Abstract
Rauwolfia species (Apocynaceae) are medicinal plants well known worldwide due to its potent bioactive monoterpene indole alkaloids (MIAs) such as reserpine, ajmalicine, ajmaline, serpentine and yohimbine. Reserpine, ajmalicine and ajmaline are powerful antihypertensive, tranquilizing agents used in hypertension. Yohimbine is an aphrodisiac used in dietary supplements. As there is no report on the comparative and comprehensive phytochemical investigation of the roots of Rauwolfia species, we have developed an efficient and reliable liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for ethanolic root extract of Rauwolfia species to elucidate the fragmentation pathways for dereplication of bioactive MIAs using high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (HPLC–ESI–QTOF–MS/MS) in positive ion mode. We identified and established diagnostic fragment ions and fragmentation pathways using reserpine, ajmalicine, ajmaline, serpentine and yohimbine. The MS/MS spectra of reserpine, ajmalicine, and ajmaline showed C-ring-cleavage whereas E-ring cleavage was observed in serpentine via Retro Diels Alder (RDA). A total of 47 bioactive MIAs were identified and characterized on the basis of their molecular formula, exact mass measurements and MS/MS analysis. Reserpine, ajmalicine, ajmaline, serpentine and yohimbine were unambiguously identified by comparison with their authentic standards and other 42 MIAs were tentatively identified and characterized from the roots of Rauwolfia hookeri, Rauwolfia micrantha, Rauwolfia serpentina, Rauwolfia verticillata, Rauwolfia tetraphylla and Rauwolfia vomitoria. Application of LC–MS followed by principal component analysis (PCA) has been successfully used to discriminate among six Rauwolfia species.
Keywords: Rauwolfia species, Monoterpene indole alkaloids (MIAs), HPLC–ESI–QTOF–MS/MS, Principal component analysis (PCA)
1. Introduction
Rauwolfia species, which belong to Apocynaceae family, are widely distributed in Asia, Africa and America [1], [2]. Apart from the common Rauwolfia serpentina, other species found in India are R. hookeri, R. micrantha, R. verticillata, R. tetraphylla and R. vomitoria [3]. Rauwolfia species have been used for the treatment of hypertension, snake bites, feverish illnesses and insanity from ancient time in Indian System of Medicine (ISM), Traditional Chinese Medicine (TCM) and Western System of Medicine (WSM) [4], [5], [6]. The ethanolic extract of the roots of Rauwolfia species has been also used to treat cardiovascular diseases [7], [8], cancer, hypertension [9], [10], [11], [12], various psychiatric diseases [1] and snake bites [13]. Dried root powder of Rauwolfia serpentina is also used for the treatment of cancer in Ayurveda [14], [15], [16]. The Rauwolfia species are a rich source of bioactive monoterpene indole alkaloids (MIAs) such as reserpine, ajmaline, ajmalicine, deserpidine, rescinnamine and yohimbine [17]. Reserpine is a powerful antihypertensive and tranquilizing agent used for the treatment of hypertension, schizophrenia, paranoia, breast cancer and Parkinson’s disease [18], [19], [20], [21], [22]. Ajmaline possesses antihypertensive and antiarrhythmic activities and is also used in high blood pressure [23], [24]. Ajmalicine is an antihypertensive drug used for treatment of cardiovascular diseases [25]. Serpentine possesses antihistaminase or antihistamine activity and is used in the treatment of snake bites [26], [27], [28]. Yohimbine has potential clinical applications in erectile dysfunction and is used as an aphrodisiac in dietary supplements [29], [30].
Qualitative and quantitative analyses of MIAs in Rauwolfia species have been carried out using high performance liquid chromatography (HPLC) [31], [32], high performance thin layer chromatography (HPTLC) [3], [33], gas chromatography mass spectrometry (GC–MS) [32], direct analysis in real time mass spectrometry (DART–MS) [5], [34], liquid chromatography/tandem mass spectrometry (LC–MS/MS) [3], [35] and Orbitrap Velos Pro mass spectrometer [36]. LC–MS/MS offers many advantages in the analysis of these types of compounds, due to its speed, sensitivity, specificity and ability to couple with high performance chromatographic techniques [37]. There are only a few reports available for the identification and characterization of MIAs by LC–MS/MS in plant extracts [35], [36], [38], [39], [40], [41], [42]. However, there is no report on the comparative and comprehensive phytochemical investigation of the roots of Rauwolfia species. Therefore, we have developed a simple and specific high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (HPLC–ESI–QTOF–MS/MS) method to establish fragmentation pathways for the identification and characterization of bioactive compounds from ethanolic extract of the root of Rauwolfia species.
2. Experimental
2.1. Chemicals and materials
LC–MS grade solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra-pure water was produced by Milli-Q Advantage system (Millipore, USA). AR grade ethanol, purchased from Merck Millipore (Darmstadt, Germany), was used in the preparation of ethanolic extracts. Reserpine, ajmalicine, ajmaline and serpentine were purchased from Sigma-Aldrich (St. Louis, MO, USA) and yohimbine was purchased from Chem. Faces (Wuhan, Hubei, China).
2.2. Plant materials
The roots of R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata and R. vomitoria were collected from the plants growing under similar conditions in Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI) campus, Kerala, India. The plant materials were collected in September 2012 and the voucher specimens (R. hookeri-66449, R. micrantha-66450, R. serpentina-66451, R. tetraphylla-66452, R. verticillata-66453 and R. vomitoria-66454) were deposited in the Herbarium of JNTBGRI. The roots were powdered, packed in airtight containers and stored at 20 °C until analysis.
2.3. Extraction
The powdered roots (50 g each) were extracted with 250 mL of ethanol. The extractions were performed by initial sonication for 30 min at 30 °C, followed by being kept at room temperature for 24 h. The extracts were filtered through filter paper (Whatman no. 1) and the residues were re-extracted using the same method four times with fresh solvent at room temperature for 24 h. The combined filtrates were evaporated to dryness under reduced pressure at 20–50 kPa at 40 °C using a Buchi rotary evaporator. 1 mg/mL stock solutions of each species of the dried plant extracts were prepared in methanol and filtered through a 0.22µm polyvinylidene fluoride (PVDF) membrane into the HPLC auto sampler vial prior to LC–MS analysis.
2.4. HPLC–ESI–QTOF–MS/MS conditions
Analyses were carried out using an Agilent 1200 HPLC system interfaced with Agilent 6520 hybrid quadrupole time-of-flight mass spectrometer (Agilent Technologies, USA). The 1200 HPLC system was equipped with a quaternary pump (G1311A), online vacuum degasser (G1322A), autosampler (G1329A), thermostatted column compartment (G1316C) and diode-array detector (G1315D).
2.5. Chromatographic conditions
Chromatographic separations were performed using a Thermo BetaSil C8 column (250 mm×4.5 mm, 5μm) operated at 25 °C employing a gradient elution using 0.1% formic acid in water (A) and acetonitrile (B) as mobile phase at a flow rate of 0.6 mL/min. The elusion consisted of a linear gradient from 25% to 27%; 0–15 min, 27%–37%; 15–18 min, 37%–42%; 18–20 min, 42%–45%; 20–22 min, 45%–48%; 22–25 min, 48–60%; 25–30 min, then returned to the initial conditions after 5 min. The sample injection volume was 1 μL.
2.6. Mass spectrometric condition
The mass spectrometer was operated in positive electrospray ionization mode and spectra were recorded by scanning the mass range m/z 50–1500 in both MS and MS/MS modes. Nitrogen was used as drying, nebulising and collision gas. The drying gas flow rate was 12 L/min. The heated capillary temperature was set to 350 °C and nebulizer pressure at 45 psi. The source parameters capillary voltage (VCap), fragmentor, skimmer and octapole voltages were set to 3500 V, 175 V, 65 V and 750 V, respectively. For the MS/MS analysis, collision energy was set at 30 eV and 35 eV, respectively, for reserpine and ajmalicine classes of compounds whereas 40 eV for ajmaline class of compounds. The accurate mass data of the molecular ions were processed through the Mass Hunter Workstation (version B 04.00) software (Agilent Technology, USA).
