Abstract
Hyponatremia is defined by low serum sodium concentration and is the most common electrolyte disorder encountered in clinical practice. Serum sodium is the main determinant of plasma osmolality, which, in turn, affects cell volume. In the presence of low extracellular osmolality, cells will swell if the adaptation mechanisms involved in the cell volume maintenance are inadequate. The most dramatic effects of hyponatremia on the brain are seen when serum sodium concentration decreases in a short period, allowing little or no adaptation. The brain is constrained inside a nonextensible envelope; thus, brain swelling carries a significant morbidity because of the compression of brain parenchyma over the rigid skull. Serum sodium concentration is an important determinant of several biological pathways in the nervous system, and recent studies have suggested that hyponatremia carries a significant risk of neurological impairment even in the absence of brain edema. The brain can also be affected by the treatment of hyponatremia, which, if not undertaken cautiously, could lead to osmotic demyelination syndrome, a rare demyelinating brain disorder that occurs after rapid correction of severe hyponatremia. This review summarizes the pathophysiology of brain complications of hyponatremia and its treatment.
Keywords: brain edema, hyponatremia, osmolarity, serum sodium
Plasma osmolality refers to the quantity of solute dissolved into 1 kg of plasma.1, 2 The serum is separated from the inside of the cell by the cell membrane. The cell membrane is highly permeable to water but not to some ions (e.g., potassium and sodium), and is called semipermeable. The ability of water to move across the cell membrane is related to the tonicity of the plasma that determines the direction and the magnitude of water movement.3
The serum sodium (SNa) concentration is the main extracellular osmolyte, and therefore, the most important determinant of serum osmolality.4 The permeability of the cell membrane to water is due to the presence of water channels called aquaporins (AQPs) that mediate bidirectional water transport.5 In the central nervous system (CNS), the blood−brain barrier (BBB) separates the brain parenchyma from the plasmatic space. The BBB is composed of several layers; the outermost layer is astrocyte end-feet, which is responsible for water exchange between the brain parenchyma and the vascular space.6 Astrocyte end-feet express a high number of AQP 4, but other forms of AQP have been identified in astrocytes and other CNS cell types.5
At equilibrium, extracellular osmolality equals intracellular osmolality, and the net movement of water across the cell membrane is null. When the SNa concentration is reduced, hypo-osmolality and hypotonicity will ensue, and the water will flow from the extracellular space into the intracellular compartment. This movement of water into the cell will cause cell swelling, and conversely, in the presence of hypertonicity, cells will shrink because of water movement from inside to outside the cell. In the mammalian CNS, even minimal changes in the intracellular volume and the associated brain swelling or shrinking might lead to dramatic symptoms. Macromolecular crowding refers to the behavior of protein inside the cell with respect to the salt and water content of the cytoplasm.7 It has been shown that many cellular functions (e.g., enzymatic activity) depend of the ionic strength of the cytoplasm, the cell volume, and the macromolecular crowding.8 Therefore, the maintenance of a normal cell volume and normal intracellular ionic strength is essential. The ability to respond to brisk changes in extracellular osmolality has been evolutionarily conserved across species throughout the evolution process.
The present review details the mechanisms of brain adaptation to hyponatremia, the consequences of hyponatremia on the brain, and discusses the treatment of hyponatremia and the risk associated with excessive correction of SNa from a neurological perspective.
Mechanisms of Brain Cells Adaptation to Hypo-Osmotic Challenge
Cells have developed several mechanisms to counteract the deleterious effect of extracellular hypotonicity on cell volume. These mechanisms are collectively called regulatory volume decrease (RVD) because their aim is to restore the initial volume after swelling induced by hypotonicity.9 They essentially involve the extrusion of intracellular osmotically active solutes that will induce obligated intracellular loss of water and prevent or reduce cell swelling. RVD has been well studied in isolated brain cells, mostly in astrocytes and neurons.10, 11, 12, 13 Neuronal cell lines exposed to hypotonicity quickly experience an increase in their volume up to 2-fold in the first minutes, and after a slow decrease in the volume, this reaches a plateau at approximately 60% to 80% of volume recovery in the first 15 minutes.11 The same is true for glial cell lines.10 However, these observations should be interpreted along with some caveats. First, not all neuronal cells react alike during hypo-osmolality; some differences have been noted across different neuronal cells.14 Second, most studies done on RVD in brain cells used either cultured or immortalized cell lines that displayed notable differences with in vivo cells. Lastly, regional variability and interspecies variability in glial phenotype have been described, which might have an impact on some biological functions.15, 16, 17 Therefore, it is possible that not only do in vivo astrocytes not exhibit the exact same behavior as in vitro astrocytes when confronted with hypotonic stress, but also that not all astrocytes in the brain exhibit the same pattern of changes during hyponatremia.
Cellular Mechanisms of RVD, Osmotic Sensing, Signal Transduction, and Efflux Pathways
An osmolyte is a noncell membrane permeable substance that can exert a net movement of water across a semipermeable membrane. Osmolytes are categorized as electrolytes and nonelectrolyte or organic osmolytes. The most common electrolyte osmolytes present in the mammalian brain are sodium, potassium, and chloride ions, and the most common organic osmolytes are myoinositol, betaine, glutamine, taurine, and γ-aminobutyric acid.
The occurrence of RVD implies a sensor for extracellular osmolality, a signal transducer that will translate the information on extracellular osmolality to the channels responsible for intracellular osmolyte depletion. The word osmosensor refers to a sensory element that can detect changes in plasma osmolality.18 In the mammalian brain, the true nature of the osmosensor is still elusive. The transient receptor potential vanilloid 4 (TRPV4) channel is a member of the broader class of the TRPV channel family that has been shown to be essential for tonicity sensing and transduction trough modulation of calcium influx in several cell lines.19 In cortical astrocytes and muller glia (retinal glial cells), TRPV4 forms a molecular complex with AQP 4, the main water channel present in astrocyte end-feet. The integrity of that molecular complex is necessary for calcium influx, which has been linked to RVD with an hypo-osmotic challenge.20, 21 Other researchers have suggested that calcium influx is not essential for RVD, and also that inhibition of the TRPV channel does not significantly affect RVD in astrocytes.22 Because of the complexity and the importance of the process, it is likely that osmosensing operates through at least a few redundant pathways that might not be identical for all brain cells.
