Abstract
Introduction
Contrast-induced nephropathy is a complication following coronary angiography and percutaneous coronary intervention. Because contrast-induced nephropathy is a predictor of long-term mortality in patients with ischemic heart disease undergoing percutaneous coronary intervention, preventive strategies are required. We assessed the effects of periprocedural oxygenation on contrast-induced nephropathy among patients with pre-existing renal dysfunction.
Methods
A total of 200 consecutive patients with impaired renal function (estimated glomerular filtration < 60 ml/min per 1.73 m2) undergoing elective cardiovascular angiography were randomly assigned to an oxygenation treatment (n = 100) or control group (n = 100). In oxygenation treatment, pure oxygen (2 L/min) was administered for 10 minutes before exposure to contrast medium. The primary endpoint was the incidence of contrast-induced nephropathy, defined as a ≥ 25% increase in serum creatinine levels from baseline within 48 hours of exposure.
Results
In the oxygenation treatment group, partial pressure of arterial oxygen was higher (135 ± 25 mm Hg vs. 84 ± 10 mm Hg, P < 0.001); contrast-induced nephropathy incidence was lower (1% vs. 8%, odds ratio [OR] = 0.12, 95% confidence interval [CI] = 0.01–0.95, P = 0.02); and partial pressure of arterial carbon dioxide and bicarbonate base lactate levels were similar compared with those in the control group. Upon univariate analysis, excess and absence of oxygenation treatment (OR = 9.18, CI = 1.13–74.86, P = 0.03) and anemia (OR = 4.30, CI = 1.04–17.78, P = 0.04) were shown to be associated with contrast-induced nephropathy incidence.
Conclusion
Oxygenation, a simple, nonpharmacological strategy, may be beneficial when using contrast media in patients with impaired renal function from noninvasive angiography to emergency catheterization.
Keywords: acute kidney injury, cardiovascular disease, chronic kidney disease, hypoxia, nephrotoxicity
Contrast-induced nephropathy (CIN), a complication following coronary angiography and percutaneous coronary intervention (PCI),1 can progress to acute kidney injury and irreversible chronic kidney disease (CKD).2 CIN has been recognized as a significant predictor of long-term mortality in patients with ischemic heart disease who are undergoing PCI.3, 4 Consequently, several preventive strategies have been developed; however, to date, only isotonic saline hydration has been accepted and applied as a standard preventive measure.5 CIN is caused by a combination of renal ischemia and direct toxic effects of contrast media on renal tubular cells.6 The renal medulla is uniquely susceptible to ischemic injury.7 Contrast media may cause medullary hypoxia by shunting blood flow to the renal cortex.8, 9 Although hydration exerts a degree of protection by maintaining renal plasma flow and improving intrarenal hypoxia, hydration alone is insufficient to prevent CIN, particularly in high-risk patients.
A more effective approach toward CIN prevention may be achieved by combining isotonic saline hydration with oxygenation prior to contrast medium exposure. We recently reported the Option CIN study, showing that oxygenation treatment decreased CIN incidence in patients undergoing elective cardiac catheterization angiography.10 The majority of patients enrolled in the Option CIN study had normal renal function; 122 of 349 patients (35%) had an estimated glomerular filtration rate (eGFR) < 60/ml/min/1.73 m2. The incidence of CIN is widely recognized to be higher in patients with reduced eGFR.11 Indeed, CIN occurred more frequently in patients with eGFR < 60 ml/min/1.73 m2 compared to those with eGFR ≥ 60 ml/min/1.73 m2 (6.5% vs. 0.8%, P < 0.01) in our previous study.10 Moreover, renal damage from CIN is more severe in individuals with pre-existing impaired renal function than in those with normal renal function. Accordingly, we sought to determine whether oxygenation treatment is effective among patients with pre-existing renal dysfunction, who tend to have a higher incidence of CIN.
Our proposed non-pharmacological preventive strategy using oxygen is safe, cost-saving, and widely applicable in either the elective or emergency setting for both outpatients and critically ill patients. The Extended-Option CIN study, a single-center, prospective, randomized controlled study, aimed to extend our previous findings to patients with reduced eGFR, a relatively high-risk subset of CIN, who underwent cardiac catheterization using contrast media.