2.7. Statistical analysis
HPLC–ESI–QTOF–MS data obtained from three repeats of all the samples were subjected to statistical analysis. Principal component analysis (PCA) was performed on statistical software STATISTICA Version 7.0 (StatSoft, Inc., USA).
3. Results and discussion
3.1. Analysis of standards (templates)
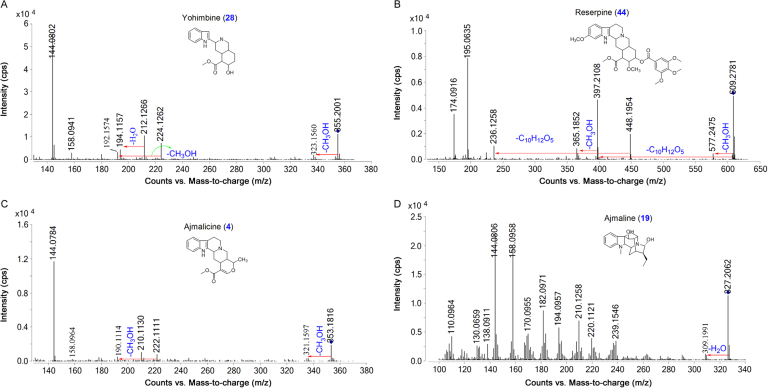
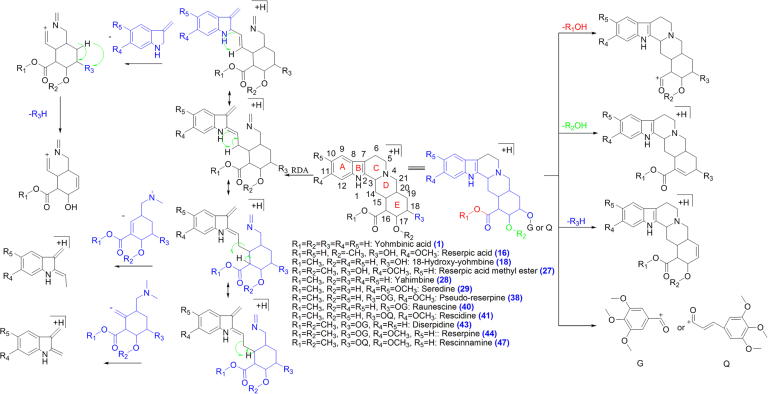
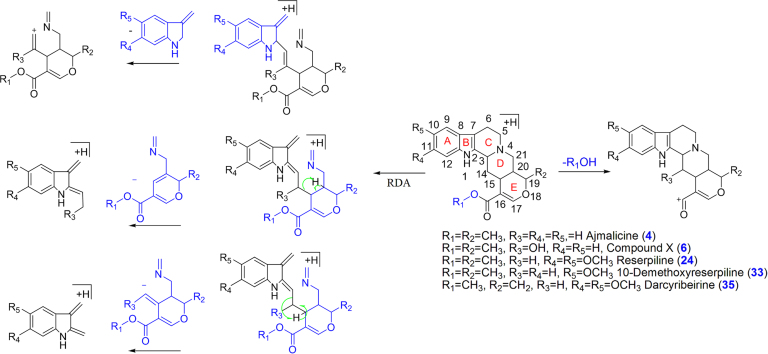
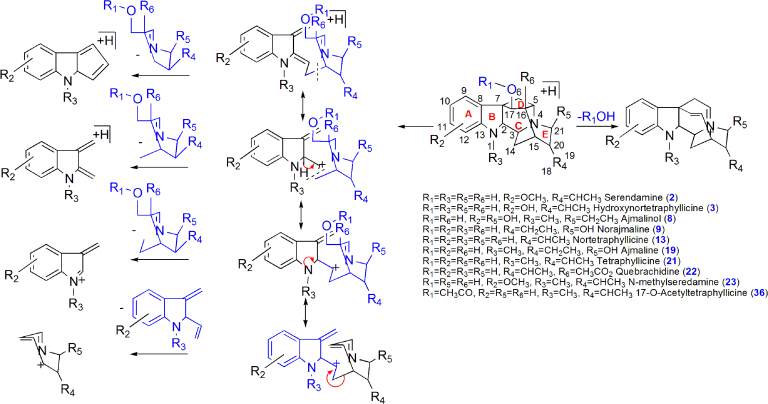
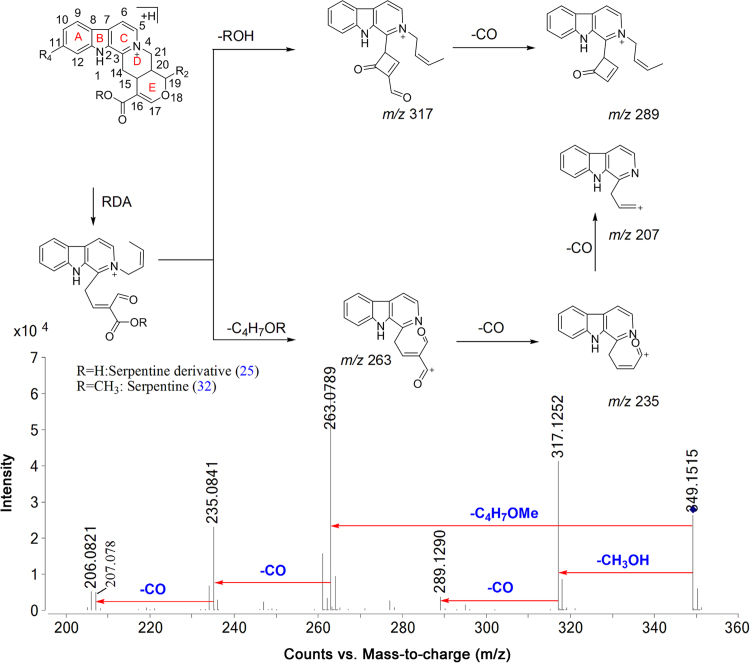
Major bioactive MIAs such as ajmalicine (4), ajmaline (19) serpentine (32), yohimbine (28) and reserpine (44) were selected as templates to construct the diagnostic fragmentation pathways for identification and characterization of MIAs from plant extracts. Yohimbine and reserpine showed fragment ions at m/z 323.1560 and 577.2475, respectively, due to losses of CH3OH. Both compounds also showed fragment ions at m/z 337.1912 and 397.2108 due to losses of H2O and C10H12O5, respectively. Fragment ions at m/z 224.1266, 212.1262, 158.0941 and 144.0802 were observed in yohimbine, whereas MS/MS spectrum of reserpine showed fragment ions at m/z 448.1954, 436.1950, 188.1070 and 174.0916 due to retro Diels Alder (RDA) cleavage of the C-ring. The fragment ion at m/z 448.1950 from reserpine gave product ion at m/z 236.1255 due to loss of C10H12O5. Similarly, the fragment ions at m/z 224.1266 and 212.1262 gave product ions at m/z 192.1574 and 194.1157 due to losses of CH3OH and H2O, respectively, in yohimbine (Fig. 1A and B, Scheme 1). The MS/MS spectrum of ajmalicine showed fragmention ion at m/z 321.1597 due to loss of CH3OH. RDA cleavage produced the fragment ions at m/z 222.1111, 210.1130, 158.0964 and 144.0784. The product ion at m/z 190.1114 was formed by loss of CH3OH from the ion at m/z 222.1111 (Fig. 1C and Scheme 2). Successive losses of H2O gave rise to the ions at m/z 309.1991 and 291.1881 in ajmaline. RDA cleavage of the C-ring gave characteristic fragment ions at m/z 170.0955, 158.0958 and 144.0806. The fragment ion at m/z 120.0795 was produced by loss of H2O from the ion at m/z 138.0911 (Fig. 1D and Scheme 3). The MS/MS spectrum of serpentine showed fragment ions at m/z 317.1290 and 263.0789 due to losses of CH3OH and C4H7OCH3, respectively, whereas fragment ions at m/z 289.1314, 235.0826 and 207.0858 were formed due to successive losses of CO (Scheme 4).
Fig. 1.
MS/MS spectra of yohimbine, reserpine, ajmalicine and ajmaline.
Scheme 1.
Proposed fragmentation pathways of reserpine class of MIAs.
Scheme 2.