Upon sensing, the hypo-osmotic signal must be transduced inside the cell, and protein kinases and calcium are believed to be involved in the transduction of the signal of RVD in astrocytes and neurons. For example, inhibition of protein kinase C can significantly reduce the efflux of potassium and taurine in hypo-osmotically challenged glial cells, which suggests that G-coupled protein receptor with protein kinase C activity is a likely transducer for hypo-osmotic stimuli.23 After signal transduction, the osmolyte must flow inside or outside the cell trough special channels. The responsible channels for intracellular electrolytes depletion are collectively called volume sensitive channels (for review, see Strange et al.24). They have been identified in nearly all CNS cells.25, 26, 27 Although these channels can be blocked pharmacologically, their precise identities remain elusive. As for organic osmolytes, several organic osmolyte transporters have been identified in the mammalian brain. For example, these include the γ-aminobutyric acid−betaine transporter and the sodium myoinositol transporter.28 The channels are bidirectional, and the movement of organic osmolytes through them is dependent on the net concentration gradient. Some interconnections between the volume sensitive channel and the organic osmolyte channels have been described.29
Brain Adaptation to Hyponatremia
After onset of systemic hypotonicity from hyponatremia, the brain water content will increase to commensurate the extent of the hyponatremia if the brain behaves like a perfect osmometer. However, studies have shown that after either chronic or acute hyponatremia, the brain water content does not increase as predicted. For instance, after 6 hours of hyponatremia, the brain only increases by 40% of what is predicted, and after 4 days of hyponatremia, there is only a 0.6% of increase in the brain water content.30 These observations point to the fact that the brain possesses some defense mechanisms that minimize organ swelling upon plasma hypotonicity. Although sometimes presented as distinct, brain adaptation to acute and chronic hyponatremia belongs to the same physiological spectrum, but some of the mechanisms are of early onset, with rapid exhaustion, whereas others occur in a prolonged timeframe. The clinical distinction between acute and chronic hyponatremia is often set between 24 and 48 hours. This distinction is arbitrary, but it is believed that the reasons for such a cutoff reflect the time frame after which complete mechanisms of brain adaptation are in place, thus making correction of hyponatremia potentially harmful for the brain. Figure 1 depicts brain mechanisms of adaptation to hyponatremia.
Figure 1.
Mechanisms of brain adaptation to hyponatremia. Hyponatremia induces an increase in the intracellular fluid (ICF) and interstitial fluid (ISF). During hyperacute adaptation, water moves from the ISF compartment to systemic circulation. In the following hours, the depletion of intracellular electrolytes and nonelectrolyte osmolytes is responsible for the movement of water from the intracellular space in the extracellular space and later into the systemic circulation. This will ultimately decrease the brain water content.
Adapted from Sterns, RH. The management of symptomatic hyponatremia. Semin Nephrol. 1990;10:503–514,42 with permission from Elsevier.
Early Mechanisms of Brain Adaptation to Hyponatremia
One of the first mechanisms of defense of the brain against hypotonicity is the water flow from brain parenchyma into the cerebrospinal fluid (CSF) and later into systemic circulation.32, 33 Within the first minutes of hyponatremia, it is believed that the increased pressure inside the brain will drive a hydrostatic water movement inside the CSF first and then into the systemic circulation. This will work as a first guard to prevent rapid brain edema. For example, in a new-born rat pup with a soft skull in which brain edema does not cause an increase in hydrostatic pressure, there is no water flow from the brain to the CSF.34
Another step of brain cell adaptation to hyponatremia involves movement of electrolytes from inside the cell to the extracellular compartment. Within the first hours of hyponatremia, there is a significant decrease in the intracellular content of sodium, chloride, and potassium.31, 35 The kinetics of brain electrolytes depletion during acute hyponatremia revealed that after 3 hours of hyponatremia, brain depletion in electrolytes reaches a plateau, and the depletion of sodium is believed to be primarily from the CSF, which occurs together with intracellular depletion of chloride faster than the intracellular depletion of potassium.30 The total brain ion depletion is roughly similar (∼18%) within a large range of hyponatremia (72−116 mEq/l), which strongly suggests the brain can lose no more than 18% of its ion content. Because of this limited nature of the electrolyte depletion of the brain, it is excepted that by the time the mechanisms behind electrolyte loss are exhausted, severe continued hyponatremia will inevitably cause significant brain edema. There are 2 pieces of evidence here to consider. On the one hand, it has clearly been shown experimentally and observed in clinical practice that the occurrence of severe hyponatremia within few hours will cause death from brain swelling.36 (One of the first reports of evidence of brain edema secondary to hypo-osmolality occurred in a patient who underwent proctoclysis after an uneventful cholecystectomy who started to develop severe neurological symptoms 12 hours after the surgery and later succumbed from brain edema.37) On the other hand, when hyponatremia develops slowly, experimentally and in humans, even to a level of <100 mEq/l, there is no brain edema or immediately increased mortality.38 This apparently contradictory evidence can be reconciled if one considers that there are other mechanisms, not yet active during acute hyponatremia, that help to prevent brain edema when hyponatremia becomes chronic.