Materials and Methods
Study Design
The Option CIN study was a prospective open-label, randomized, single-center study of patients who underwent scheduled cardiovascular angiography, percutaneous coronary intervention (PCI), or both (n = 349) from 29 April 2011 to 16 August 2011. The Extended-Option CIN study extended the recruitment period of the Option CIN study to evaluate the preventive effect of oxygenation on CIN in patients with impaired renal function, as a subpopulation analysis of the previous study. In the interim analysis of the previous study, 200 patients were enrolled (control group, n = 100; oxygen group, n =100). CIN occurred in 5 control-group patients and 1 treatment-group patient, all of whom demonstrated impaired renal function. Thus the rate of CIN among patients with impaired renal function was 14.7% (5 of 34) in the control group and 2.8% (1 of 36) in the treatment group. In the present study, sample size calculation was conducted based on this interim analysis using a CIN incidence of 2.8% in the oxygenation treatment group and 14.7% in the control group. To detect a difference in CIN incidence between the 2 groups using the Fisher exact probability test at a power of 80%, α error of 0.5 (2-sided), and β error of 0.05, we calculated that 89 patients per control group and treatment group would need to be enrolled. Factoring in a 10% dropout rate, we estimated that 98 patients were needed per group. Therefore, we enrolled and evaluated 200 new patients with impaired renal function in the present study.
Study Population
Between 1 September 2011 and 30 June 2012, a total of 200 consecutive patients with an eGFR < 60 ml/min/1.73 m2 (mean, 48.4 ± 9.3 ml/min/1.73 m2, range 18.4–59.9 ml/min/1.73 m2) who underwent elective cardiovascular angiography, PCI, or both were enrolled. Exclusion criteria were acute coronary syndrome, end-stage renal failure requiring dialysis, cardiogenic shock, symptomatic and congestive heart failure, pregnancy, a history of hypersensitivity to contrast media, metformin ingestion within 48 hours of study entry, chronic obstructive pulmonary disease, oxygen saturation level <90% (indicating respiratory failure, necessitating a supply of oxygen), use of an antioxidant drug such as N-acetylcysteine to prevent CIN, severe infection, and severe malnutrition. Patients in whom CIN occurred were followed up in terms of clinical outcomes and renal function for 1 month after the procedure, with some followed up for as long as 1 year.
Study Protocol
Eligible patients were randomly assigned in a 1:1 ratio to receive either supplemental oxygen (oxygenation treatment group) or room air (control group). All patients received i.v. hydration with 0.9% isotonic saline at a rate of 1 ml/kg per hour (or 0.5 ml/kg per hour in cases with an ejection fraction < 40%) for 12 hours before and after the procedure as a standard preventive measure. Randomization of patients was performed by a computer in blocks of 4. Groups were sequentially assigned by a research pharmacist who dispensed medications but was not otherwise involved in the study.
Blood oxygenation levels were assessed using arterial blood gas samples obtained before and after the procedure through an arterial sheath. Serum creatinine and blood urea nitrogen levels were measured before angiography (during hydration before contrast) and 48 hours after the procedure to determine the development of CIN. Catheterization was performed via the radial artery, or the femoral artery in difficult cases, without sedation. Low osmolality, non-ionic contrast agent iopamidol (Iopamiron, Osaka, Japan) was used. The eGFR was calculated using a level-modified Japanese adaptation of the Modification of Diet in Renal Disease formula12: eGFR in men = 0.741 × 175 × (age in years) − 0.203 × (serum creatinine in mg/dl) − 1.154, and eGFR in women = (eGFR in men) × 0.742. The primary endpoint of the study was CIN incidence. Athough CIN has also been defined as an increase in the absolute creatinine level by 44 μmol/l (0.5 mg/dl) from baseline, for the purpose of this study, CIN was defined as an increase in serum creatinine levels of ≥ 25% from baseline within 48 hours of contrast medium administration. We also evaluated the occurrence of acute kidney injury, defined as an increase in serum creatinine of > 0.3 mg/dl compared to baseline within 48 hours, and sustained kidney injury defined was as eGFR reduction of > 30% for 6 to 12 months after the procedure.
Oxygenation Treatment
In the oxygenation treatment group, patients received 2 L/min of pure oxygen intranasally from 10 minutes prior to contrast medium exposure until the end of the procedure. Oxygen administration was terminated at the end of the procedure. In addition, our previous study showed that a low partial pressure of arterial oxygen (PaO2) (< 100 mm Hg) was significantly associated with a high incidence of CIN. Therefore, the present study sought to determine the efficacy of oxygen administration at a concentration of 2 L/min, which is sufficient to increase the PaO2 levels > 100 mm Hg in most stable patients. Administration commenced 10 minutes prior to contrast medium exposure because oxygen levels usually plateau within 3 to 10 minutes of oxygenation.