Proposed fragmentation pathways of ajmalicine class of MIAs (X: Methyl-14-hydroxy-19-methyl-16,17-didehydro-18-oxayohimban-16 carboxylate).
Scheme 3.
Proposed fragmentation pathways of ajmaline class of MIAs.
Scheme 4.
Proposed fragmentation pathways of serpentine class of MIAs.
3.2. Metabolic profiling using LC–MS
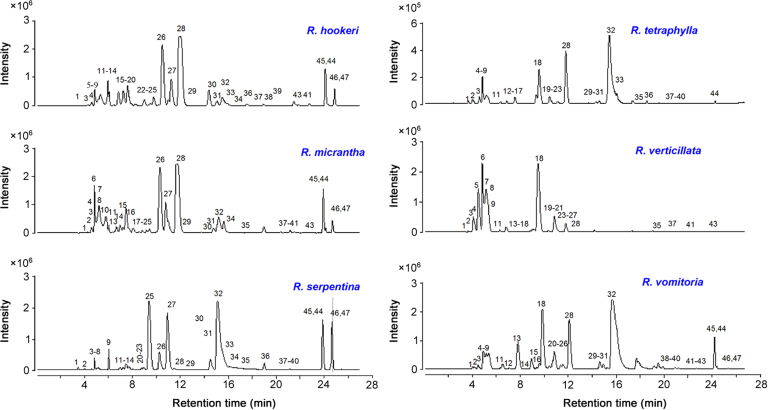
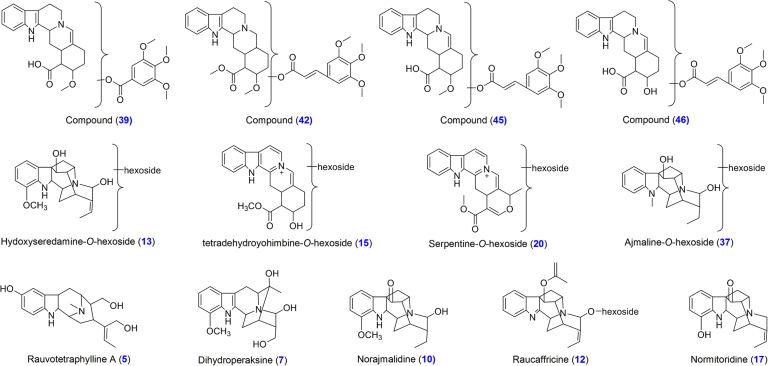
All the six Rauwolfia species were cultivated in similar environmental conditions to study phytochemical variations. Ethanolic extract of root was analyzed using gradient mobile phase consisting of acetonitrile and 0.1% aqueous formic acid. Parameters such as column type, column temperature, mobile phase, elution conditions, flow rate and MS conditions were optimized. Base peak chromatograms (BPCs) of ethanolic extracts of R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata and R. vomitoria in positive-ion mode are shown in Fig. 2. Retention time (RT), observed [M+H]+, molecular formula, error (Δppm), major fragment ions and their relative abundance and distribution along with assignment are presented in Table 1. All these compounds were identified based on their exact mass, molecular formula, and fragmentation pattern. A total of 47 known/unknown MIAs were tentatively identified and characterized. The proposed structures of the unknown compounds are shown in Fig. 3. Yohimbine, reserpine, ajmalicine, ajmaline and serpentine were unambiguously identified and characterized by comparison with their authentic standards.
Fig. 2.
Base peak chromatograms (BPCs) of ethanolic extracts of Rauwolfia species.
Table 1.
Chromatographic and spectrometric characteristics of monoterpene indole alkaloids (MIAs) in ethanolic extracts of six Rauwolfia species (root) by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry.
| Compound (peak) no. | RT (min) | Error (Δppm) | Obs. m/z | Molecular formula | MS/MS fragment ions (Relative abundance, %) | Compounds | Distribution |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root |
||||||||||||
| Rh | Rm | Rs | Rt | Rv | Rvm | |||||||
| Reserpine class | ||||||||||||
| 1 | 3.40 | 1.49 | 341.1867 | C20H24N2O3 | 323.1764 (5), 210.1119 (9), 192.1019 (4), 158.0932 (15), 144.0794 (100) | Yohimbinic acid | + | + | + | + | + | + |
| 11 | 6.95 | −0.21 | 341.1865 | C20H24N2O3 | 323.1762 (3), 210.1153 (11), 192.1018 (5), 158.0953 (15), 144.0797(100), | Yohimbinic acid (isomer) | + | + | + | + | + | + |
| 16 | 7.81 | 0.03 | 401.2071 | C22H28N2O5 | 383.1965 (2), 383.1965 (2), 369.1809 (2), 321.1598 (5), 240.1200 (32), 222.1125 (1), 188.1045 (5), 174.0907 (100) | Reserpic acid | + | + | + | + | + | + |
| 18 | 8.20 | −0.29 | 371.1966 | C21H26N2O4 | 339.1703 (3), 353.1857 (21), 240.1230 (12), 222.1125 (2), 158.0964 (10), 144.0798 (100), 228.1223 (17) | 18-Hydroxy-yohmbine | + | + | + | + | + | + |
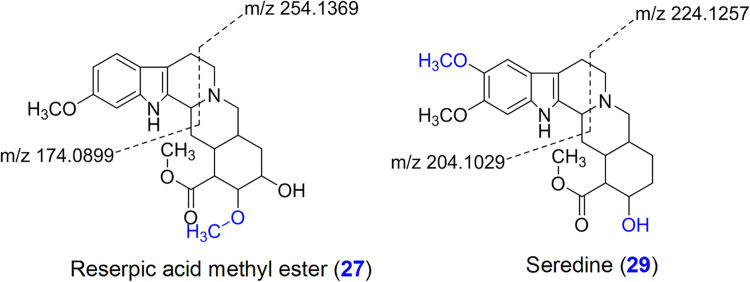
| 27 | 10.75 | 1.44 | 415.2217 | C23H30N2O5 | 383.1916 (4), 397.2122 (2), 254.1369 (51), 236.1281 (2), 222.1116 (7), 188.1059 (7), 174.0899 (100), 160.0743 (4) | Reserpic acid methyl ester | − | + | − | − | + | − |
| 28 | 10.91 | −0.79 | 355.2019 | C21H26N2O3 | 323.1560 (10), 224.1285(19), 337.1920 (3), 212.1282 (24), 158.0959 (1), 144.0808 (100) | Yohimbinea | + | + | + | + | + | + |
| 29 | 11.70 | 1.44 | 415.2217 | C23H30N2O5 | 383.1935 (3), 224.1257 (9), 204.1029 (100), 218.1176 (1), 189.0771 (21), 173.0845 (45) | Seredine | + | + | + | + | + | + |
| 38 | 20.82 | −0.49 | 595.2651 | C32H38N2O9 | 434.1748 (15), 383.1941 (30), 351.1773 (5), 222.1110 (10), 195.0631 (100), 188.10 (3), 174.0880 (43) | Pseudo-reserpine | + | + | + | + | + | + |
| 39 | 21.20 | 0.14 | 563.239 | C31H34N2O8 | 351.1711 (60), 333.1535 (42), 319.1463 (55), 222.1244 (32), 195.0672 (21), 170.0939 (34), 144.0800 (100) | Reserpine class (unknown) | + | + | + | + | − | + |
| 40 | 21.50 | 0.19 | 565.2544 | C31H36N2O8 | 533.2282 (2), 434.1714 (3), 353.1805 (57), 321.1559 (5), 222.1115 (5), 158.063 (2), 195.0622 (100), 144.0777(28), | Raunescine | − | + | + | + | + | + |
| 41 | 22.90 | 0.4 | 621.2805 | C34H40N2O9 | 589.2544 (2), 460.1930 (4), 383.1925 (11), 351.1703 (3), 221.0794 (100), 222.1115 (98), 188.1070 (5), 174.0804 (19) | Rescidine | + | + | + | − | + | + |
| 42 | 23.48 | 1.45 | 591.271 | C33H38N2O8 | 371.1896 (21), 353.1847 (28), 221.0808 (100), 144.0771 (17) | Reserpine class (unknown) | − | − | + | − | − | + |
| 43 | 23.80 | 0.03 | 579.2702 | C32H38N2O8 | 547.2413 (12), 367.2009 (79), 448.1907 (1), 335.1730 (24), 236.1262 (10), 195.0649 (100), 144.0817 (38) | Deserpidine | + | + | + | − | + | + |
| 44 | 24.31 | −0.25 | 609.2807 | C33H40N2O9 | 577.2492 (5), 448.1930 (24), 436.1934 (5), 397.2090 (66), 365.1835 (13), 236.1261 (15), 195.0640 (100), 174.0905 (55) | Reserpinea | + | + | + | + | − | + |
| 45 | 24.54 | 1.87 | 607.2634 | C33H38N2O9 | 367.2017 (17), 335.1765 (11), 236.1272 (7), 221.0820 (100), 206.0577 (10), 190.0621 (14), 144.0815 (35) | Reserpine class (unknown) | + | + | + | − | − | + |
| 46 | 25.02 | 1.78 | 605.2857 | C34H40N2O8 | 369.1805 (20), 221.1805 (100), 206.0598 (11), 190.0618 (27), 174.0910 (40) | Reserpine class (unknown) | + | + | + | − | − | + |
| 47 | 25.22 | 0.08 | 635.2963 | C35H42N2O9 | 603.2779 (1), 474.2066 (5), 397.2085 (22), 236.1281 (2), 221.0791 (100), 188.1070 (2), 174.0881 (11) | Rescinnamine | + | + | + | − | − | + |
| Ajmalicine class | ||||||||||||
