. R. Soc. N. Z. 2013;4:211–230. doi: 10.1080/03036758.2013.825300. [DOI] [Google Scholar]
- 35.Schwarzhans W, Scofield RP, Tennyson AJD, Worthy JP, Worthy TH. Fish remains, mostly otoliths, from the non-marine Early Miocene of Otago, New Zealand. Acta Palaeontol. Pol. 2012;57:319–350. doi: 10.4202/app.2010.0127. [DOI] [Google Scholar]
- 36.De Pietri VL, Scofield RP, Hand SJ, Tennyson AJD, Worthy TH. Sheathbill-like birds (Charadriiformes: Chionoidea) from the Oligocene and Miocene of Australasia. J. R. Soc. N. Z. 2016;46:181–199. doi: 10.1080/03036758.2016.1194297. [DOI] [Google Scholar]
- 37.De Pietri VL, Scofield RP, Tennyson AJD, Hand SJ, Worthy TH. Wading the lost connection: New Zealand Miocene fossils reveal a new lineage of shorebirds (Aves, Charadriiformes) linking Gondwanan avifaunas. J. Syst. Palaeontol. 2016;14:603–616. doi: 10.1080/14772019.2015.1087064. [DOI] [Google Scholar]
- 38.Gunnell GF, Worsham SR, Seiffert ER, Simons EL. Vampyravus orientalis Schlosser (Chiroptera) from the Early Oligocene of Egypt – body mass, humeral morphology and affinities. Acta Chiropt. 2009;11:271–278. doi: 10.3161/150811009X485512. [DOI] [Google Scholar]
- 39.Smith FA, et al. Body mass of Late Quaternary mammals. Ecological archives E084-094. Ecology. 2003;84:3403. doi: 10.1890/02-9003. [DOI] [Google Scholar]
- 40.Czaplewski, N. J. & Morgan, G. S. New basal noctilionoid bats (Mammalia: Chiroptera) from the Oligocene of subtropical North America in Evolutionary history of bats: fossils, molecules and morphology (ed. Gunnell, G. F. & Simmons, N. B.) 162–209 (Cambridge University Press, 2012).
- 41.Simmons, N. B., Czaplewski, N. J. & Morgan, G. S. Oligocene mormoopids from Florida: surprising ancient diversity in a Neotropical bat family. Abstracts, 17th International Bat Research Conference, Durban, 128 (2016).
- 42.Savage DE. A Miocene phyllostomatid bat from Colombia, South America. Univ. Calif. Publ. Bull. Dept. Geol. Sci. 1951;28:357–366. [Google Scholar]
- 43.Dávalos LM, Velazco PM, Warsi O, Smits P, Simmons NB. Integrating incomplete fossils by isolating conflictive signal in saturated and non-independent morphological characters. Syst. Biol. 2014;63:582–600. doi: 10.1093/sysbio/syu022. [DOI] [PubMed] [Google Scholar]
- 44.Morgan GS. Late Rancholabrean mammals from southernmost Florida, and the Neotropical influence in Florida Pleistocene faunas. Smithson. Contrib. Paleobiol. 2002;93:15–38. [Google Scholar]
- 45.Archer M. Australia’s oldest bat. Proc. Roy. Soc. Qd. 1978;89:23–24. [Google Scholar]
- 46.Morgan GS, Czaplewski NJ, Rincon AF, Wood AR, MacFadden BJ. An early Miocene bat (Chiroptera: Phyllostomidae) from Panama and mid Cenozoic chiropteran dispersals between the Americas. J. Vertebr. Paleontol. Prog. Abstr. 2013;2013:180. [Google Scholar]
- 47.Czaplewski, N. J. Chiroptera in Vertebrate paleontology in the Neotropics: the Miocene fauna of La Venta, Colombia (ed. Kay, R. F., Madden, R. H., Cifelli, R. L. & Flynn, J. J.) 410–431 (Smithsonian Institution Press, 1997).