Late Mechanisms of Brain Adaptation to Chronic Hyponatremia
It has been shown that after 4 days of hyponatremia, the brain electrolyte content in rats is reduced by 33%, 11%, and 17% for chloride, sodium, and potassium, respectively, but the brain water content only increases by 0.6%.38 The loss of electrolytes during chronic severe hyponatremia does not account for the magnitude of the brain water changes. In other words, the absence of brain edema or the minimal increase in brain water content, despite excessive depletion of ionic electrolytes, suggest that the brain does not behave as a perfect osmometer during chronic hyponatremia. This shows that other osmotically active substances must be taken into account when explaining the minimal brain water changes during chronic hyponatremia. Studies in the early 1990s confirmed that organic osmolytes play a significant role in brain volume regulation during chronic hyponatremia. The importance of organic osmolyte is already evident even after 24 hours.39, 40, 41 Quantitatively, it has been shown that the contribution of electrolytes in brain volume regulation in humans during chronic hyponatremia is approximately 70%, with the remaining 30% as the contribution of organic osmolyte loss.42 Many of the identified organic osmolytes that are lost during chronic hyponatremia also play a role in vital cell functions such as neurotransmission and protein-folding pathways; therefore, their depletion might not be inconsequential and could be related to the neurological abnormalities observed in patients with chronic “asymptomatic” hyponatremia. It is now clear that osmolytes that are lost during chronic hyponatremia reaccumulate upon correction of hyponatremia. At least 2 studies have shown that the reaccumulation of electrolytes occurs faster with correction of hyponatremia, whereas organic osmolytes take much longer (>5 days) to return to baseline levels.43, 44 This observation carries weight because some have suggested that this delayed reaccumulation of organic osmolytes might play a role in the pathophysiology of osmotic demyelination syndrome.45
Hyponatremic Encephalopathy
Hyponatremic encephalopathy (HNE) refers to the neurological dysfunction observed during hyponatremia. The clinical manifestations of HNE are related to the brain adaptation capacities to a hypo-osmotic challenge.
Risk Factors for HNE
The clinical manifestations of HNE are dependent on several factors, including the cause and the magnitude of hyponatremia, sex, age, and rapidity of onset.
The clinical picture of HNE resulting from acute hyponatremia might be different from the symptoms brought on by chronic hyponatremia. Acute hyponatremia with the same magnitude as chronic hyponatremia is likelier to induce more drastic symptoms, such as seizures or coma. During acute hyponatremia, when brain electrolyte content has reached its maximal point of depletion (∼18%), and if organic osmolyte extrusion is not yet complete, brain edema will invariably occur. Clinically, this situation will happen if the SNa drops over a short period of time by a large magnitude. One study reported that if the rate of SNa decrease is <0.5 mEq/l per hour over 24 hours, then the clinical course is likely to be uncomplicated, whereas neurological sequalae and death are more common if the rate of sodium drop is >1 mEq/l per hour.46
HNE is more severe in preadolescents. This simply reflects the fact that most of the dramatic symptoms of HNE are related to brain edema.47, 48 The brain reaches it maximal size by 6 years of age, which is approximately 10 years earlier than the skull, which reaches its maximal size by 16 years of age. Therefore, during hyponatremia, brain edema will be more pronounced in younger individuals because their skulls pose more steric constraints than in adults.
The role of sex in HNE remains unclear. Experimental in vitro studies suggested that estrogens might affect astrocyte brain volume regulation,49 but in vivo studies in rodents did not confirm that there was a different susceptibility to hyponatremia with regard to sex.50, 51 The clinical data regarding female predisposition to HNE are also conflictual because the initial studies by Ayus et al. pointed to a female susceptibility,52, 53 but a large review did not confirm these findings.54
Hypoxia is undoubtedly a worsening factor in HNE; this has been documented by experimental and clinical studies.55, 56 The relationship between hyponatremia and hypoxia is complex. Swelling of the brain parenchyma compresses the brain vasculature and contributes to brain hypoxia that will cause neurogenic pulmonary edema, which, in turn, will reduce oxygen delivery to the brain.57 However, others have shown that the occurrence of severe systemic hypoxia in the setting of severe acute hyponatremia itself is uncommon.58
Symptoms of HNE
The most striking and severe symptoms of HNE are related to the compression of the brain parenchyma against the rigid skull. In severe cases, brain herniation and death often occurs preceded by seizures and coma.59 As discussed earlier, these symptoms often occur during acute and profound hyponatremia because the brain has no or little time to adjust to hypo-osmolality. Severe symptoms can also occur after acute on chronic hyponatremia, or even after moderate acute hyponatremia. For instance, marathon runners with moderate hyponatremia were reported to experience nausea and vomiting, and sometimes acute confusion, which were treated effectively by correction of SNa.60 The largest series of patients studied with HNE revealed that acute severe hyponatremia might be fatal, with abrupt respiratory arrest in up to 60% of cases, but prompt reversal of the prognosis can occur with correction of SNa.61
Chronic hyponatremia will usually manifest as malaise, weakness, and confusion.62 Until the last decade, it was believed that mild chronic hyponatremia was asymptomatic and carried little neurological dysfunction. Studies by our team and others revealed that chronic hyponatremia is associated with significant subtle neurological abnormalities, including attention deficit, falls, and gait imbalance. Patients with chronic hyponatremia have a much higher risk of falls, which seems to more marked in older adult patients.63, 64 Some of these manifestations are reversible upon improvement in SNa.63, 64, 65, 66 Table 1 shows the manifestations of HNE.
Table 1.
Manifestations of hyponatremic encephalopathy
| Acute severe | Chronic |
|---|---|
| Nausea and vomiting | Nausea |
| Headaches | Fatigue |
| Seizures | Gait and attention deficit |
| Coma | Falls and bone fractures |
| Death | |
| Respiratory arrest | |
| Noncardiogenic pulmonary edema |
The physiological basis of chronic HNE is starting to be elucidated. One group recently demonstrated that the extracellular glutamate content in chronic hyponatremic rats was increased, and that the astrocytic glutamate uptake in low sodium medium was also unpaired.67 These results are consistent with the fact that glutamate is a significant organic osmolyte that is extruded from the cell. Because other osmolytes have important functions in brain physiology (e.g., taurine was studied as anticonvulsant in rats), it is likely that the brain depletion of these organic osmolytes might play a role in HNE.
