To the Editor:
Patients with end-stage kidney disease (ESKD) are at increased risk for cardiovascular disease (CVD) compared to the general population, because of a number of factors including endothelial dysfunction.1 Endothelial cells (ECs) arise from progenitors that reside in both the bone marrow and vascular niches, including that of the kidney,2 and are classified according to specific morphological and functional differences.3 Endothelial progenitor cells (EPCs) can form “blood-like” islands, which can be measured in a colony-forming unit (CFU) assay4 in vitro, and late outgrowth ECs (OECs) can home to sites of ischemic injury and contribute to neovascularization.5, 6 EPCs identified in peripheral blood can be used as indicators of risk of CVD in dialysis patients7, 8 and play a role in vascular repair by releasing pro-angiogenic growth factors9; yet the effect of clinical parameters on OEC appearance in culture has been lacking. OECs have angiogenic properties and as such have been investigated as a source for cell therapy10; however, their success has been limited because of their early senescence in culture. In this study, we investigate the effect of patient clinical parameters on OEC appearance as a means of furthering the understanding of OECs as a source of cell therapy for vascular injury.
Patients, Materials, and Methods
ESKD patients, treated with hemodialysis were recruited (n = 20) from Monash Medical Centre and participated in this study under informed consent. Patients were excluded from the study if their original diagnosis of ESKD was type I or II diabetes, were on antibiotics, or had a recent infection or inflammatory flare-up. Parameters such as patient age, time of dialysis, erythropoietin (EPO) and statin use, and smoking status were collected, as these are known to influence the percentage of circulating EPCs, in addition to height, weight, and blood pressure. All human studies were approved by the Monash Health Human Research Ethics Committee (CF16/402-2016000182). A 10-ml quantity of blood was collected prior to a single hemodialysis session, and the peripheral blood mononuclear cell (PBMC) fraction was isolated,11 seeded onto fibronectin (2 μg/cm2)−coated, 6-well plates and cultured in Endothelial Growth Media (EGM)−2 Microvascular Bullet Kit medium (Lonza, Mount Waverly, Australia) containing 5% fetal bovine serum, 0.04% hydrocortisone, 0.4% human fibroblast growth factor, and 0.1% of vascular endothelial growth factor (VEGF), R3−insulin-like growth factor−1, human epidermal growth factor, gentamicin, and amophotericin-B (catalog no. CC-3202, Lonza). Medium was changed after 72 hours and every second day thereafter. At 7 days after PBMC seeding, a CFU assay was performed. Cell culture continued for a total of 21 days or until OECs appeared, as identified by their extensive proliferation and cobblestone morphology. A small volume of whole blood (100 μl) was analyzed by flow cytometry for markers of EPCs, as identified by a subpopulation of CD31+CD34+KDR+/−CD45− cells.5
Results
Clinical Data
The patient cohort had a mean age of 64.2 (± 15.5) years; 80% were male and 20% female. The mean blood pressure of patients prior to dialysis was 139.1 (± 27.1)/75.2 (± 18.9), and the mean time on hemodialysis was 46 months (± 69.5). Half of the patients received EPO, 30% were administered statins, 15% were taking other medication including anticoagulants or blood thinners, and 65% had a history of smoking or were current smokers (Table 1).
Table 1.
Clinical characteristics of all patients recruited to this study
| Characteristic | All subjects |
|---|---|
| N | 20 |
| Male | 16 (80%) |
| Female | 4 (20%) |
| Age, yr | 64 ± 15 |
| Months on dialysis | 36 ± 56 |
| Height, cm | 172 ± 9 |
| Weight, kg | 78 ± 18 |
| Systolic BP, mm Hg | 139 ± 27 |
| Diastolic BP, mm Hg | 75 ± 19 |
| EPO use | 10 (50%) |
| Statin use | 6 (30%) |
| Other drugs | 3 (15%) |
| Smoking status | |
| Never smoked | 7 (35%) |
| Previous smoker | 11 (45%) |
| Current smoker | 2 (10%) |
ACEi, angiotensin-converting enzyme inhibitor; BP, blood pressure; EPO, erythropoietin.