Statistical Analysis
All continuous data were analyzed using the t test (a paired t test for before-and-after comparison within a single group, and an unpaired t test for comparisons between experimental groups), or using the Mann–Whitney U test or Wilcoxon signed-rank test if data were skewed. Categorical data were compared using the χ2 test or Fisher exact test. We referred to 2 risk score models. In a report by Morcos et al., CIN occurred in 10% to 30% of participants with risk factors, and in a study by Rich et al., CIN occurred in patients with high serum creatinine (> 1.5 mg/dl), high contrast volume (> 200 ml), diabetes, low serum albumin (< 3.5 g/dl), or hyponatremia (< 135 mM). CIN predictors such as sex, age > 70 years, hypertension, diabetes mellitus, dyslipidemia, history of myocardial infarction, brain natriuretic peptide > 100 pg/dl, anemia, use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, or statins, contrast media > 150 ml, and oxygenation treatment were first evaluated using logistic regression.
All statistical analyses were performed using SPSS statistical software (version 17.0,; SPSS Institute, Chicago, IL). P values < 0.05 were considered statistically significant. Continuous data are reported as mean ± SD, skewed variables as median and interquartile range, and categorical data as absolute values and percentages.
Ethical Considerations
The study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The ethics committee of our institution approved the study protocol (NHO11-11), and written informed consent was obtained from all patients before study entry.
Results
Patient Demographics
A total of 200 participants were enrolled in the study, with a mean eGFR of 48.4 ± 9.3 ml/min per 1.73 m2 (range, 18.4–59.9 ml/min per 1.73 m2). The patients’ mean age was 74.2 ± 8.2 years, and 77% (153 of 200) were > 70 years of age. Table 1 presents the patients’ baseline characteristics. Pre-existing renal dysfunction, diabetes mellitus, and heart failure are possible risk factors for CIN.13 However, no significant differences were observed between groups in terms of indices related to these risk factors or with regard to other factors such as baseline eGFR, cystatin C, creatinine, blood urea nitrogen, hemoglobin A1c (HbA1c), and brain natriuretic peptide levels. In addition, the proportion of patients receiving calcium channel blockers, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, or statins was equivalent between groups. The volume of contrast medium and procedure duration in the oxygenation treatment group were equivalent to those in the control group (109 ± 48 ml vs. 110 ± 56 ml, P = 0.89, and 47 ± 35 minutes vs. 48 ± 34 minutes, P = 0.84, respectively) (Table 1).
Table 1.
Baseline characteristics of study patients
| Clinical characteristics | Oxygenation treatment (n = 100) | Control (n = 100) | P value |
|---|---|---|---|
| Age, yr | 73.8 ± 8.3 | 74.6 ± 8.1 | 0.49 |
| Male sex, n (%) | 66 (66.0%) | 64 (64.0%) | 0.77 |
| Body mass index | 23.1 ± 4.0 | 24.0 ± 4.1 | 0.12 |
| Diabetes, n (%) | 30 (30.0%) | 38 (38.0%) | 0.23 |
| Hypertension, n (%) | 85 (85.0%) | 86 (86.0%) | 0.84 |
| Dyslipidemia, n (%) | 63 (63.0%) | 66 (66.0%) | 0.66 |
| Current smoking, n (%) | 6 (6.0%) | 6 (6.0%) | 1.00 |
| Prior CABG, n (%) | 4 (4.0%) | 4 (4.0%) | 1.00 |
| Prior PCI, n (%) | 27 (27.0%) | 31 (31.0%) | 0.53 |
| History of myocardial infarction, n (%) | 15 (15.0%) | 19 (19.0%) | 0.45 |
| Volume of contrast medium (ml) | 109 ± 48 | 110 ± 56 | 0.89 |
| Procedure time (min) | 47 ± 35 | 48 ± 34 | 0.84 |
| Medications | |||
| ACE inhibitors, n (%) | 9 (9.0%) | 10 (10.0%) | 0.81 |
| Angiotensin II receptor inhibitors, n (%) | 49 (49.0%) | 54 (54.0%) | 0.48 |
| Calcium channel blocker, n (%) | 31 (31.0%) | 38 (38.0%) | 0.29 |
| β-Blockers | 36 (36.0%) | 34 (34.7%) | 0.77 |
| Aspirin, n (%) | 59 (59.0%) | 60 (60.0%) | 0.89 |
| Statins, n (%) | 49 (49.0%) | 53 (53.0%) | 0.57 |
| Diuretics, n (%) | 35 (35.0%) | 37 (37.0%) | 0.77 |
| Laboratory tests | |||
| Serum creatinine (mg/dl) | 1.08 ± 0.21 | 1.08 ± 0.25 | 1.00 |
| Cystatin C (mg/dl) | 1.11 ± 0.32 | 1.13 ± 0.27 | 0.63 |
| eGFR (ml/min per 1.73 m2) | 48.4 ± 9.1 | 48.6 ± 9.0 | 0.88 |
| eGFR 30–60 (%) | 95 (95.0%) | 96 (96.0%) | 0.73 |
| eGFR < 30 (%) | 5 (5.0%) | 4 (4.0%) | 0.73 |
| Blood urea nitrogen (mg/dl) | 19.6 ± 5.9 | 19.4 ± 6.8 | 0.82 |
| HbA1c (%) | 6.2 ± 3.7 | 5.9 ± 1.0 | 0.43 |
| BNP (ng/ml) | 211.7 ± 315.5 | 167.5 ± 332.2 | 0.34 |
| Procedure | |||
| Coronary artery angiography | 78 (78%) | 78 (78%) | 1.00 |
| PCI | 22 (22%) | 22 (22%) | 1.00 |
| Mehran’s risk score | 7.6 ± 3.1 | 7.0 ± 3.4 | 0.19 |
ACE, angiotensin-converting enzyme; BNP, brain natriuretic peptide; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Effects of Oxygenation Treatment on CIN Incidence
As presented in Table 2, the initial arterial blood gas levels were significantly higher in the oxygenation treatment group than in the control group (135 ± 25 mm Hg vs. 84 ± 10 mm Hg, P < 0.001). The total duration of oxygenation in the oxygenation treatment group was 62 ± 35 minutes. No statistically significant difference was observed in the levels of partial pressure of arterial carbon dioxide (PCO2), bicarbonate (HCO3−), base excess, or lactate between groups. Therefore, systemically higher PaO2 levels in the arterial blood were achieved prior to contrast medium administration at oxygen levels of 2 L/min.