Treatment of HNE
Severe HNE
The criteria for severe HNE includes altered mental status, seizures, focal neurological damage, coma, and other signs or symptoms of brain herniation. Severe HNE is a medical emergency and the treatment should be undertaken promptly. Chronic hyponatremia, if not complicated by an acute episode, rarely presents as severely symptomatic HNE. To date, few life-threatening manifestations have been associated with chronic hyponatremia even if severe; this reflects the almost completed adaptation of the brain during chronic hyponatremia with a negligible brain volume increase.
In all cases, the treatment should aim to reverse severe neurological manifestations that the clinician believes to be secondary to hyponatremia. Therefore, prompt correction of hyponatremia should be undertaken in patients who present with severe symptoms, regardless of the chronicity and the magnitude of the decrease in SNa.68, 69
The mainstay of treatment of HNE is the reduction of intracranial pressure by decreasing brain water content. The neurosurgery literature has suggested that in normonatremic patients with others causes of brain edema, an increase in the plasma sodium of approximately 5 to 6 mEq/l is sufficient to decrease the intracranial pressure by 5 to 10 mm Hg, which would prevent brain herniation.70, 71, 72 Based on these findings, it could be postulated that a similar increase in SNa in patients with hyponatremia would be enough to reduce intracranial pressure to nonlife-threatening levels. This can be achieved by rapid infusion of hypertonic saline. The suggested dose and administration scheme varies according to the different guidelines, but it is well accepted that boluses of 100 to 300 ml of 3% sodium chloride are effective. It should be remembered that the boluses must be repeated until symptoms of brain edema regress. Importantly, frequent sodium monitoring is mandatory.
It has recently been shown that a single dose of enteral urea (15 g) urea can decrease intracranial pressure by up to 8 mm Hg in neurotrauma patients with an intracranial pressure of >15 mm Hg.73
Moderate HNE
The treatment options for mild and moderate HNE depend on the cause of hyponatremia. The cornerstone of treatment is to adjust the numerator or the denominator of Edelman’s equation to restore normal SNa concentrations. In patients with salt depletion from renal or extrarenal causes, replacement of salt stock will normalize SNa. This could be achieved with normal saline infusion or oral salt tablets for salt-depleted patients. Patients with syndrome of inappropriate antiduiretic hormone secretion who have excessive total body water could benefit from water restriction and aquaretics such as V2 receptors antagonist or urea.
Brain Complications Following Treatment of Hyponatremia
Rapid correction of chronic hyponatremia can sometimes lead to neurological damage. From the late 1950s to the early 1970s, several authors described case reports of hyponatremic alcoholic patients who died during treatment and who had necropsy findings consistent with central pontine myelinolysis (CPM). It was not clear at that time if SNa levels per se or if it was rather the correction of serum sodium that was the cause of the neurological deterioration.74, 75, 76 The most definitive evidence of the relationship between correction of chronic hyponatremia and CPM came from experimental studies that showed significant demyelination in rats, rabbits, and dogs after rapid correction of chronic hyponatremia.77, 78, 79 Sterns et al.80, 81, 82 studied patients with severe hyponatremia and found that permanent neurological damage occurred only in patients who had their hyponatremia corrected too rapidly. That publication contrasted with a previous study by Arieff56 who suggested that either acute or chronic hyponatremia should be corrected rapidly to avoid permanent neurological damage.56 The demyelination seen after correction of chronic hyponatremia is now called osmotic demyelination syndrome (ODS). CPM is a more generic term that reflects the loss of myelin in the pons. CPM occurs in several other conditions aside from rapid correction of chronic hyponatremia, and ODS can also affect extrapontine regions. The experimental model of ODS has shed light on the pathophysiology of the disorder.83, 84, 85, 86, 87, 88, 89, 90, 91
ODS is a rarely reported disorder because the disease is rare; however, there is also a possibility that the reporting rate might be lower than the actual prevalence rate because some clinicians might be reluctant to report a potentially preventable complication of hyponatremia treatment. It is also possible that some cases of ODS92, 93 can be clinically silent, and total recovery has been described in some patients.
Risk Factors for ODS
Some risk factors have been associated with development of ODS, but due to the rare nature of the disease, they have not been studied systematically. The most important predisposing factors for ODS are the chronicity of hyponatremia and the final increment of SNa achieved over 24 hours. It is now believed that the 24-hour sodium increment as opposed to the hourly increment of SNa is more important.94, 95 Other risk factors include malnutrition, liver disease, and alcoholism.96 Hypokalemia has also been mentioned in some reports but has not been studied experimentally.97 It should be mentioned that the correction of hypokalemia might increase SNa as predicted by Edelman’s equation. ODS is seldom seen in patients with acute hyponatremia, and experimental evidence has shown that chronicity of hyponatremia is an important prerequisite.98 One of the explanations might be that during acute hyponatremia, the loss of organic osmolytes is less important because they seem to play a protective role in the development of the disease.45
Over the last 4 decades, the cutoff of the sodium increment in 24 hours, at which the correction of chronic hyponatremia is deemed safe, has varied significantly (reviewed by Martin96). The largest series reported in experimental research that addressed the topic of threshold for demyelination lesions revealed that no animal had clinical evidence of neurological impairment of displayed myelin loss at a cutoff of 16 mEq/l per 24 hours.95 However, it should be noted that clinical manifestations of neurological disturbance are difficult to appreciate in animals, and these early experiments did not use the most sensitive techniques for determination of myelin damage. In clinical practice, it is believed that increments of <8 to 10 mEq/l per day are nevertheless associated with low risk of significant symptoms from ODS if there is no concomitant hypokalemia or alcoholism. Although there are still some case reports that describe a diagnosis of ODS after correction of SNa with an increment of <10 mEq/l per 24 hours over a 24-hour period,99, 100 the real increment of SNa might have been underscored because the SNa before the hospital admission and before the initiation of correction is often unknown. Therefore, some patients could have started to self-correct their hyponatremia before they presented to the hospital, which made their real sodium levels higher than reported.