- 48.Czaplewski NJ, Takai M, Naeher TM, Shigehara N, Setoguchi N. Additional bats from the middle Miocene La Venta fauna of Colombia. Rev. Acad. Colomb. Cienc. Exactas, Fis. Nat. 2003;27:263–282. [Google Scholar]
- 49.Morgan GS, Czaplewski NJ. First fossil record of Amorphochilus schnablii (Chiroptera: Furipteridae) from the late Quaternary of Peru. Acta Chiropt. 1999;1:75–79. [Google Scholar]
- 50.Ali JR, Krause DW. Late Cretaceous bioconnections between Indo-Madagascar and Antarctica: refutation of the Gunnerus Ridge causeway hypothesis. J. Biogeogr. 2011;38:1855–1872. doi: 10.1111/j.1365-2699.2011.02546.x. [DOI] [Google Scholar]
- 51.Pierson ED, Sarich VM, Lowenstein JM, Daniel MJ, Rainey WE. A molecular link between the bats of New Zealand and South America. Nature. 1986;324:60–63. doi: 10.1038/323060a0. [DOI] [PubMed] [Google Scholar]
- 52.Sigé B. Rhinolophoidea et Vespertilionoidea (Chiroptera) du Chambi (Eocène inférieur de Tunisie). Aspects biostratigraphique, biogéographique et paléoécologique de l’origine des chiroptères modernes. Neues Jahrb. Geol. Paläontol. 1991;182:355–376. [Google Scholar]
- 53.Gunnell GF, Simons EL, Seiffert ER. New bats (Mammalia: Chiroptera) from the late Eocene and early Oligocene FayumDepression, Egypt. J. Vertebr. Paleontol. 2008;28:1–11. doi: 10.1671/0272-4634(2008)28[1:NBMCFT]2.0.CO;2. [DOI] [Google Scholar]
- 54.Ravel A, et al. Origine et radiation initiale des chauves-souris modernes: nouvelles découvertes dans l’Éocène d’Afrique du Nord. Geodiversitas. 2016;38:355–434. doi: 10.5252/g2016n3a3. [DOI] [Google Scholar]
- 55.Yu W, Wu Y, Yang G. Early diversification trend and Asian origin for extent bat lineages. J. Evol. Biol. 2014;27:2204–2218. doi: 10.1111/jeb.12477. [DOI] [PubMed] [Google Scholar]
- 56.Kirsch JAW, Hutcheon JM, Byrnes DGP, Lloyd BD. Affinities and historical zoogeography of the New Zealand short-tailed bat, Mystacina tuberculata Gray, 1843, inferred from DNA-hybridization comparisons. J. Mammal. Evol. 1998;5:33–64. doi: 10.1023/A:1020519019830. [DOI] [Google Scholar]
- 57.Pross J, et al. Persistent near-tropical warmth on the Antarctic continent during the early Eocene epoch. Nature. 2012;488:73–77. doi: 10.1038/nature11300. [DOI] [PubMed] [Google Scholar]
- 58.Wilf P, Cunéo NR, Escapa IH, Pol D, Woodburne MOW. Splendid and seldom isolated: the palaeobiogeography of Patagonia. Annu. Rev. Earth Planet. Sci. 2013;41:561–603. doi: 10.1146/annurev-earth-050212-124217. [DOI] [Google Scholar]
- 59.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 60.Hand SJ, Novacek MJ, Godthelp H, Archer M. First Eocene bat from Australia. J. Vertebr. Paleontol. 1994;14:375–381. doi: 10.1080/02724634.1994.10011565. [DOI] [Google Scholar]
- 61.Beck, R. M. D. The biogeographical history of non-marine mammaliaforms in the Sahul region in Handbook ofAustralasian biogeography (ed. Ebach, M. C.) (CRC Press, in press).
- 62.Hand, S. J. Bat beginnings and biogeography: the Australasian record in Evolution and biogeography ofAustralasian vertebrates (ed. Merrick, J. R., Archer, M., Hickey, G. & Lee, M. S. Y.) 673–705 (Auscipub, 2006).
- 63.Long, J., Archer, M., Flannery, T. F. & Hand, S.J. Prehistoric mammals of Australia and New Guinea. UNSW Press, Sydney, 244 pp (2002).
- 64.Black K. H., Archer, M., Hand, S. J. & Godthelp, H. The rise of Australian marsupials: A synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding in Earth and life: global biodiversity, extinction intervals and biogeographic perturbations through time (ed. Talent, J. A.) 983–1078 (Springer Verlag, 2012).