Data are mean ± SD, or number with percentage in parentheses.
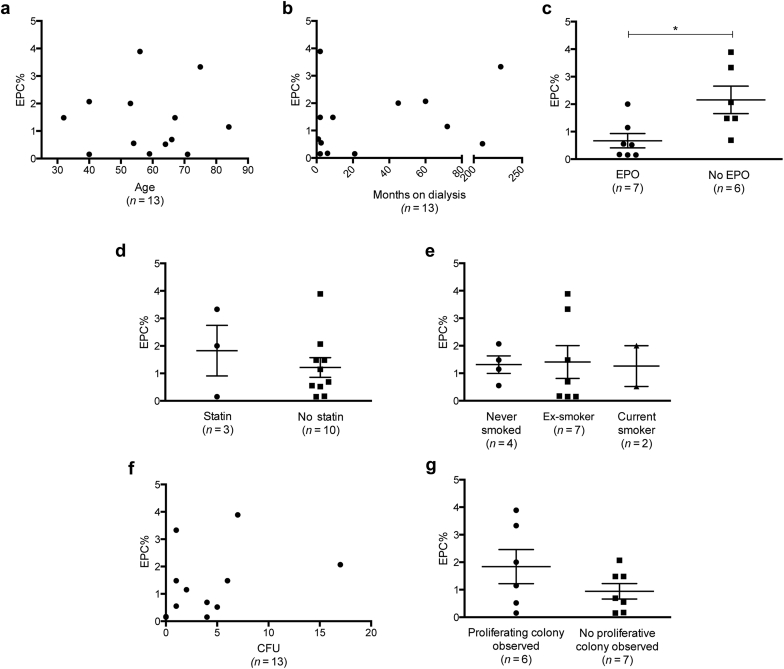
Circulating EPC Number Negatively Correlates With Systolic Blood Pressure and Does Not Affect CFU or OEC Transformation
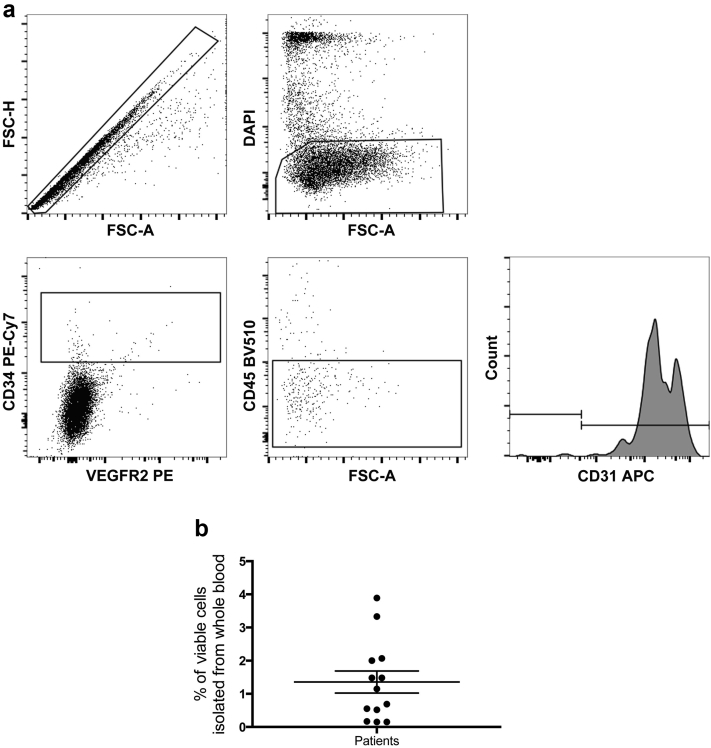
The percentage of EPCs was identified using flow cytometry. EPCs were classified according to positive expression of both CD31 and CD34, in addition to positive or negative expression of vascular endothelial growth factor receptor. EPCs also had dim expression of CD45 (Figure 1a).12 The percentage of circulating EPCs in patients with ESKD ranged from 0.15% to 3.8% (Figure 1b). Because of the impact that circulating EPC percentage has on the health of hemodialysis patients,13 we investigated whether any clinical parameters had an effect on patient EPC levels in our cohort. Although patient age14 and time on dialysis15 have previously correlated with a decreased circulating EPC percentage, we did not find that these parameters influenced circulating EPC percentage in this cohort (Figure 2a and b). However, patients receiving EPO had a significantly lower percentage of circulating EPCs compared to patients who were not administered EPO (mean difference 1.487 ± 0.538, 95% confidence interval [CI] = 0.3−2.7, P = 0.0184; Figure 2c), and systolic blood pressure negatively correlated with EPC percentage, whereby, as systolic pressure increased, the percentage of EPCs decreased (r = −0.59, P = 0.033; Figure 2d). There were no correlations observed between the starting EPC percentage and the number of CFUs formed (P = 0.27; Figure 2d), nor was the circulating EPC percentage a factor in determining whether patient cells successfully formed OEC colonies (P = 0.19; Figure 2e).