Table 2.
Comparison of preprocedural arterial blood gas between the patients in the oxygenation treatment and the control group
| Oxygenation treatment (n = 100) | Control Group (n = 100) | P value | |
|---|---|---|---|
| pH | 7.416 ± 0.061 | 7.418 ± 0.039 | 0.78 |
| PaO2 (mm Hg) | 134.5 ± 25.2 | 83.8 ± 10.0 | < 0.001 |
| PaCO2 (mm Hg) | 38.2 ± 5.6 | 39.5 ± 5.2 | 0.09 |
| HCO3− (mEq/L) | 24.0 ± 2.9 | 24.8 ± 3.0 | 0.06 |
| Base excess (mEq/L) | 0.3 ± 2.6 | 0.4 ± 2.8 | 0.79 |
| Hemoglobin (mg/dl) | 12.3 ± 1.8 | 12.5 ± 1.9 | 0.45 |
| Hematocrit (%) | 36.6 ± 4.8 | 37.1 ± 5.4 | 0.49 |
| Na (mEq/L) | 139.7 ± 2.8 | 139.6 ± 2.8 | 0.80 |
| K (mEq/L) | 4.3 ± 0.4 | 4.3 ± 0.5 | 1.00 |
| Cl (mEq/L) | 106.3 ± 3.9 | 105.9 ± 3.9 | 0.47 |
| Lactate (mg/dl) | 8.1 ± 2.5 | 7.9 ± 2.5 | 0.57 |
HCO3−, bicarbonate; Pa02, partial pressure of arterial oxygen.
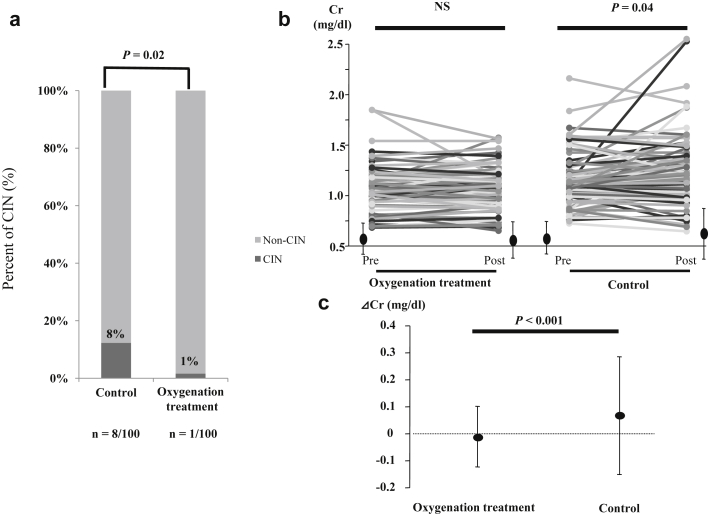
Overall, CIN developed in 9 patients (4.5%). CIN incidence was significantly lower in the oxygenation treatment group than in the control group (1 of 100 [1%] vs. 8 of 100 [8%]; odds ratio [OR] = 0.12, 95% confidence interval [CI] = 0.01–0.95, P = 0.02) (Figure 1a). However, there was no difference in CIN incidence between the 2 groups, when the definition of CIN was an increase in the serum creatinine level 0.5 mg/dl above baseline. Serum creatinine levels increased in the control group (P = 0.04) but remained unchanged in the oxygenation treatment group (P = 0.714) (Figure 1b). Moreover, the mean change in serum creatinine levels was lower in the oxygenation treatment group (−0.01 ± 0.12 mg/dl) than in the control group (0.07 ± 0.24 mg/dl; P < 0.001) (Figure 1c). Similarly, the baseline eGFR was equivalent between groups.