| 4 | 5.52 | −0.36 | 353.1861 | C21H24N2O3 | 321.1598 (1), 222.1124 (1), 210.111 (7), 144.0808 (100), 158.0964 (2) | Ajmalicinaa | + | + | + | + | + | + |
| 6 | 6.01 | 0.05 | 369.1809 | C21H24N2O4 | 337.1558 (19), 222.1275 (16), 351.1703 (37), 291.1496 (5), 158.0963 (30), 144.0808 (100) | Methyl-14-hydroxy-19-methyl-16,17-didehydro-18-oxayohimban-16 carboxylate | + | + | + | + | + | + |
| 24 | 10.52 | 1.07 | 413.2076 | C23H28N2O5 | 397.1763 (3), 323.1560 (3), 222.1106 (28), 218.1176 (18), 204.1005 (100) | Reserpiline | + | + | + | + | − | + |
| 31 | 14.81 | 0.02 | 413.2072 | C23H28N2O5 | 397.1766 (10), 323.1560 (2), 222.1125 (29), 204.1005 (100), 218.1176(30) | Reserpiline (isomer) | + | + | + | + | + | + |
| 33 | 15.50 | 0.01 | 383.1965 | C22H26N2O4 | 351.1677 (2), 222.1110 (10), 159.0675 (3), 188.1053 (2), 174.0895 (100) | 10-Demethoxyreserpiline | + | + | + | + | − | + |
| 35 | 17.50 | 0.23 | 411.1914 | C23H26N2O5 | 379.1652 (1), 222.1110 (16), 204.1007 (100), 218.1181 (12) 173.0818 (11) | Darcyribeirine | − | + | + | + | − | + |
| Ajmaline class | ||||||||||||
| 2 | 4.80 | 0.31 | 325.1910 | C20H24N2O2 | 307.1715 (5), 186.0896 (12), 174.0913 (57), 160.0805 (100), 146.0935 (22), 138.0954 (12) | Seredamine | + | + | + | + | + | + |
| 3 | 5.01 | 0.76 | 311.1752 | C19H22N2O2 | 293.1752 (2), 172.0763 (9), 160.0768 (21), 146.0960 (100), 136.0791 (10) | Hydroxynortetraphyllicine | + | + | + | + | + | + |
| 8 | 6.55 | 1.4 | 343.2005 | C20H27N2O3 | 325.1927 (8), 307.1830 (15), 186.0906 (54), 174.0917 (60), 160.0766 (22), 138.0912 (100) | Ajmalinol | + | + | + | + | + | + |
| 9 | 6.60 | 0.09 | 313.191 | C19H24N2O2 | 295.1806 (5), 196.1123 (21), 156.0813 (16), 144.0821 (55), 130.0664 (100) | Norajmaline | + | + | + | + | + | + |
| 13 | 7.45 | 1.52 | 503.2386 | C26H34N2O3 | 341.1898 (10), 323.1752 (62), 309.1595 (27), 291.1487 (100) | Hydoxyseredamine-O- hexoside | + | + | + | + | − | + |
| 14 | 7.52 | 1.26 | 295.1801 | C19H22N2O | 277.1666 (4), 156.0799 (7), 144.0811 (27), 138.0474 (47), 130.0656 (100), 120.0791 (24) | Nortetraphyllicine | + | + | + | + | + | + |
| 19 | 8.91 | 0.13 | 327.2067 | C20H26N2O2 | 309.1991 (3), 291.1839 (3), 170.0974 (45), 158.0964 (100), 144.0812 (82) | Ajmalinea | + | + | + | + | + | + |
| 21 | 9.53 | 0.6 | 309.196 | C20H24N2O | 291.1780 (4), 263.1536 (31), 170.0950 (19), 158.0965 (77), 144.0817 (100), 120.0822 | Tetraphyllicine | − | + | + | + | − | − |
| 22 | 9.84 | 1.4 | 353.186 | C21H24N2O3 | 335.1758 (3), 275.1501 (2), 156.0808 (4), 144.0890 (100), 130.0693 (7) | Quebrachidine | + | + | + | + | + | + |
| 23 | 10.11 | 0.11 | 355.2017 | C21H26N2O3 | 309.2031 (5), 277.1667 (13), 260.1360 (12), 170.0955 (78), 158.0956 (98), 144.0805 (78) | Hydroxymethylseredamine | + | + | + | + | + | + |
| 36 | 19.31 | 0.28 | 351.2066 | C22H26N2O2 | 291.1827 (17),182.962 (78), 170.0956 (45), 158.0962 (45), 144.0803 (100), 120.0802 (10) | 17-O-Acetyltetraphyllicine | + | − | + | + | − | − |
| 37 | 20.76 | 0.45 | 489.2595 | C26H36N2O7 | 327.2073 (100), 309.1663 (80), 291.1554 (47) | Ajmaline-O-hexoside | + | + | − | + | ||
| Quaternary alkaloids | ||||||||||||
| 15 | 7.70 | 1.25 | 513.2232 | C27H32N2O8 | 333.1615 (14), 274.1199 (16), 246.1299 (53), 230.0986 (87), 220.1137 (67), 204.0823 (100), 195.0926 (65), | Tetradehydroyohimbine-O-hexoside | + | + | + | + | + | + |
| 20 | 9.17 | 0.25 | 511.2373 | C37H30N2O8 | 349.1548 (100), 317.1326 (24), 289.1313 (10) | Serpentine-O-hexoside | + | + | + | + | + | + |
| 25 | 10.60 | 0.85 | 335.1389 | C20H18N2O3 | 317.1296 (100), 289.1492 (7), 263.0808 (71), 235.0863 (34), 307.0858 (5) | Serpentine derivative | − | + | + | + | + | + |
| 26 | 10.70 | −1.54 | 351.1707 | C21H22N2O3 | 351.1674 (100), 333.1570 (4), 219.1440 (5), 271.1470 (20), 248.1024 (4), 195.0890 (6), 168.0788 (13) | Tetradehydroyohimbine | + | + | + | + | + | + |
| 32 | 15.40 | 0.47 | 349.1547 | C21H20N2O3 | 317.1290 (66), 289.1314 (7), 263.0796 (100), 235.0826 (34), 307.0858 (6) | Serpentinea | + | + | + | + | − | + |
| 34 | 16.62 | 0.45 | 685.3375 | C42H45N4O5 | 653.3131 (13), 435.1947 (23), 403.1516 (3), 349.1235 (2), 375.1701 (23), 251.1558 (7) | Serpentinine | + | + | + | − | − | − |
| Other compounds | ||||||||||||
| 5 | 5.93 | 1.47 | 343.2015 | C20H26N2O3 | 326.1972 (47), 307.1794 (16), 182.0968 (57), 170.0945 (36), 158.0962 (81), 144.0795 (100), 132.0757 (11) | Rauvotetraphylline A | + | + | + | + | + | + |
| 7 | 6.33 | 1.25 | 313.1917 | C19H24N2O2 | 295.1845 (7), 144.0819 (70), 138.0933 (100), 122.0869 (61) | Dihydroperaksine | + | + | + | + | + | + |
| 10 | 6.90 | 0.89 | 311.1755 | C19H22N2O2 | 293.1641 (8), 156.0808 (17), 144.0804 (52), 138.0919 (10), 130.0650 (100), 120.0805 (28) | Norajmalidine | − | + | + | + | − | − |
| 12 | 7.12 | 0.12 | 513.2231 | C27H32N2O8 | 351.1994 (100), 291.1480 (16) | Raucaffricine | + | − | + | + | + | + |
| 17 | 8.02 | 0.15 | 309.1598 | C19H20N2O2 | 291.1492, 172.0782 (24), 160.0757 (51), 146.0612 (100), 172.0782 (24), 136.0780 (38), 120.0803(15) | Normitoridine | + | + | + | + | + | + |
| 30 | 12.04 | 0.74 | 429.2018 | C23H28N2O6 | 397.1781 (87), 369.1759 (10), 339.1340 (14), 220.0972 (100), 210.1124 (35), 205.0732 (9), 189.0772 (21), 178.0845 (13), 150.0913 (21) | Carapanaubine | + | + | + | − | + | + |
Matched with the standards. Rh:R. hookeri; Rm: R. micrantha; Rs: R. serpentina; Rt: R. tetraphylla; Rv: R. verticillata; Rvm: R. vomitoria. RT: Retention time; Obs: observed.