Figure 1.
Frequency analysis of endothelial progenitor cells (EPCs) from patients with end-stage kidney disease (ESKD). (a) Flow-cytometric protocol used to determine the percentage of circulating EPCs from whole blood. All cells were visualized on a forward versus side scatter plot and assessed for viability by gating on DAPI-negative cells. Viable vascular endothelial growth factor receptor-2 (VEGFR2+/−) cells subgated with CD34+ cells. Of these, the CD45dim population was subgated, and CD31+ expression on the resulting cells was confirmed on a CD31 histogram. This flow-cytometric protocol was used and established by Yoder et al.12 Analysis was conducted using FlowJo software, and isotypes were used to for compensation. (b) Percentage of circulating EPCs for all patients in whole blood. The range of EPCs is spread from 3.8% down to 0.15% of whole blood in these patients (n = 13) with ESKD. Data are mean ± SD. APC, allophycocyanin fluorescent protein; FSC-A, forward scatter area; FSC-H, forward scatter height; PE, phycoerythrin fluorescent protein.
Figure 2.
Circulating endothelial progenitor cell (EPC) percentage negatively correlates with erythropoietin (EPO) use. The percentage of circulating EPCs was compared against clinical parameters from patients participating in the study (n = 13). (a) Age and (b) months on dialysis were not correlated with the percentage of circulating EPCs. There was a correlation observed between patients who did not receive EPO administration by which they had significantly higher circulating EPCs compared to patients who did not receive EPO (c, P < 0.05). The percentage of EPCs in the peripheral circulation was also investigated against (d) statin use, (e) smoking status, (f) colony-forming unit (CFU) formation, and (g) late outgrowth endothelial cell (EC) formation, with no significant differences found. Data are mean ± SD and were analyzed using the Student t-test. *P < 0.05.