Figure 1.
(a) Contrast-induced nephropathy (CIN) incidence between study groups. CIN incidence was significantly lower in the oxygenation treatment group than in the control group (1/100 [1%] vs. 8/100 [8%], odds ratio = 0.12, 95% confidence interval = 0.01–0.95, P = 0.02). (b) Changes in serum creatinine levels following contrast exposure. Postprocedural serum creatinine levels increased in the control group (1.08 ± 0.25 mg/dl to 1.15 ± 0.31 mg/dl, P = 0.04) but remained unchanged in the oxygenation treatment group (1.08 ± 0.21 mg/dl to 1.07 ± 0.19 mg/dl, P = 0.714). (c) Median and interquartile range of serum creatinine levels before and after cardiovascular angiography. The average change in serum creatinine levels was significantly lower in the oxygenation treatment group than in the control group (−0.01 ± 0.12 mg/dl vs. 0.07 ± 0.24 mg/dl, P < 0.001). Data are presented as mean ± SD. NS, not significant.
The occurrence of acute kidney injury was also lower in the oxygenation treatment group than in the control group (1 of 100 [1%] vs. 11 of 100 [11%]; OR = 0.08, 95% CI = 0.01–0.65, P = 0.0029).
Risk for CIN
In the univariate analysis, 2 factors were significantly associated with a higher CIN incidence: no oxygenation treatment (OR = 9.18, 95% CI = 1.13–74.86, P < 0.05) and anemia, defined as a hemoglobin level < 12.0 mg/dl in men and < 11.0 mg/dl in women (OR = 4.30, 95% CI = 1.04–17.78, P < 0.05). However, diabetes mellitus, the volume of contrast medium (≥ 150 ml), hypertension, brain natriuretic peptide level > 100 pg/dl, and medication use (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, statins, and diuretics) were not associated with CIN incidence in this study population (Table 3).
Table 3.
Univariate analysis for the occurrence of contrast-induced nephropathy (CIN)
| CIN |
OR | P value | ||
|---|---|---|---|---|
| + (n = 9) | − (n = 191) | |||
| Male | 6 (66.7%) | 124 (64.9%) | 1.08 | 0.92 |
| Age > 70 yr | 7 (77.8%) | 146 (76.4%) | 1.08 | 0.93 |
| Hypertension | 8 (88.9%) | 156 (81.6%) | 1.79 | 0.58 |
| Diabetes mellitus | 2 (22.2%) | 68 (35.6%) | 0.52 | 0.41 |
| Dyslipidemia | 6 (66.7%) | 127 (66.5%) | 1.01 | 0.99 |
| History of myocardial infarction | 0 (0.0%) | 34 (17.8%) | 0.24 | 0.16 |
| BNP > 100 pg/dl | 6 (66.7%) | 67 (35.1%) | 3.70 | 0.05 |
| Anemiaa | 6 (66.7%) | 60 (20.1%) | 4.37 | 0.03 |
| ACE inhibitors or ARBs | 4 (44.4%) | 118 (61.8%) | 0.49 | 0.30 |
| Diuretics | 4 (44.4%) | 68 (35.6%) | 1.45 | 0.59 |
| Statins | 4 (44.4%) | 98 (51.3%) | 0.76 | 0.69 |
| Contrast media > 150 ml | 3 (33.3%) | 26 (13.6%) | 3.17 | 0.10 |
| No oxygenation treatment | 8 (88.9%) | 92 (48.1%) | 8.61 | 0.02 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; OR, odds ratio.
Anemia was defined as a hemoglobin concentration of < 12 mg/dl for men or < 11 mg/dl for women.
Follow-up of Patients With CIN
Over the course of this study, no patient died, and none developed end-stage kidney disease requiring dialysis. The rate of patients with 30% eGFR reduction at 6 to 12 months after the procedure was significantly lower in the oxygenation treatment group (10%) compared to the control group (21%) (OR = 2.37, 95% CI = 1.05–5.33, P = 0.034).
Discussion
Our study findings indicated that the addition of oxygenation treatment to standard hydration decreased the CIN incidence in patients with reduced eGFR undergoing cardiovascular angiography. Therefore, oxygenation treatment may protect against CIN in patients with impaired renal function during the scheduled cardiovascular angiography.