Mechanisms of ODS
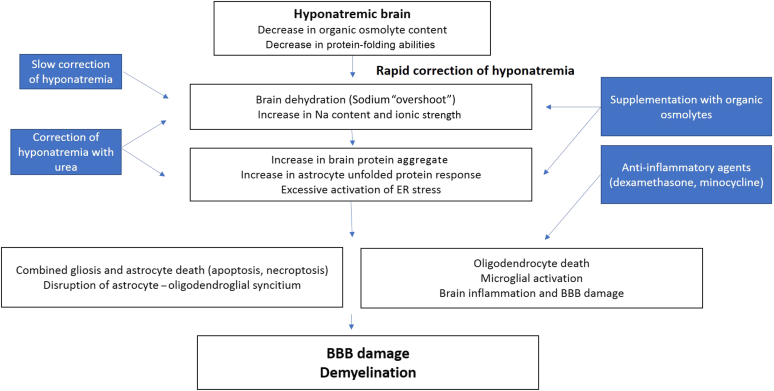
Several factors contribute to the apparition of demyelinative brain lesions upon correction of chronic hyponatremia. It was initially suggested that BBB breakdown allowed invasion of the brain parenchyma with myelinolytic substances, including cytokines and complement factors.90 Microglial activation was also suggested to play a role.87, 88 Recent reports clearly established that one of the first events after rapid correction of chronic hyponatremia is astrocyte damage.83 It was further demonstrated that rapid correction of chronic hyponatremia induces protein aggregation in astrocytes, together with unfolded protein response and an exaggerated endoplasmic reticulum stress that will culminate into astrocyte death.85 These events occur before any histological evidence of myelin damage, and they all take place in regions prone to demyelination. The relationship between astrocyte death and myelin breakdown is explained by the fact that astrocytes provide trophic support to oligodendrocytes, and astrocytes are required for maintenance of normal myelin. Figure 2 illustrates the current knowledge on the pathophysiology of the disease.
Figure 2.
Schematic representation of the pathophysiology of osmotic demyelination after rapid correction of chronic hyponatremia. Therapeutic approaches are depicted in the blue boxes. BBB, blood−brain barrier; ER, endoplasmic reticulum.
Prevention of Brain Edema and Avoidance of ODS in the Treatment of HNE
Strategies to minimize the risk of ODS while treating severe HNE should focus on several goals: (i) identification of patients who need rapid correction of hyponatremia; (ii) identification of patients at risk of ODS; and (iii) minimizing the increment of SNa and achieving the necessary increment to reverse the life-threatening manifestations of HNE (Figure 3).
Figure 3.
Proposed algorithm for the management of hyponatremia with regard to central nervous system. This algorithm is given as a first basis for management and should integrate the particularities of each patient. The most important parameter in determining the need of urgent treatment should be the presence of neurological symptoms attributable to hyponatremia and not the chronicity of hyponatremia or the magnitude of hyponatremia. Chronic hyponatremia with limited neurological symptoms is a risk factor for osmotic demyelination syndrome (ODS) and dictates slow correction of serum sodium, regardless the correction method selected. Acute hyponatremia with no neurological symptoms should not be corrected rapidly because there are many caveats in the evaluation of the duration of hyponatremia, and little is known about the duration that poses a risk for ODS. These limits are based on the current state of the literature, but unpublished evidence suggests that the lower increment is the better. ICU, intensive care unit; Na, sodium; NaCl, sodium chloride.
Depending on the cause of hyponatremia, an increase in SNa can be achieved using several therapeutic agents, including hypertonic saline, normotonic saline, diuretics, urea, or V2 receptor antagonists. The rapidity of onset, the reversibility and the duration of the effect might be different for these agents. For example, hypertonic saline will produce a more brisk increment of SNa than normal saline or urea, and treatment with V2 receptor antagonists could produce a long lasting aquaresis and brisk correction of SNa depending on the half-life and the dose of the chosen agent. Experimental evidence has suggested that the use of urea is less prone to induce ODS compared with hypertonic saline and vaptans.84 It was found that animals treated with urea had minimal experimental demyelinative lesions despite an increment of >25 mEq/l per 24 hours. A follow-up study confirmed that urea decreased the unfolded protein response and endoplasmic reticulum stress in the brain after correction of hyponatremia.85
The recommended scheme for severely symptomatic hyponatremia includes administration of hypertonic saline (100 ml boluses of sodium chloride 3%) until symptoms abate. Patients who need urgent correction of SNa, as mentioned previously, are patients who present with severe symptoms related to brain edema. Although to date no study has looked at the net brain volume decrease brought on by a given increment of SNa, in neurosurgery patients with normonatremia and impending brain herniation from trauma or brain tumor, increasing SNa to approximately 5 to 6 mEq /l is sufficient to lower the intracranial pressure by approximately 10 to 15 mm Hg and prevent brain herniation.70, 72 Therefore, it could be speculated that such a small increment of SNa might be enough to stop the symptoms of brain edema associated with hyponatremia. Sterns et al. have proposed that the SNa should not be corrected by >6 mEq/l in the first 24 hours.101 To date, there are no experimental data or large clinical data to support this recommendation, and experimental studies are still needed to determine if correction of SNa by >6 mEq/l and <10 mEq/l is associated with some neurological impairment.