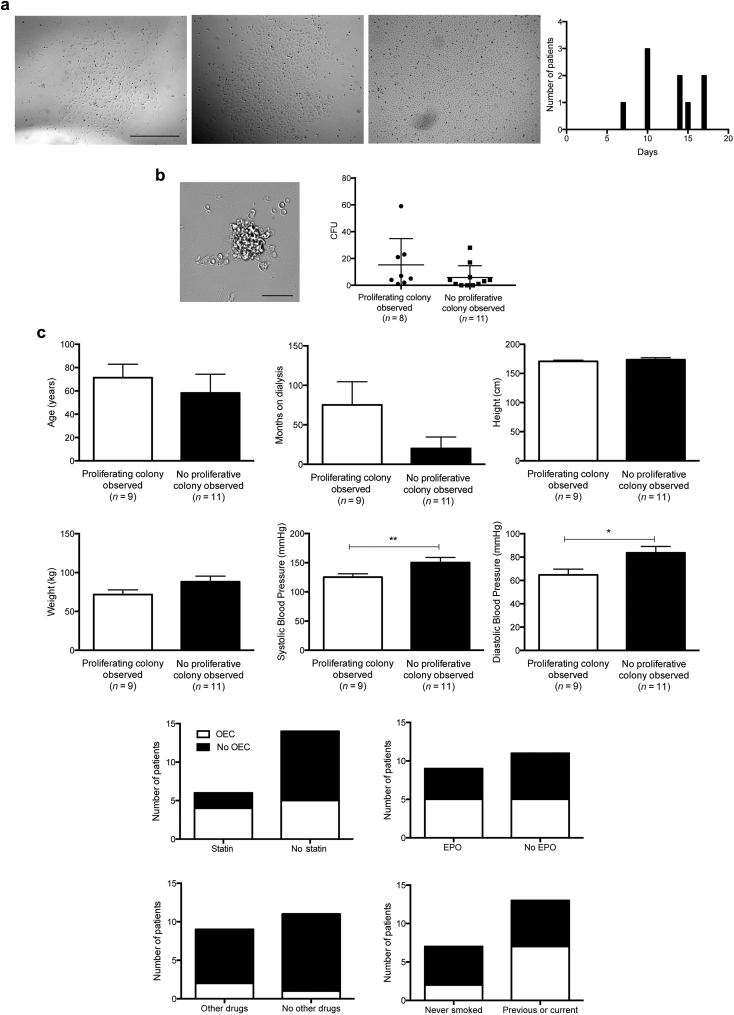
The Best Predictor of OEC Appearance in Culture Is Blood Pressure
We observed a 45% conversion of EPCs to OECs, which were identified according to their cobblestone morphology and high proliferation rate. OECs appeared as early as 7 days and as late as 17 days in culture (Figure 3a). The number of CFUs counted at 7 days did not affect the appearance of OECs (P = 0.24; Figure 3b), nor did patient age, time on dialysis, or any other clinical parameter collected except for blood pressure (Figure 3c). Patient cells that transformed into OECs had significantly lower systolic (mean difference 24.95 mm Hg, 95% CI = 1.7−48.1, P = 0.0365) and diastolic (mean difference 18.9 mm Hg, 95% CI = 3.2−34.6, P = 0.0208) blood pressures compared to patient cells that did not (Figure 3c).
Figure 3.
Observations of a late-outgrowth endothelial cell (OEC) colony correlate with low blood pressure. (a) OECs were identified by their cobblestone morphology and proliferative potential and converted in 9 of 20 patients from 7 to 17 days in culture. (b) Investigations between an observed proliferative colony and colony-forming units (CFUs) at 7 days and (c) clinical parameters were investigated for correlations (n = 20). Blood pressure was significantly lower in patients whose cells went on to form OECs (systolic blood pressure, P < 0.0365; diastolic blood pressure, P < 0.0208). Data are mean ± SD and were analyzed using the Student t-test. Bar = a: 500 μm; b: 50 μm. **P < 0.01, *P < 0.05.
Discussion
The primary aim of this study was to investigate whether patient clinical parameters affected circulating EPC percentage and the ability of isolated PBMCs to differentiate into angiogenic OECs. Interestingly, we found that patients administered EPO had a significantly lower percentage of circulating EPCs than patients who were not, and that high blood pressure was negatively correlated with EPC percentage. The overall number and function of EPCs was reduced in patients with ESKD16 compared to healthy controls, which was suggested to be related to exposure to uremic toxins.17, 18, 19 In addition, it has been reported that patients with hypertension have reduced EPC numbers and limited vascular regenerative potential when isolated EPCs are challenged in vitro,20 which has also been found in patients who are obese or diabetic.21 However, the use of angiotensin-converting enzyme inhibitors has previously been shown to reduce oxidative stress, increase EPC number and function, and reduce vascular damage in hypertensive patients.22, 23 There is evidence to suggest that dialysis modality can affect circulating EPC numbers,24 with hemodialysis significantly reducing EPCs compared to continuous peritoneal dialysis. Furthermore, patients who have controlled blood pressure on hemodialysis have a reduced risk of cardiovascular events.25 Our findings further suggest that high blood pressure reduces EPC numbers, and therefore may contribute to negative vascular health outcomes in the hemodialysis population, although this needs to be tested further in the clinical setting.