Fig. 4.
Structures of isomeric compounds.
3.2.1. Reserpine class of compounds
Seventeen compounds were tentatively identified and characterized on the basis of their fragmentation pathways of yohimbine and reserpine. Compounds 28 and 44 were identified and characterized as yohimbine and reserpine, respectively, by comparison with their authentic standards. Fragmentation pathways of reserpine class of compounds are shown in Scheme 1. Compounds 1 and 16 were tentatively identified as yohimbine acid and reserpic acid, respectively, which showed characteristic fragment ions at m/z 323.1754 and 383.1965 respectively, due to the losses of H2O whereas fragment ion at m/z 369.1809 was observed due to loss of CH3OH in compound 16. Compounds 1 (RT 3.4 min) and 11 (RT 6.9 min) gave the same fragment ions with different relative abundances in their MS/MS spectra. Both compounds 1 and 11 were tentatively identified as an isomeric pair. Compounds 16, 18, 27, 29, 38, 40, 41, 43 and 47 were identified as 18-hydroxy-yohmbine, reserpic acid methyl ester, seredine, pseudo-reserpine, raunescine, rescidine, deserpidine and rescinnamine, respectively. Compounds 18, 27, 29, 38, 40, 41, 43 and 47 showed fragment ions at m/z 339.1703, 383.1916, 383.1935, 563.2394, 533.2282, 589.2544, 547.2413 and 603.2779 due to loss of CH3OH from [M+H]+ ions, respectively. Similarly, all these compounds also showed fragment ions at m/z 353.1857, 397.2122, 383.1941, 353.1805, 383.1925, 367.2009 and 397.2085, respectively due to loss of H2O (compounds 18 and 27), C10H12O5 (compounds 38, 40, 43 and 47) and C12H14O5 (compounds 41 and 47) from [M+H]+ ions. Fragment ion at m/z 158.0932 was observed in compounds 1, 18, 40 and 43, whereas compounds 16, 27, 38, 40, 41 and 43 showed fragment ion at m/z 188.1045 due to loss of terpene moiety via C-ring cleavage followed by bond breaking between C14 and C15. Similarly, compound 29 gave fragment ion at m/z 218.1178. Compounds 1, 18, 40 and 43 provided RDA fragment ions at m/z 144.0794, whereas fragment ions at m/z 174.0907 (143 Da+OCH3) was observed in compounds 16, 27, 38, 41 and 47 due to loss of the terpene moiety via C-ring cleavage by RDA followed by bond breaking between C3 and C14. Similarly, the compound 29 showed fragment ion at m/z 204.1029 (142 Da+2OCH3) due to loss of terpene moiety. Compounds 38, 40 and 43 showed fragment ions at m/z 195.06 whereas fragment ion at m/z 221.0790 was observed in compounds 41 and 47 as base peak. Compounds 27 and 29 may be positional isomers distinguished by RDA fragment ions. Compound 27 showed fragment ions at m/z 174.0899, 188.1059 and 254.1369 whereas fragment ions at m/z 204.1029, 218.1176 and 224.1257 were observed in compound 29. Fragments of compounds 27 and 29 showed 30 Da difference indicating -OCH3 group on terpene and indole moiety, respectively (Fig. 4). The characteristic RDA fragment ions at m/z 210.1119 (compounds 1), 240.1200 (compounds 16), 254.1369 (compound 27), 224.1257 (compound 29), 434.1748 (compounds 38 and 40), 460.1930 (compound 41), 448.1907 (compound 43) and 474.2066 (compound 47) were observed due to losses of indole moiety via RDA cleavage followed by bond cleavage between C2-C3. Fragment ion at m/z 222.1110 was observed due to loss of H2O (compounds 16), C10H12O5 (compounds 38 and 40) and C12H14O5 (compound 41) from fragment ions at m/z 240.1200, 434.1748 and 460.1930, respectively. Similarly, compounds 27, 29, 43 and 47 showed fragmention at m/z 236.1262 due to loss of H2O (compound 27), C10H12O5 (compound 43) and C12H14O5 (compound 47) from fragment ions at m/z 254.1369, 448.1907 and 474.2066, respectively. Compounds 39, 42, 45 and 46 may be reserpine class of compounds because all these compounds followed similar fragmentation pathways with the reserpine and provided the characteristic fragment ions (Fig. 3).
Fig. 3.
Structures of unknown and other class of compounds.
3.2.2. Ajmalicine class of compounds
Six compounds 4, 6, 24, 31, 33 and 35 were identified and characterized as ajmalicine, methyl-14-hydroxy-19-methyl-16,17-didehydro-18-oxayohimban-16 carboxylate, reserpiline, reserpiline (isomer), 10-demethoxyreserpiline and darcyribeirine, respectively (Scheme 2). Compound 4 was identified and characterized by comparison with the authentic standard of ajmalicine. Compounds 6, 24, 33 and 35 showed fragment ions at m/z 337.1558, 323.1560, 351.1677 and 379.1652 due to loss of CH3OH, respectively. Fragment ions at m/z 158.0963 (compound 6), 218.1174 (compounds 24 and 35) and 188.1053 (compound 33) were observed due to loss of terpene moiety via RDA cleavage followed by bond cleavage between C14 and C15. Similarly, the fragment ions at m/z 144.0808 (compound 6), 174.0895 (143 Da+OCH3) (compound 33) and 204.1005 (142 Da+2OCH3) (compounds 24 and 35) were observed as base peak due to loss of terpene moiety via RDA followed by bond cleavage between C3 and C14. Compounds 24 and 31 may be isomeric pair, showing similar fragment at retention time 10.52 min and 14.81 min with different relative abundances of fragment ions. RDA fragment ion at m/z 222.1124 was observed in all compounds due to loss of indole moiety via RDA cleavage followed by bond cleavage between C2 and C3.