Accumulating evidences have supported the experimental findings102, 103, 104, 105 that relowering of SNa could prevent or mitigate the disease when rapid correction has been undertaken.106 Relowering of SNa should ideally be performed within 12 to 24 hours of overcorrection and can be achieved using 1-desamino-8-d-arginine vasopressin and dextrose infusion.106 Controlled increased of SNa can be performed by combining 1-desamino-8-d-arginine vasopressin infusion along with hypertonic saline.101, 107
Conclusion and Future Perspectives
The regulation of body fluid osmolality is of a crucial importance, and throughout evolution, this property has been conserved in fish to mammals. The cell volume and macromolecular crowding, and hence, essential biological functions are closely dependent on extracellular tonicity.
Despite being the most common electrolyte disorder in clinical practice, the treatment of hyponatremia still represents a challenge for the clinician, and this has not changed much since back in the 1990. Berl summarized this conundrum by the famous phrase: “Treating hyponatremia, damned if you do and damned if you don’t.”108 If severe and of rapid onset, hyponatremia could induce brain edema with potentially lethal consequences. Mild to moderate hyponatremia has also been associated with various degrees of neurological dysfunction. In contrast, rapid correction of chronic hyponatremia is linked to brain demyelination.
To date, although experimental findings have suggested potential benefit in treating mild hyponatremia on outcomes such as gait and memory,67 there have been no large-scale clinical data suggesting that the treatment of mild to moderate hyponatremia is associated with a better neurological outcome.
Despite the significant amount of research on the topic, there are still several areas of uncertainty, and there is a crucial need of more studies to answer the following questions: How does the brain sense hyponatremia? What is the minimal increment of SNa that will safely prevent brain edema in a severely symptomatic hyponatremia patient, and conversely, how much of an increment of SNa poses a risk of brain damage from ODS? What is the role of the vasopressin receptor antagonist in the prevention of neurological complications of hyponatremia? Hopefully, future research will help to address these issues.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by a grant from FNRS Grant number J0137.14 and a grant from the Fondation Erasme. The authors are indebted to Bruno Couturier and Alain Soupart for their helpful revision of this manuscript. The authors would like to apologize to those researchers whose work could not be cited due to space limitations.
References
- 1.Gennari F.J. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. 1984;310:102–105. doi: 10.1056/NEJM198401123100207. [DOI] [PubMed] [Google Scholar]
- 2.Rasouli M. Basic concepts and practical equations on osmolality: biochemical approach. Clin Biochem. 2016;49:936–941. doi: 10.1016/j.clinbiochem.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Pasantes-Morales H., Lezama R.A., Ramos-Mandujano G., Tuz K.L. Mechanisms of cell volume regulation in hypo-osmolality. Am J Med. 2006;119:S4–S11. doi: 10.1016/j.amjmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Edelman I.S., Leibman J., O'Meara M.P., Birkenfeld L.W. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Investig. 1958;37:1236–1256. doi: 10.1172/JCI103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agre P., Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 6.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 7.Pasantes-Morales H., Korpi E.R., Simo S. Oja–amino acids all along as building blocks of brain and life. Neurochem Res. 2005;30:1463–1464. doi: 10.1007/s11064-005-8822-z. [DOI] [PubMed] [Google Scholar]
- 8.Franco R., Rodriguez R., Pasantes-Morales H. Mechanisms of the ATP potentiation of hyposmotic taurine release in Swiss 3T3 fibroblasts. Pflugers Arch: Eur J Physiol. 2004;449:159–169. doi: 10.1007/s00424-004-1322-1. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann E.K., Lambert I.H., Pedersen S.F. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 10.Pasantes Morales H., Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988;20:503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- 11.Pasantes-Morales H., Maar T.E., Moran J. Cell volume regulation in cultured cerebellar granule neurons. J Neurosci Res. 1993;34:219–224. doi: 10.1002/jnr.490340209. [DOI] [PubMed] [Google Scholar]
- 12.Pasantes-Morales H., Murray R.A., Lilja L., Moran J. Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability. Am J Physiol. 1994;266:C165–C171. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- 13.Pasantes-Morales H., Murray R.A., Sanchez-Olea R., Moran J. Regulatory volume decrease in cultured astrocytes. II. Permeability pathway to amino acids and polyols. Am J Physiol. 1994;266:C172–C178. doi: 10.1152/ajpcell.1994.266.1.C172. [DOI] [PubMed] [Google Scholar]
- 14.Andrew R.D., Labron M.W., Boehnke S.E., Carnduff L., Kirov S.A. Physiological evidence that pyramidal neurons lack functional water channels. Cerebral Cortex. 2007;17:787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- 15.Lange S.C., Bak L.K., Waagepetersen H.S., Schousboe A., Norenberg M.D. Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res. 2012;37:2569–2588. doi: 10.1007/s11064-012-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LoVerso P.R., Wachter C.M., Cui F. Cross-species transcriptomic comparison of in vitro and in vivo mammalian neural cells. Bioinform Biol Insights. 2015;9:153–164. doi: 10.4137/BBI.S33124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberheim N.A., Goldman S.A., Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol (Clifton, NJ) 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verney E.B. Croonian lecture: the antidiuretic hormone and the factors which determine its release. Proc Royal Soc London. Series B, Biol Sci. 1947;135:25–106. [PubMed] [Google Scholar]