- 65.Simmons NB, Seiffert ER, Gunnell GF. A new family of large omnivorous bats (Mammalia, Chiroptera) from the Late Eocene of the Fayum Depression, Egypt, with comments on use of the name “Eochiroptera”. Am. Mus. Novit. 2016;3857:1–43. doi: 10.1206/3857.1. [DOI] [Google Scholar]
- 66.Hunter JP, Jernvall J. The hypocone as a key innovation in mammalian evolution. Proc. Natl. Acad. Sci. USA. 1995;92:10718–10722. doi: 10.1073/pnas.92.23.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norberg UM, Fenton MB. Carnivorous bats? Biol. J. Linn. Soc. 1988;33:383–394. doi: 10.1111/j.1095-8312.1988.tb00451.x. [DOI] [Google Scholar]
- 68.Dondini G, Vergari S. Carnivory in the greater noctule bat (Nyctalus lasiopterus) in Italy. J. Zool. 2000;251:233–236. doi: 10.1111/j.1469-7998.2000.tb00606.x. [DOI] [Google Scholar]
- 69.Santana SE, Strait S, Dumont ER. The better to eat you with: functional correlates of tooth structure in bats. Funct. Ecol. 2011;25:839–847. doi: 10.1111/j.1365-2435.2011.01832.x. [DOI] [Google Scholar]
- 70.Santana SE, Geipel I, Dumont ER, Kalka MB, Kalko EK. All you can eat: high performance capacity and plasticity in the common big-eared bat, Micronycteris microtis (Chiroptera: Phyllostomidae) PLoS ONE. 2011;6:e28584. doi: 10.1371/journal.pone.0028584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freeman PW. Frugivorous and animalivorous bats (Microchiroptera): dental and cranial adaptations. Biol. J. Linn. Soc. 1988;33:249–272. doi: 10.1111/j.1095-8312.1988.tb00811.x. [DOI] [Google Scholar]
- 72.Arkins AM, Winnington AP, Anderson S, Clout MN. Diet and nectarivorous foraging behaviour of the short-tailed bat (Mystacina tuberculata) J. Zool. 1999;247:183–187. doi: 10.1111/j.1469-7998.1999.tb00982.x. [DOI] [Google Scholar]
- 73.Lloyd BD. Advances in New Zealand mammalogy 1990– 2000: short-tailed bats. J. R. Soc. N. Z. 2001;31:59–81. doi: 10.1080/03014223.2001.9517639. [DOI] [Google Scholar]
- 74.Rojas D, Vale A, Ferrero V, Navarro L. When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Mol. Ecol. 2011;20:2217–2228. doi: 10.1111/j.1365-294X.2011.05082.x. [DOI] [PubMed] [Google Scholar]
- 75.Datzmann, T., von Helversen, O. & Mayer, F. Evolution of nectarivory in phyllostomid bats (Phyllostomidae Gray, 1825, Chiroptera: Mammalia). BMC Evol. Biol. 10, 165, 14 pp. (2010). [DOI] [PMC free article] [PubMed]
- 76.Baker, R., Bininda-Emonds, O. R. P., Mantilla-Meluk, H., Porter, C. A. & van den Bussche, R. Molecular timescale of diversification of feeding strategy and morphology in New World leaf-nosed bats (Phyllostomidae): a phylogenetic perspective in Evolutionary history of bats: fossils, molecules and morphology (ed. Gunnell, G. F. & Simmons, N. B.) 385–409 (Cambridge University Press, 2012).
- 77.Dumont ER, et al. Morphological innovation, diversification and invasion of a new adaptive zone. Proc. R. Soc. Lond. B. 2012;279:1797–1805. doi: 10.1098/rspb.2011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giannini, N. P., Gunnell, G. F., Habersetzer, J. & Simmons, N. B. Early evolution of body size in bats in Evolutionary history of bats: fossils, molecules and morphology (ed. Gunnell, G. F. & Simmons, N. B.) 530–555 (Cambridge University Press, 2012).
- 79.Pole M, Douglas BJ, Mason G. The terrestrial Miocene biota of southern New Zealand. J. R. Soc. N. Z. 2003;33:415–426. doi: 10.1080/03014223.2003.9517737. [DOI] [Google Scholar]
- 80.Lee DE, Conran JG, Lindqvist JK, Bannister JM, Mildenhall DC. New Zealand Eocene, Oligocene and Miocene macrofossil and pollen records and modern plant distributions in the Southern Hemisphere. Bot. Rev. 2012;78:235–260. doi: 10.1007/s12229-012-9102-7. [DOI] [Google Scholar]
- 81.Molnar RE, Pole MA. Miocene crocodilian from New Zealand. Alcheringa. 1997;21:65–70. doi: 10.1080/03115519708619185. [DOI] [Google Scholar]
- 82.Pole, M. The Miocene climate in New Zealand: Estimates from paleobotanical data. Palaeontol. Electron. 17, 2, 27A,79 pp. (2014).
- 83.Kück P, Struck TH. BaCoCa–a heuristic software tool for the parallel assessment of sequence biases in hundreds of gene and taxon partitions. Mol. Phylogenet. Evol. 2014;70:94–98. doi: 10.1016/j.ympev.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Ronquist F, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017;34:772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 86.Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- 87.Worthy TH, Scofield RP. Skeletal and dental variation within and between Mystacina species in southern New Zealand. N. Z. J. Zool. 2004;31:351–361. doi: 10.1080/03014223.2004.9518388. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.