3.2.3. Ajmaline class of compounds
Twelve ajmaline class of compounds 2, 3, 8, 9, 13, 14, 19, 21, 22, 23, 36 and 37 were tentatively identified and characterized as seredamine, hydroxynortetraphyllicine, ajmalinol, norajmaline, hydoxyseredamine-O-hexoside, nortetraphyllicine, ajmaline, tetraphyllicine, quebrachidine, hydroxymethylseredamine, 17-O-acetyltetraphyllicine and ajmaline-O-hexoside, respectively (Scheme 3). Compound 19 was identified and characterized by comparison with ajmaline standard. Compounds 2, 3, 8, 9, 14, 21, 22, 23 and 36 showed fragment ions at m/z 307.1715, 293.1752, 325.1927, 295.1806, 277.1666, 291.1780, 335.1758, 309.2031 and 291.1827 due to loss of H2O except compound 36 (-CH3COOH). Fragment ions at m/z 186.0896 [155 Da+OMe] (compound 2), 172.0763 [155 Da+OH] (compound 3 and 8), 156.0813 (compounds 9, 14 and 22) and 170.0974 [155 Da+CH3] (compounds 21, 23, and 36) were observed due to loss of terpene moiety via RDA cleavage followed by bond cleavage between C14 and C15. Similarly, fragment ions at m/z 174.0913 [143 Da+OMe] (compound 2), 160.0768 [143 Da+OH] (compounds 3 and 8), 144.0821 (compounds 9, 14, and 22) and 158.09 [143 Da+CH3] (compounds 21, 23 and 36) formed due to loss of terpene moiety via RDA cleavage followed by bond cleavage between C3 and C14. All compounds showed RDA fragment ions at m/z 160.0805 [129 Da+OMe] (compounds 2 and 8), 146.0960 (129 Da+OH) (compound 3), 130.0656 (compounds 9, 14 and 22) and 144.0817 [129 Da+CH3] (compounds 21, 23 and 36) due to loss of terpene moiety via RDA cleavage followed by bond cleavage between C2 and C3. Compounds 13 and 37 were identified as hydoxyseredamine-O-hexoside and ajmaline-O-hexoside which showed a characteristic loss of 162 Da and gave fragment ions at m/z 341.1898 and 327.2073, respectively.
3.2.4. Quaternary indole alkaloids
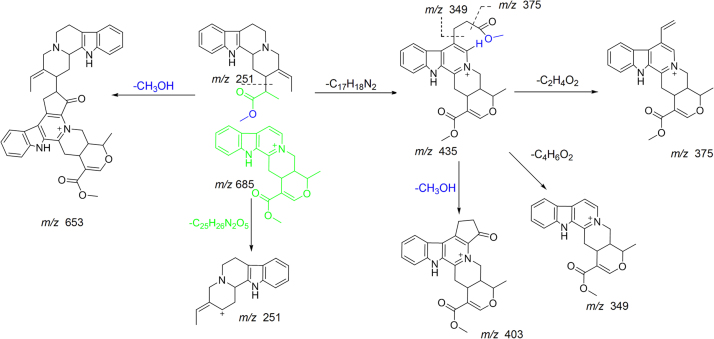
Six quaternary indole alkaloids compounds 15, 20, 25, 26, 32 and 34 were identified and characterized as tetradehydroyohimbine-O-hexoside, serpentine-O-hexoside, serpentine derivative, tetradehydroyohimbine, serpentine and serpentinine. Compound 32 was identified and characterized as serpentine which also matched with the standard. Compound 15 was identified as a hexoside of tetradehydroyohimbine and it showed fragment ion at m/z 351.1674 due to the loss of C6H10O5. Fragment ion at m/z 351.1674 gave product ions at m/z 333.1615 and 319.1440 due to further losses of H2O and CH3OH, respectively. Compound 20 showed fragment ion at m/z 349.1548 due to characteristic loss of 162 Da (C6H10O5). Compound 25 was identified as serpentine derivative which showed 15 Da lower molecular weight compared to serpentine and followed similar fragmentation pattern. It showed fragment ions at m/z 317.1296 and 289.1492 due to losses of H2O and CO, respectively. Compound 34 showed fragment ions at m/z 653.3131, 435.1956 and 251.1546 due to losses of CH3OH, C25H26N2O5 and C17H18N2, respectively. Fragment ion m/z 435.1956 showed product ions at m/z 375.1732, 349.1235, and 403.1516 due to losses of C2H4O2, C4H6O2 and CH3OH, respectively (Scheme 5).
Scheme 5.
Proposed fragmentation pathways of serpentinine.
3.2.5. Other indole alkaloids
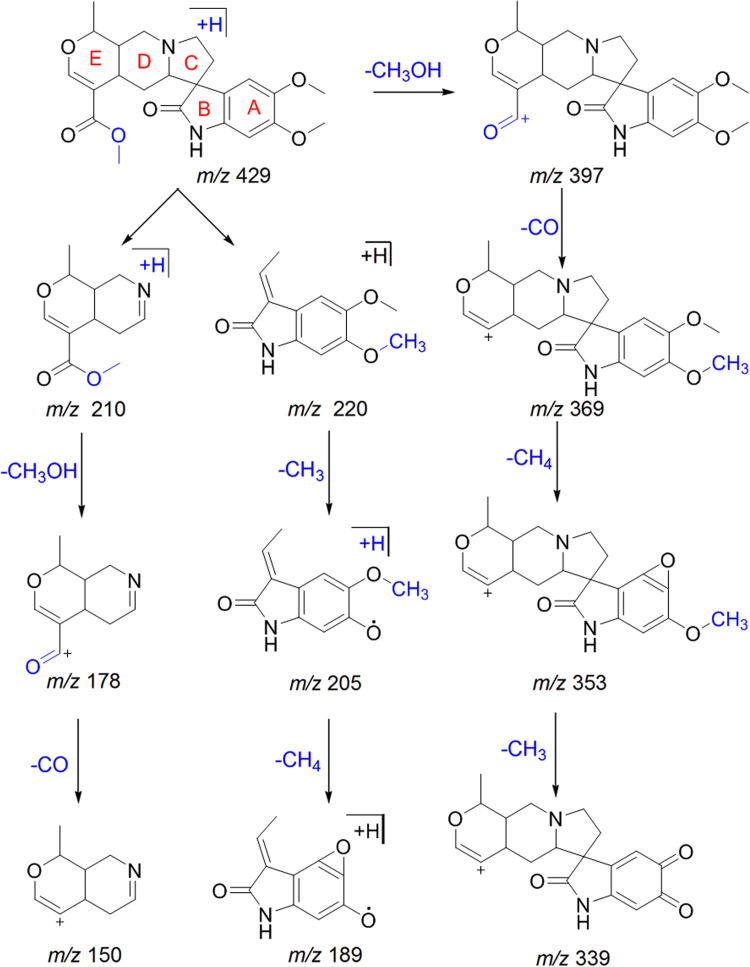
Six indole alkaloids 5, 7, 10, 12, 17 and 30 were tentatively identified and characterized as rauvotetraphylline A, dihydroperaksine, norajmalidine, raucaffricine, normitoridine, and carapanaubine, respectively. Compounds 5, 7, 10 and 17 showed fragment ions at m/z 326.1972, 295.1845, 293.1641 and 291.1492, respectively due to loss of H2O from precursor. The RDA fragment ions at m/z 158.0962 [143+CH3], 144.0795, 156.0808 and 172.0782 were observed in compounds 5, 7, 10 and 17 due to C-ring cleavage via RDA. Similarly, RDA fragment ions at m/z 130.0650 and 146.0612 [129+OH] were observed as base peaks in compounds 10 and 17, respectively. Compound 12 showed fragment ions at m/z 351.1994 and 291.1480 due to losses of C6H10O5 and C2H4O2, respectively. Compound 30 showed fragment ions at m/z 397.1781, 369.1759, 353.1501 and 339.1340 due to successive losses of CH3OH, CO, CH4 and CH3, respectively. Fragment ions at m/z 210.1124 and 220.0972 were observed as base peaks due to C-ring cleavage. Further fragment ions at m/z 210.1124 and 220.0972 produced fragment ions at m/z 178.0845 and 205.0732 due to losses of CH3OH and CO, respectively. Fragment ions at m/z 150.0913 and 189.0772 formed due to losses of CO and CH4 from m/z 178.0845 and 205.0732, respectively (Scheme 6).
Scheme 6.
Proposed fragmentation pathways of carapanaubine.