- 19.Gradilone S.A., Masyuk A.I., Splinter P.L. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benfenati V., Caprini M., Dovizio M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A. 2011;108:2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo A.O., Ryskamp D.A., Phuong T.T. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J Neuroscience. 2015;35:13525–13537. doi: 10.1523/JNEUROSCI.1987-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mola M.G., Sparaneo A., Gargano C.D. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins. GLIA. 2016;64:139–154. doi: 10.1002/glia.22921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher S.K., Heacock A.M., Keep R.F., Foster D.J. Receptor regulation of osmolyte homeostasis in neural cells. J Physiol. 2010;588:3355–3364. doi: 10.1113/jphysiol.2010.190777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strange K., Emma F., Jackson P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 25.Jalonen T. Single-channel characteristics of the large-conductance anion channel in rat cortical astrocytes in primary culture. GLIA. 1993;9:227–237. doi: 10.1002/glia.440090308. [DOI] [PubMed] [Google Scholar]
- 26.Jackson P.S., Strange K. Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J Gen Physiol. 1995;105:661–676. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel A.J., Lauritzen I., Lazdunski M., Honore E. Disruption of mitochondrial respiration inhibits volume-regulated anion channels and provokes neuronal cell swelling. J Neurosci. 1998;18:3117–3123. doi: 10.1523/JNEUROSCI.18-09-03117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussy N., Deleuze C., Desarmenien M.G., Moos F.C. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 29.Kirk K. Swelling-activated organic osmolyte channels. J Membrane Biol. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- 30.Melton J., Patlak C., Pettigrew K., Cserr H. Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am J Physiol Renal Physiol. 1987;252:F661–F669. doi: 10.1152/ajprenal.1987.252.4.F661. [DOI] [PubMed] [Google Scholar]
- 31.Arieff A.I., Guisado R. Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int. 1976;10:104–116. doi: 10.1038/ki.1976.82. [DOI] [PubMed] [Google Scholar]
- 32.Melton J.E., Nattie E.E. Brain and CSF water and ions during dilutional and isomotic hyponatremia in the rat. Am J Physiol. 1983;244:R724–R732. doi: 10.1152/ajpregu.1983.244.5.R724. [DOI] [PubMed] [Google Scholar]
- 33.Hochwald G.M., Wald A., Malhan C. The sink action of cerebrospinal fluid volume flow. Effect on brain water content. Arch Neurol. 1976;33:339–344. doi: 10.1001/archneur.1976.00500050025005. [DOI] [PubMed] [Google Scholar]
- 34.Nattie E.E., Edwards W.H. Brain and CSF water in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol. 1981;51:1086–1091. doi: 10.1152/jappl.1981.51.5.1086. [DOI] [PubMed] [Google Scholar]
- 35.Holliday M.A., Kalyci M.N., Harrah J. Factors that limit brain volume changes in response to acute and sustained hyper- and hyponatremia. J Clin Invest. 1968;47:1916–1928. doi: 10.1172/JCI105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arieff A.I., Llach F., Massry S.G. Neurological manifestations and morbidity of hyponatremia. Correlation with brain water and electrolytes. Medicine. 1976;55:121–129. doi: 10.1097/00005792-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Helwig F.C., Schutz C.B., Curry D.E. Water intoxication. Report of a fatal human case, with clinical, pathologic and experimenal studies. JAMA. 1935;104:1569–1575. [Google Scholar]
- 38.Verbalis J.G., Drutarosky M.D. Adaptation to chronic hypoosmolality in rats. Kidney Int. 1988;34:351–360. doi: 10.1038/ki.1988.188. [DOI] [PubMed] [Google Scholar]
- 39.Thurston J.H., Hauhart R.E., Nelson J.S. Adaptive decreases in amino acids (taurine in particular), creatine, and electrolytes prevent cerebral edema in chronically hyponatremic mice: rapid correction (experimental model of central pontine myelinolysis) causes dehydration and shrinkage of brain. Metab Brain Dis. 1987;2:223–241. doi: 10.1007/BF00999694. [DOI] [PubMed] [Google Scholar]
- 40.Verbalis J.G., Gullans S.R. Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res. 1991;567:274–282. doi: 10.1016/0006-8993(91)90806-7. [DOI] [PubMed] [Google Scholar]
- 41.Malhotra I., Gopinath S., Janga K.C. Unpredictable nature of tolvaptan in treatment of hypervolemic hyponatremia: case review on role of vaptans. Case Rep Endocrinol. 2014:4. doi: 10.1155/2014/807054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterns R.H. The management of symptomatic hyponatremia. Semin Nephrol. 1990;10:503–514. [PubMed] [Google Scholar]
- 43.Lien Y.H., Shapiro J.I., Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest. 1991;88:303–309. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbalis J.G., Gullans S.R. Rapid correction of hyponatremia produces differential effects on brain osmolyte and electrolyte reaccumulation in rats. Brain Res. 1993;606:19–27. doi: 10.1016/0006-8993(93)91564-9. [DOI] [PubMed] [Google Scholar]
- 45.Silver S.M., Schroeder B.M., Sterns R.H., Rojiani A.M. Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. J Neuropathol Exp Neurol. 2006;65:37–44. doi: 10.1097/01.jnen.0000195938.02292.39. [DOI] [PubMed] [Google Scholar]
- 46.Cluitmans FHM, Meinders AE. Management of severe hyponatremia: rapid or slow correction? Am J Med. 88:161−166. [DOI] [PubMed]
- 47.Ayus J.C., Achinger S.G., Arieff A. Brain cell volume regulation in hyponatremia: role of gender, age, vasopressin and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 48.Halberthal M., Halperin M.L., Bohn D. Acute hyponatraemia in children admitted to hospital: retrospective analysis of factors contributing to its development and resolution. BMJ. 2001;322:780–782. doi: 10.1136/bmj.322.7289.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraser C.L., Swanson R.A. Female sex hormones inhibit volume regulation in rat brain astrocyte culture. Am J Physiol Cell Physiol. 1994;267:C909–C914. doi: 10.1152/ajpcell.1994.267.4.C909. [DOI] [PubMed] [Google Scholar]