OECs are being investigated as a source for cell therapy with limited success due to senescence of cells in culture.26 Here, we observed a 45% conversion rate of EPCs to OECs after an average of 12 days of PBMC culture. The success of conversion is thought to be associated with the starting volume of blood, and because EPCs are rare in the circulation, it has been suggested that large volumes of blood are required to obtain proliferative OEC colonies.27 Unlike previous studies,28 in our study we did not observe a correlation between starting EPC numbers and CFU after 7 days of culture. However, we showed that OEC colony transformation did occur if the blood pressure of the patient was lower before dialysis. A controlled blood pressure is linked with reduced cardiovascular events and a decrease in all-cause mortality in patients with stages 1 to 5 CKD,29 and evidence suggests that controlling blood pressure, and therefore pulse pressure, in the dialysis population has similar outcomes.30 There are limited data available about the characteristics of OECs isolated from patients with ESKD who are dialysis dependent and the clinical factors influencing their appearance in culture. The functional characteristics of OECs isolated from ESKD patients have been previously described,31 including demonstration that OECs proliferate and form tubes in vitro, as well as contribute to in vivo neovascularization. Interestingly, this previous study demonstrated that increased age and high blood pressure contributed to the formation of mesenchymal stem cell colonies after isolation of the PBMC fraction from ESKD patients, yet had no effect on the appearance on OEC formation.31 This, in combination with our findings, suggest that blood pressure may play a role in the formation of specific cell types in culture, which may influence the future use of autologous cell therapy. To further elucidate the full effect of blood pressure on circulating EPC percentage and OEC transformation in vitro, future studies with larger patient numbers are required. In our study, we were unable to determine whether there were any sex, age, and ethnicity differences that may be affecting both EPCs and OECs, due to the number of patients recruited to this study. Additionally, the patients recruited to this study were all using the same mode of dialysis, hemodialysis, and since there has been some suggestion that nocturnal dialysis normalizes blood pressure, increases the baroreflex sensitivity, and reduces cardiovascular mortality,32 it would be interesting to investigate and compare the appearance of OECs isolated from patients undertaking different dialysis modalities.
In conclusion, our study suggests that a reduced blood pressure is beneficial for the vascular health of hemodialysis patients, increasing the number of circulating endothelial progenitor cells and the appearance of angiogenic OEC colonies in culture.
Disclosure
All the authors declared no competing interests.
Acknowledgments
BMH was supported by an Australian Postgraduate Award. CSS is supported by a National Health & Medical Research Council of Australia Senior Research Fellowship (GNT1041766).