3.3. Identification of markers using PCA
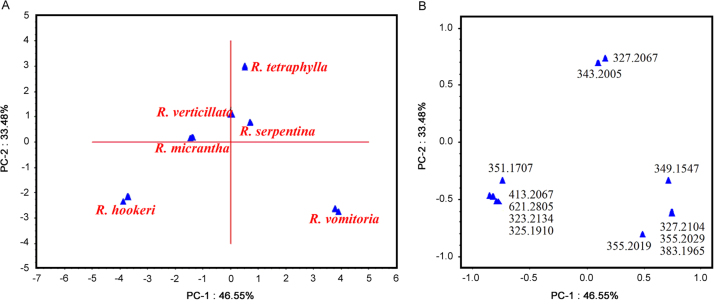
PCA converts a large number of data sets to a smaller number of variables. It produces overall discrimination between the closely related samples for quality control and authentication [43], [44], [45]. LC–MS data in combination with a data reduction technique such as PCA serves as an efficient and powerful tool to identify the chemical markers [34], [43], [44]. The LC–MS chemical fingerprints of R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata, and R. vomitoria roots were analyzed by PCA to identify the chemical markers for discrimination amongst these species.
A total of 66 peaks from mass range m/z 179.0708 to 635.2984, peak area ≥1000, were taken from the HPLC–ESI–QTOF–MS fingerprints (n=3) of roots and PCA was run. The PC1 and PC2 together were able to explain 57.96% of variance information. To obtain the best expression, peaks with lowest contribution were dropped and only twelve peaks at m/z 323.2134 (unknown), 325.1910 (seredamine), 343.2005 (ajmalinol), 327.2067 (ajmaline), 327.2104 (isomer of ajmaline), 355.2019 (yohimbine), 355.2029 (isomer of yohimbine), 349.1547 (serpentine), 383.1965 (10-demethoxyreserpiline), 351.1707 (tetradehydroyohimbine), 413.2067 (reserpiline) and 621.2805 (rescidine) were identified as marker peaks which were responsible for discrimination of six Rauwolfia species. Using these chemical markers PCs could explain 80.03% of the variance information as shown in the score and loading plots (Fig. 5). Peak at m/z 343.2005 (46.55%) showed a higher contribution followed by m/z 327.2104 (33.47%) and 327.2067 (12.28%), respectively. Similar report with contribution of peak area has already been reported in chromatographic fingerprinting and quantitative analysis of Xinkeshu tablet [46]. The PCA plots afforded useful qualitative information of six Rauwolfia species roots and revealed similarities and dissimilarity among them shown in Fig. 5A. It showed that R. serpentina. R. micrantha and R. verticillata were close whereas R. hookeri, R. tetraphylla, R. vomitoria were much apart from each other. R. serpentina is commonly used in herbal formulations and endangered due to over-exploitation [47]. This study showed that R. micrantha and R. verticillata might be used as substitutes for R. serpentina in herbal formulations. Hence, this analysis will also help to use alternate plant on the basis of phytochemical investigation which may conserve the R. serpentina.
Fig. 5.
(A) PCA score plot from Rauwolfia species showing discrimination among the R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata and R. vomitoria. (B) Loading plot of the normalized LC–MS data obtained from three samples of each species showing possible ions of interest including those predominantly responsible for the discrimination of R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata and R. vomitoria.
4. Conclusions
A simple, sensitive, reproducible HPLC–ESI–QTOF–MS/MS method was developed in positive ion mode for the dereplication of MIAs. The diagnostic fragmentation pathways were established with the help of MS/MS spectra or diagnostic fragment ions of standard compounds. Reserpine, ajmalicine, ajmaline and yohimbine classes of MIAs showed two types of fragment ions, namely due to loss of substituents which were attached with the terpene moiety and RDA fragment ions. The established diagnostic fragmentation pathways were applied for identification and characterization of MIAs. 47 compounds were tentatively identified and characterized in ethanolic extract of roots of R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata and R. vomitoria. Hydoxyseredamine-O-hexoside (13), ajmaline-O-hexoside (37), tetradehydroyohimbine-O-hexoside (15), serpentine-O-hexoside (25) and 4 reserpine class compounds 39, 42, 45 and 46 were identified as unknown/new for the first time. The isomeric compounds reserpic acid methyl ester (27) and seredine (29) were successfully distinguished by MS/MS analysis. The marker peaks were successfully identified by PCA, which can discriminate the R. hookeri, R. micrantha, R. serpentina, R. tetraphylla, R. verticillata and R. vomitoria for quality control and authentication. Present information showed utility of accurate mass measurements which will also speed up dereplication of MIAs.
Acknowledgments
Grateful acknowledgement is given to Sophisticated Analytical Instrument Facility, CSIR-Central Drug Research Institute, Lucknow, India where all mass spectral studies were done. We are also thankful to Dr. K.B. Rameshkumar, Scientist Jawaharlal Nehru Tropical Botanic Garden and Research Institute Palode, Thiruvananthapuram, Kerala, India (JNTBGRI), for providing plant materials. Sunil Kumar is thankful to Council of Scientific Industrial Research, India for providing financial support. CDRI communication number is 9229.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Kline N.S. Use of Rauwolfia serpentina Benth. in neuropsychiatric conditions. Ann. N. Y. Acad. Sci. 1954;59:107–132. doi: 10.1111/j.1749-6632.1954.tb45922.x. [DOI] [PubMed] [Google Scholar]
- 2.Mabberley D.J. Cambridge University Press; London: 2008. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications, and Uses. [Google Scholar]
- 3.Bindu S., Rameshkumar K.B., Kumar B. Distribution of reserpine in Rauwolfia species from India–HPTLC and LC–MS studies. Ind. Crop. Prod. 2014;62:430–443. [Google Scholar]
- 4.Itoh A., Kumashiro T., Yamaguchi M. Indole alkaloids and other constituents of Rauwolfia serpentine. J. Nat. Prod. 2005;68:848–852. doi: 10.1021/np058007n. [DOI] [PubMed] [Google Scholar]
- 5.Madhusudanan K.P., Banerjee S., Khanuja S.P. Analysis of hairy root culture of Rauvolfia serpentina using direct analysis in real time mass spectrometric technique. Biomed. Chromatogr. 2008;22:596–600. doi: 10.1002/bmc.974. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava A., Tripathi A.K., Pandey R. Quantitative determination of reserpine, ajmaline, and ajmalicine in Rauvolfia serpentina by reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 2006;44:557–560. doi: 10.1093/chromsci/44.9.557. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt R., Arif M., Gaur A.K. Rauwolfia serpentina: protocol optimization for in vitro propagation. Afr. J. Biotechnol. 2008;7:4265–4268. [Google Scholar]
- 8.Vakil R.J. Rauwolfia Serpentina in the treatment of high blood pressure a review of the literature. Circulation. 1955;12:220–229. doi: 10.1161/01.cir.12.2.220. [DOI] [PubMed] [Google Scholar]
- 9.Achor R.W.P., Norbert O.H., Ray W.G. Hypertension treated With Rauwolfia serpentina (whole root) and with reserpine: controlled study disclosing occasional severe depression. J. Am. Med. Assoc. 1955;159:841–845. doi: 10.1001/jama.1955.02960260011004. [DOI] [PubMed] [Google Scholar]
- 10.Lemieux G., Davignon A., Genest J. Depressive states during Rauwolfia therapy for arterial hypertension. Can. Med. Assoc. J. 1956;74:522–526. [PMC free article] [PubMed] [Google Scholar]
- 11.Quetsch R.M., Achor R.W., Litin E.M. Depressive reactions in hypertensive patients a comparison of those treated with Rauwolfia and those receiving no specific antihypertensive treatment. Circulation. 1959;19:366–375. doi: 10.1161/01.cir.19.3.366. [DOI] [PubMed] [Google Scholar]
- 12.Vakil R.J. A clinical trail of Rauwolfia Serpentina in essential hypertension. Br. Heart J. 1949;11:350–355. doi: 10.1136/hrt.11.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathi P., Kumari R., Rajput C.S. Therapeutic characterstics of Rauwolfia Serpentina. Int. J. Pharm. Chem. Sci. 2013;2:1038–1042. [Google Scholar]