CIN occurs at an incidence of 10% to 30%, inclusive of patients with pre-existing renal dysfunction or diabetes mellitus, in whom a standard hydration protocol is not administered.3, 14 In patients without these risk factors, the CIN incidence decreases to approximately 3.3% following coronary angiography. Although several preventive strategies have been identified and investigated, a definitive solution remains to be established.
The pathogenesis of CIN is not fully understood, and little is known regarding cellular mechanisms underlying this condition.15, 16 To date, contrast medium−induced direct tubular and mesangial toxicity,17 intraluminal obstruction,18 and immunological injury19 have been explored. Renal hypoxia8 and the concomitant release of reactive oxygen are also considered important mechanisms of renal injury following contrast medium administration.20 Thus, CIN is currently believed to result from renal damage caused by a combination of both direct toxicity of contrast agents and renal ischemia associated with reduced renal blood flow.15
Liss et al. demonstrated that contrast medium administration decreased oxygen tension in the renal medulla 10 minutes after injection, and caused renal parenchymal PaO2 levels to decline to critically low levels (∼10 mm Hg) within medullary structures extending to the renal cortex.21 Therefore, because of the relationship between systematic oxygenation and contrast media exposure described by Heyman et al.,22 we thought that oxygen administration might prevent this drop in inner medullary oxygen.
Interestingly, a reduction of approximately 5% in systemic PaO2 levels was observed after the procedure in both groups in the present study. This decrease was statistically significant only in the control group following angiographic procedures (135 ± 25 mm Hg to 130 ± 47 mm Hg in the oxygenation treatment group, P = 0.35; 84 ± 10 mm Hg to 80 ± 10 mm Hg in the control group, P < 0.01), suggesting that renal hypoxia was additively enhanced following contrast medium administration, and that oxygenation can suppress the reduction in PaO2 levels. Although isotonic saline hydration maintains renal plasma flow, when used alone it may be insufficient to resolve systemic and renal ischemia, which may explain the 5% to 10% incidence of CIN associated with the use of isotonic saline hydration.23 In addition, the finding that hyperbaric oxygen treatment improves the glomerular filtration rate in rats with renal ischemic reperfusion injury24 supports the crucial role of enhanced oxygenation in preserving renal function.
Under physiological conditions, the renal cortex contains the majority of glomeruli and therefore receives most of the renal blood flow, whereas the medulla receives approximately 10%.25 During ischemia, the decrease in blood flow is regional rather than uniform throughout the kidney, and is more prominent in the outer medulla than in the cortex.26 The corticomedullary region is most vulnerable to tubular injury, inflammation, and vascular alterations, which can extend cellular injury beyond the initial insult and propagate continued hypoperfusion.27 The fact that patients with unstable hemodynamics (i.e., myocardial infarction and systemic hypoxia due to congestive heart failure) are at high risk for CIN suggests that ischemic acute tubular necrosis or acute cardiorenal syndrome exacerbates contrast-induced renal ischemia.
Despite some negative effects of oxygen such as the potential generation of reactive oxygen species and oxygen-mediated vasoconstriction during ischemia-reperfusion injuries, in cases of acute coronary syndrome, oxygen administration is necessary to protect the patient from myocardial ischemia,28 so the oxygen administration was class IIa according to the American Heart Association guidelines. Thus, supplemental oxygenation may be a simple method to prevent contrast-mediated hypoxic renal injury.
Several strategies have been evaluated for preventing CIN, and the following have demonstrated renoprotective effects and a reduced CIN incidence: hydration with saline solution,5, 29 the use of iso-osmolality or low-osmolality contrast agents,30 and application of remote ischemic conditioning.9 However, the efficacy of these strategies remains controversial in patients with severe renal failure undergoing angiographic procedures that require a high volume of contrast medium. Oxygenation treatment via the nasal cannula is minimally invasive and may be used in combination with any of the previously reported methods for CIN prevention. This technique is promising, particularly for high-risk patients, if oxygenation is combined with previously reported pharmacological agents to preserve renal blood flow.
Diabetes mellitus, dehydration, anemia, congestive heart failure, and high contrast volume have been reported as risk factors for CIN.13 In patients with such risk factors, previous reports have indicated that the CIN incidence increased from 7.5% to 26.1% following catheterization.4 Contrast medium directly affects endothelium-dependent vasomotion.31 Impairment of the renal endothelium results in decreased vasodilation and further enhances regional hypoxia.32 Defective endothelial-dependent vasodilation is characteristic of diabetes mellitus,33 hypertension,34 and heart failure.35 Hydration alone is insufficient to prevent CIN under these conditions, which further emphasizes the importance of sufficient renal perfusion in high-risk patients. In the present study, the physician who performed catheterization was aware of the patients’ renal function before the procedure and therefore used a reduced contrast volume in patients with impaired renal function than in patients with normal renal function, which may explain why contrast volume was not a predictor of CIN in our study.