References
- 1.Bábíčková J., Klinkhammer B.M., Buhl E.M. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017;91:70–85. doi: 10.1016/j.kint.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Sims-Lucas S., Schaefer C., Bushnell D. Endothelial progenitors exist within the kidney and lung mesenchyme. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirschi K.K., Ingram D.A., Yoder M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill J.M., Zalos G., Halcox J.P.J. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Yoder M.C., Mead L.E., Prater D. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruyama S., Taguchi A., Iwashima S. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int. 2008;74:1603–1609. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 8.Lee H.J., Kim W., Kim W.S. Circulating endothelial progenitor cell levels predict cardiovascular events in end-stage renal disease patients on maintenance hemodialysis. Nephron. 2015;130:151–158. doi: 10.1159/000430471. [DOI] [PubMed] [Google Scholar]
- 9.Rehman J. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 10.Gulati R., Jevremovic D., Peterson T.E. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 11.Kalka C., Masuda H., Takahashi T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoder M.C. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C.-L., Leu J.-G., Liu W.-C. Endothelial progenitor cells predict long-term mortality in hemodialysis patients. Int J Med Sci. 2016;13:240–247. doi: 10.7150/ijms.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aragona C.O., Imbalzano E., Mamone F. Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease. Stem Cells Int. 2016;2016:8043792. doi: 10.1155/2016/8043792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jie K.E., Lilien M.R., Goossens M.H.J. Reduced endothelial progenitor cells in children with hemodialysis but not predialysis chronic kidney disease. Pediatrics. 2010;126:e990–e993. doi: 10.1542/peds.2009-3346. [DOI] [PubMed] [Google Scholar]
- 16.Kiewisz J., Kaczmarek M.M., Pawlowska A. Endothelial progenitor cells participation in cardiovascular and kidney diseases: a systematic review. Acta Biochim Pol. 2016;63:475–482. doi: 10.18388/abp.2016_1284. [DOI] [PubMed] [Google Scholar]
- 17.de Groot K., Bahlmann F.H., Sowa J. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66:641–646. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 18.Jourde-Chiche N., Dou L., Cerini C. Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–337. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 19.Ozkok A., Aktas E., Yilmaz A. Decrease in endothelial progenitor cells associated with inflammation, but not with endothelial dysfunction in chronic hemodialysis patients. Clin Nephrol. 2013;79:21–30. doi: 10.5414/CN107318. [DOI] [PubMed] [Google Scholar]
- 20.Giannotti G., Doerries C., Mocharla P.S. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 21.Fadini G.P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scribner A.W., Loscalzo J., Napoli C. The effect of angiotensin-converting enzyme inhibition on endothelial function and oxidant stress. Eur J Pharmacol. 2003;482:95–99. doi: 10.1016/j.ejphar.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Cacciatore F., Bruzzese G., Vitale D.F. Effects of ACE inhibition on circulating endothelial progenitor cells, vascular damage, and oxidative stress in hypertensive patients. Eur J Clin Pharmacol. 2011;67:877–883. doi: 10.1007/s00228-011-1029-0. [DOI] [PubMed] [Google Scholar]
- 24.Ueno H., Koyama H., Fukumoto S. Dialysis modality is independently associated with circulating endothelial progenitor cells in end-stage renal disease patients. Nephrol Dial Transplant. 2010;25:581–586. doi: 10.1093/ndt/gfp358. [DOI] [PubMed] [Google Scholar]
- 25.Miskulin D.C., Weiner D.E. Blood pressure management in hemodialysis patients: what we know and what questions remain. Semin Dial. 2017;30:203–212. doi: 10.1111/sdi.12586. [DOI] [PubMed] [Google Scholar]
- 26.Corselli M., Parodi A., Mogni M. Clinical scale ex vivo expansion of cord blood-derived outgrowth endothelial progenitor cells is associated with high incidence of karyotype aberrations. Exp Hematol. 2008;36:340–349. doi: 10.1016/j.exphem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Ingram D.A. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 28.Bueno-Betí C., Novella S., Lázaro-Franco M. An affordable method to obtain cultured endothelial cells from peripheral blood. J Cell Mol Med. 2013;17:1475–1483. doi: 10.1111/jcmm.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minutolo R., Gabbai F.B., Agarwal R. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. Am J Kidney Dis. 2014;64:744–752. doi: 10.1053/j.ajkd.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Lertdumrongluk P., Streja E., Rhee C.M. Changes in pulse pressure during hemodialysis treatment and survival in maintenance dialysis patients. Clin J Am Soc Nephrol. 2015;10:1179–1191. doi: 10.2215/CJN.09000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J., Bolton E.M., Randle L. Functional characterization of late outgrowth endothelial progenitor cells in patients with end-stage renal failure. Transpl Int. 2014;27:437–451. doi: 10.1111/tri.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan C.T., Jain V., Picton P. Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int. 2005;68:338–344. doi: 10.1111/j.1523-1755.2005.00411.x. [DOI] [PubMed] [Google Scholar]