- 14.Bemis D.L., Capodice J.L., Gorroochurn P. Anti-prostate cancer activity of a ß-carboline alkaloid enriched extract from Rauwolfia vomitoria. Int. J. Oncol. 2006;29:1065–1073. [PubMed] [Google Scholar]
- 15.Stanford J.L., Martin E.J., Brinton L.A. Rauwolfia use and breast cancer: a case-control study. J. Natl. Cancer Inst. 1986;76:817–822. [PubMed] [Google Scholar]
- 16.Wintersteiner O. Rauwolfia: botany, pharmacognosy, chemistry and pharmacology. J. Am. Chem. Soc. 1957;7:847–848. [Google Scholar]
- 17.O’Connor S.E., Maresh J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006;23:532–547. doi: 10.1039/b512615k. [DOI] [PubMed] [Google Scholar]
- 18.Kline N.S. Uses of reserpine, the newer phenothiazines, and iproniazid. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1959;37:218–244. [PubMed] [Google Scholar]
- 19.Heinonen O.P., Shapiro S., Tuominen L. Reserpine use in relation to breast cancer. Lancet. 1974;304:675–677. doi: 10.1016/s0140-6736(74)93259-0. [DOI] [PubMed] [Google Scholar]
- 20.Barsa J.A., Kline N.S. Use of reserpine in disturbed psychotic patients. Am. J. Psychiatry. 1956;112:684–690. doi: 10.1176/ajp.112.9.684. [DOI] [PubMed] [Google Scholar]
- 21.Prys-Roberts C., Meloche R., Foex P. Studies of anaesthesia in relation to hypertension I: cardiovascular responses of treated and untreated patients. Br. J. Anaesth. 1971;43:122–137. doi: 10.1093/bja/43.2.122. [DOI] [PubMed] [Google Scholar]
- 22.Leão A.H.F.F., Aldair J.S., José R.S. Molecular, neurochemical, and behavioral hallmarks of reserpine as a model for parkinson’s disease, new perspectives to a long-standing model. Brain Pathol. 2015;25:377–390. doi: 10.1111/bpa.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellens H.J., Dirk D. Effect of procaine amide, quinidine, and ajmaline in the Wolff-Parkinson-White syndrome. Circulation. 1974;50:114–120. doi: 10.1161/01.cir.50.1.114. [DOI] [PubMed] [Google Scholar]
- 24.Wellens H.J., Bär F.W., Gorgels A.P. Use of ajmaline in patients with the Wolff-Parkinson-White syndrome to disclose short refractory period of the accessory pathway. Am. J. Cardiol. 1980;45:130–133. doi: 10.1016/0002-9149(80)90230-1. [DOI] [PubMed] [Google Scholar]
- 25.Pan Q., Mustafa N.R., Tang K. Monoterpenoid indole alkaloids biosynthesis and its regulation in Catharanthus roseus: a literature review from genes to metabolites. Phytochem. Rev. 2015;15:1–30. [Google Scholar]
- 26.Arora R.B., Madan B.R. Antiarrhythmics. VI. Ajmaline and serpentine in experimental cardiac arrhythmias. J. Pharmacol. Exp. Ther. 1956;117:62–67. [PubMed] [Google Scholar]
- 27.Bierwagen M.E., Smith D.L. A mechanism of action for antithyroid activity of reserpine. Exp. Biol. Med. 1959;100:108–110. doi: 10.3181/00379727-100-24540. [DOI] [PubMed] [Google Scholar]
- 28.Ciofalo F., Levitt B., Roberts J. Some aspects of the anti-arrhythmic activity of reserpine. Br. J. Pharmacol. 1966;28:44–50. doi: 10.1111/j.1476-5381.1966.tb01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charney D.S., Heninger G.R., Breier A. Noradrenergic function in panic anxiety: effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch. Gen. Psychiatry. 1984;41:751–763. doi: 10.1001/archpsyc.1984.01790190025003. [DOI] [PubMed] [Google Scholar]
- 30.Starke K., Borowski E., Endo T. Preferential blockade of presynaptic α-adrenoceptors by yohimbine. Eur. J. Pharmacol. 1975;34:385–388. doi: 10.1016/0014-2999(75)90268-x. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A., Tripathi A.K., Pandey R. Gupta, quantitative determination of reserpine, ajmaline, and ajmalicine in Rauvolfia serpentina by reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 2006;44:557–560. doi: 10.1093/chromsci/44.9.557. [DOI] [PubMed] [Google Scholar]
- 32.Hong B., Li W.J., Song A.H. Determination of indole alkaloids and highly volatile compounds in Rauvolfia verticillata by HPLC-UV and GC–MS. J. Chromatogr. Sci. 2013;51:929–930. doi: 10.1093/chromsci/bms191. [DOI] [PubMed] [Google Scholar]
- 33.Klyushnichenko V.E., Yakimov S.A., Tuzova T.P. Determination of indole alkaloids from R. serpentina and R. vomitoria by high-performance liquid chromatography and high-performance thin-layer chromatography. J. Chromatogr. A. 1995;704:357–362. [Google Scholar]
- 34.Kumar S., Bajpai V., Singh A. Rapid fingerprinting of Rauwolfia species using direct analysis in real time mass spectrometry combined with principal component analysis for their discrimination. Anal. Methods. 2015;7:6021–6026. [Google Scholar]
- 35.Hong B., Cheng W., Wu J. Screening and identification of many of the compounds present in Rauvolfia verticillata by use of high-pressure LC and quadrupole TOF MS. Chromatographia. 2010;72:841–847. [Google Scholar]
- 36.Kumar S., Singh A., Bajpai V. Identification, characterization and distribution of monoterpene indole alkaloids in Rauwolfia species by Orbitrap Velos pro mass spectrometer. J. Pharm. Biomed. Anal. 2015;118:183–194. doi: 10.1016/j.jpba.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Hernando M.D., Ferrer C.M., Ulaszewska J.F. Application of high-performance liquid chromatography–tandem mass spectrometry with a quadrupole/linear ion trap instrument for the analysis of pesticide residues in olive oil. Anal. Biochem. 2007;389:1815–1831. doi: 10.1007/s00216-007-1464-z. [DOI] [PubMed] [Google Scholar]
- 38.Sun J., Baker A., Chen P. Profiling the indole alkaloids in yohimbe bark with ultra-performance liquid chromatography coupled with ion mobility quadrupole time-of‐flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011;25:2591–2602. doi: 10.1002/rcm.5158. [DOI] [PubMed] [Google Scholar]
- 39.Sagi S., Avula B., Wang Y.H. Quantification and characterization of alkaloids from roots of Rauwolfia serpentina using ultra-high performance liquid chromatography-photo diode array-mass spectrometry. Anal. Bioanal. Chem. 2016;408:177–190. doi: 10.1007/s00216-015-9093-4. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Liu Y., Pan Y.J. Determination of alkaloids in Catharanthus roseus and Vinca minor by high-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 2015 [Google Scholar]
- 41.Uhlig S., Egge-Jacobsen W., Vrålstad T. Indole–diterpenoid profiles of Claviceps paspali and Claviceps purpurea from high-resolution Fourier transform Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2014;28:1621–1634. doi: 10.1002/rcm.6938. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q., Zhang W., Zhang Y. Identification and quantification of active alkaloids in Catharanthus roseus by liquid chromatography–ion trap mass spectrometry. Food Chem. 2013;139:845–852. doi: 10.1016/j.foodchem.2013.01.088. [DOI] [PubMed] [Google Scholar]
- 43.Ian J. John Wiley & Sons, Ltd. Hoboken, New Jersey, United States; 2002. Principal Component Analysis. [Google Scholar]
- 44.Bajpai V., Singh A., Arya K.R. Rapid screening for the adulterants of Berberis aristata using direct analysis in real-time mass spectrometry and principal component analysis for discrimination. Food Addit. Contam. Part A. 2015;32:799–807. doi: 10.1080/19440049.2015.1022885. [DOI] [PubMed] [Google Scholar]
- 45.D’Urso G., d’Aquino L., Pizza C. Integrated mass spectrometric and multivariate data analysis approaches for the discrimination of organic and conventional strawberry (Fragaria ananassa Duch.), crops. Food Res. Int. 2015;77:264–272. [Google Scholar]
- 46.Wang P., Li L., Yang H. Chromatographic fingerprinting and quantitative analysis for the quality evaluation of Xinkeshu tablet. J. Pharm. Anal. 2012;2:422–430. doi: 10.1016/j.jpha.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma N., Chandel K.P.S. Low-temperature storage of Rauvolfia serpentina Benth. ex Kurz.: an endangered, endemic medicinal plant. Plant Cell Rep. 1992;11:200–203. doi: 10.1007/BF00232533. [DOI] [PubMed] [Google Scholar]