In our study population, oxygenation treatment effectively decreased the risk of CIN (1% vs. 8%) among patients with an eGFR < 60 ml/min per 1.73 m2. Therefore, this approach may benefit patients with mild CKD. Diabetes mellitus was not associated with CIN in our study cohort. This discrepancy between the results of this study and others might be caused by differences in baseline renal function. Thus, in our patients, the baseline CKD was mild, and in other studies, the baseline CKD was more severe.
Study Limitations
First, our cohort of elective cases with stable hemodynamics represents a population that has a relatively low risk of developing CIN. The optimal preventive threshold of oxygenation requires further examination, particularly in hemodynamically unstable patients or those with acute coronary syndrome, who are highly susceptible to CIN despite the routine use of oxygenation. On the other hand, the AVOID trial36 showed no benefit of oxygen in MI and in fact, the size of the MI was larger in oxygenation group than in the room air group. Therefore, we must consider these aspects before applying our treatment method to patients with acute coronary syndrome. Second, for practical reasons, oxygen administration was terminated at the end of the procedure. Prolonged oxygenation may be effective in patients with critical renal dysfunction in whom the elimination of contrast medium is delayed. Nevertheless, the observed CIN incidence of 1% using our oxygen protocol appears to be promising for patients with mild CKD in the elective setting. Therefore, it is necessary to investigate patients who are at higher risk for CIN, such as those who receive a high volume of contrast medium or who have more severe CKD, so that the protocol can be adapted for patients with more severe CKD. Third, postprocedural creatinine levels were evaluated only 48 hours after contrast exposure, and because creatinine levels typically peak 3 to 5 days after contrast medium administration, we cannot exclude the possibility that oxygenation merely delayed the development of contrast-induced renal dysfunction. Fourth, our study did not include any novel biomarkers of kidney damage, nor did we capture other urinary measures such as proteinuria or urine volume. Fifth, our study presents only initial findings, and additional research is required to establish the benefit of oxygen before treatment.
This was an open-label study. Although the outcomes were objective measurements of serum creatinine changes, the open-label study may affect patient bias somewhat in this case. Therefore, a single-blinded study is needed to reduce bias, such as by using a nasal delivery device to administer room air to a control group.
Finally, the present study was conducted at a single center, and the number of patients reaching the primary endpoint (CIN incidence) was small. Although univariate analyses identified the absence of oxygenation treatment as significant in predicting CIN incidence, our study may have been inadequate to support multivariate testing. However, it does provide some interesting preliminary data. Additional factors predictive of CIN should be evaluated in a larger study population. A large multicenter study is also needed to evaluate the safety and preventive effects of oxygen in the emergency setting and among patients with acute coronary syndrome, congestive heart failure, diabetes mellitus, and stage IV or higher CKD, who were excluded from the present study.
In conclusion, the addition of oxygenation treatment to conventional isotonic saline hydration decreased the CIN incidence in patients with impaired renal function undergoing elective cardiovascular angiography and PCI. This finding adds to existing data suggesting the importance of renal hypoxia in the pathogenesis of CIN. The benefit of this simple and minimally invasive method was demonstrated among patients with mild CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) with the identifier UMIN000007125. The authors thank Editage (www.editage.jp) for English language editing.
This work was supported by funds from the National Hospital Organization Yokohama Medical Center Clinical Research Division, Yokohama, Japan. The sponsors of this study were not involved in the study design, collection, analysis, data interpretation, or writing of this report.
References
- 1.Rihal C.S., Textor S.C., Grill D.E. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 2.Cerda J., Lameire N., Eggers P. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 3.McCullough P.A., Wolyn R., Rocher L.L. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Mehran R., Aymong E.D., Nikolsky E. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 5.Mueller C., Buerkle G., Buettner H.J. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg I., Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461–1471. doi: 10.1503/cmaj.1040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przyklenk K., Whittaker P. Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther. 2011;16:255–259. doi: 10.1177/1074248411409040. [DOI] [PubMed] [Google Scholar]
- 8.Heyman S.N., Brezis M., Epstein F.H. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991;40:632–642. doi: 10.1038/ki.1991.255. [DOI] [PubMed] [Google Scholar]
- 9.Whittaker P., Przyklenk K. Remote-conditioning ischemia provides a potential approach to mitigate contrast medium-induced reduction in kidney function: a retrospective observational cohort study. Cardiology. 2011;119:145–150. doi: 10.1159/000330930. [DOI] [PubMed] [Google Scholar]
- 10.Sekiguchi H., Ajiro Y., Uchida Y. Oxygen preconditioning prevents contrast-induced nephropathy (OPtion CIN Study) J Am Coll Cardiol. 2013;62:162–163. doi: 10.1016/j.jacc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Lameire N., Adam A., Becker C.R. Baseline renal function screening. Am J Cardiol. 2006;98:21K–26K. doi: 10.1016/j.amjcard.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Imai E., Horio M., Nitta K. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50. doi: 10.1007/s10157-006-0453-4. [DOI] [PubMed] [Google Scholar]
- 13.McCullough P.A., Adam A., Becker C.R., CIN Consensus Working Panel Risk prediction of contrast-induced nephropathy. Am J Cardiol. 2006;98:27K–36K. doi: 10.1016/j.amjcard.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Solomon R., Werner C., Mann D. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 15.Tumlin J., Stacul F., Adam A. Pathophysiology of contrast-induced nephropathy. Am J Cardiol. 2006;98:14K–20K. doi: 10.1016/j.amjcard.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Maeder M., Klein M., Fehr T. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44:1763–1771. doi: 10.1016/j.jacc.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 17.Moreau J.F., Droz D., Noel L.H. Tubular nephrotoxicity of water-soluble iodinated contrast media. Invest Radiol. 1980;15:S54–S60. doi: 10.1097/00004424-198011001-00014. [DOI] [PubMed] [Google Scholar]
- 18.Heyman S.N., Fuchs S., Jaffe R. Renal microcirculation and tissue damage during acute ureteral obstruction in the rat: effect of saline infusion, indomethacin and radiocontrast. Kidney Int. 1997;51:653–663. doi: 10.1038/ki.1997.95. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein R.S., Hook J.B., editors. Toxicology of the Kidney. 2nd ed. Raven Press; New York, NY: 1993. [Google Scholar]
- 20.Katholi R.E., Woods W.T., Jr., Taylor G.J. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64–71. doi: 10.1053/ajkd.1998.v32.pm9669426. [DOI] [PubMed] [Google Scholar]
- 21.Liss P., Nygren A., Erikson U. Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int. 1998;53:698–702. doi: 10.1046/j.1523-1755.1998.00811.x. [DOI] [PubMed] [Google Scholar]
- 22.Heyman S.N., Rosenberger C., Rosen S. Regional alterations in renal haemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol Dial Transplant. 2005;20(suppl 1):i6–i11. doi: 10.1093/ndt/gfh1069. [DOI] [PubMed] [Google Scholar]
- 23.Dussol B., Morange S., Loundoun A. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrol Dial Transplant. 2006;21:2120–2126. doi: 10.1093/ndt/gfl133. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein I., Abassi Z., Milman F. Hyperbaric oxygen treatment improves GFR in rats with ischaemia/reperfusion renal injury: a possible role for the antioxidant/oxidant balance in the ischaemic kidney. Nephrol Dial Transplant. 2009;24:428–436. doi: 10.1093/ndt/gfn511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen W.M., Beekhuis H., de Bruin R. Noninvasive measurement of intrarenal blood flow distribution: kinetic model of renal 123I-hippuran handling. Am J Physiol. 1995;269:F571–F580. doi: 10.1152/ajprenal.1995.269.4.F571. [DOI] [PubMed] [Google Scholar]
- 26.Dagher P.C., Herget-Rosenthal S., Ruehm S.G. Newly developed techniques to study and diagnose acute renal failure. J Am Soc Nephrol. 2003;14:2188–2198. doi: 10.1097/01.asn.0000079790.91292.4a. [DOI] [PubMed] [Google Scholar]
- 27.Sutton T.A. Alteration of microvascular permeability in acute kidney injury. Microvasc Res. 2009;77:4–7. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fillmore S.J., Shapiro M., Killip T. Arterial oxygen tension in acute myocardial infarction. Serial analysis of the clinical state and blood gas changes. Am Heart J. 1970;79:620–629. doi: 10.1016/0002-8703(70)90281-4. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi H.S., Moore H., Nasr S. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29–C34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 30.Heinrich M.C., Haberle L., Muller V. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009;250:68–86. doi: 10.1148/radiol.2501080833. [DOI] [PubMed] [Google Scholar]
- 31.Klause N., Arendt T., Lins M. Hypoxic renal tissue damage by endothelin-mediated arterial vasoconstriction during radioangiography in man. Adv Exp Med Biol. 1998;454:225–234. doi: 10.1007/978-1-4615-4863-8_27. [DOI] [PubMed] [Google Scholar]
- 32.Brenner B.M., Rector F.C., Jr. 4th edition. WB Saunders; Philadelphia, PA: 1991. The Kidney. [Google Scholar]
- 33.Calver A., Collier J., Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panza J.A., Quyyumi A.A., Brush J.E., Jr. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 35.Kubo S.H., Rector T.S., Bank A.J. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 36.Stub D., Smith K., Bernard S. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]