- 50.Silver S.M., Schroeder B.M., Bernstein P., Sterns R.H. Brain adaptation to acute hyponatremia in young rats. Am J Physiol. 1999;276:R1595–R1599. doi: 10.1152/ajpregu.1999.276.6.r1595. [DOI] [PubMed] [Google Scholar]
- 51.Verbalis J. Hyponatremia induced by vasopressin or desmopressin in female and male rats. J Am Soc Nephrol. 1993;3:1600–1606. doi: 10.1681/ASN.V391600. [DOI] [PubMed] [Google Scholar]
- 52.Ayus J.C., Krothapalli R.K., Arieff A.I. Sexual differences in survival with severe symptomatic hyponatremia (abstract) Kidney Int. 1988;34:180A. [Google Scholar]
- 53.Ayus J.C., Wheeler J.M., Arieff A.I. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992;117:891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- 54.Wijdicks E.F., Larson T.S. Absence of postoperative hyponatremia syndrome in young, healthy females. Ann Neurol. 1994;35:626–628. doi: 10.1002/ana.410350520. [DOI] [PubMed] [Google Scholar]
- 55.Ayus J.C., Armstrong D., Arieff A. Hyponatremia with hypoxia: effects on brain adaptation, perfusion, and histology in rodents. Kidney Int. 2006;69:1319–1325. doi: 10.1038/sj.ki.5000187. [DOI] [PubMed] [Google Scholar]
- 56.Arieff A.I. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314:1529–1535. doi: 10.1056/NEJM198606123142401. [DOI] [PubMed] [Google Scholar]
- 57.Vexler Z.S., Ayus J.C., Roberts T.P. Hypoxic and ischemic hypoxia exacerbate brain injury associated with metabolic encephalopathy in laboratory animals. J Clin Invest. 1994;93:256–264. doi: 10.1172/JCI116953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soupart A., Penninckx R., Stenuit A., Decaux G. Lack of major hypoxia and significant brain damage in rats despite dramatic hyponatremic encephalopathy. J Lab Clin Med. 1997;130:226–231. doi: 10.1016/s0022-2143(97)90100-1. [DOI] [PubMed] [Google Scholar]
- 59.Arieff A.I., Llach F., Massry S.G. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine (Baltimore) 1976;55:121–129. doi: 10.1097/00005792-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Hew T.D., Chorley J.N., Cianca J.C., Divine J.G. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin J Sport Med. 2003;13:41–47. doi: 10.1097/00042752-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Ayus J.C., Krothapalli R.K., Arieff A.I. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317:1190–1195. doi: 10.1056/NEJM198711053171905. [DOI] [PubMed] [Google Scholar]
- 62.Videen J.S., Michaelis T., Pinto P., Ross B.D. Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest. 1995;95:788–793. doi: 10.1172/JCI117728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renneboog B., Musch W., Vandemergel X., Manto M.U., Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:e1–8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 64.Renneboog B., Sattar L., Decaux G. Attention and postural balance are much more affected in older than in younger adults with mild or moderate chronic hyponatremia. Eur J Intern Med. 2017;41:e25–e26. doi: 10.1016/j.ejim.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Tachi T., Yokoi T., Goto C. Hyponatremia and hypokalemia as risk factors for falls. Eur J Clin Nutr. 2015;69:205–210. doi: 10.1038/ejcn.2014.195. [DOI] [PubMed] [Google Scholar]
- 66.Gunathilake R., Oldmeadow C., McEvoy M. Mild hyponatremia is associated with impaired cognition and falls in community-dwelling older persons. J Am Geriatr Soc. 2013;61:1838–1839. doi: 10.1111/jgs.12468. [DOI] [PubMed] [Google Scholar]
- 67.Fujisawa H., Sugimura Y., Takagi H. Chronic hyponatremia causes neurologic and psychologic impairments. J Am Soc Nephrol. 2016;27:766–780. doi: 10.1681/ASN.2014121196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoorn E.J., Zietse R. Diagnosis and treatment of hyponatremia: compilation of the guidelines. J Am Soc Nephrol. 2017;28:1340–1349. doi: 10.1681/ASN.2016101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagler E.V., Vanmassenhove J., van der Veer S.N. Diagnosis and treatment of hyponatremia: a systematic review of clinical practice guidelines and consensus statements. BMC Med. 2014;12:1. doi: 10.1186/s12916-014-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bentsen G., Breivik H., Lundar T., Stubhaug A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit Care Med. 2006;34:2912–2917. doi: 10.1097/01.CCM.0000245665.46789.7C. [DOI] [PubMed] [Google Scholar]
- 71.Tseng M.Y., Al-Rawi P.G., Czosnyka M. Enhancement of cerebral blood flow using systemic hypertonic saline therapy improves outcome in patients with poor-grade spontaneous subarachnoid hemorrhage. J Neurosurg. 2007;107:274–282. doi: 10.3171/JNS-07/08/0274. [DOI] [PubMed] [Google Scholar]
- 72.Koenig M.A., Bryan M., Lewin J.L., 3rd Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–1029. doi: 10.1212/01.wnl.0000304042.05557.60. [DOI] [PubMed] [Google Scholar]
- 73.Annoni F., Fontana V., Brimioulle S. Early effects of enteral urea on intracranial pressure in patients with acute brain injury and hyponatremia. J Neurosurg Anesthesiol. 2017;29:400–405. doi: 10.1097/ANA.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 74.Adams R.D., Victor M., Mancall E.L. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959;81:154–172. [PubMed] [Google Scholar]
- 75.Tomlinson B.E., Pierides A.M., Braddley W.G. Central pontine myelinolysis. Am J Med. 1968;45:373–386. [PubMed] [Google Scholar]
- 76.Tomlinson B.E., Pierides A.M., Bradley W.G. Central pontine myelinolysis: two cases with associated electrolyte disturbance. Quart J Med. 1976;45:373–386. [PubMed] [Google Scholar]
- 77.Kleinschmidt-DeMasters B.K., Norenberg M.D. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science. 1981;211:1068–1070. doi: 10.1126/science.7466381. [DOI] [PubMed] [Google Scholar]
- 78.Kleinschmidt-DeMasters B.K., Norenberg M.D. Neuropathologic observations in electrolyte-induced myelinolysis in the rat. J Neuropathol Exp Neurol. 1982;41:67–80. doi: 10.1097/00005072-198201000-00007. [DOI] [PubMed] [Google Scholar]
- 79.Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol. 1983;13:232–242. doi: 10.1002/ana.410130303. [DOI] [PubMed] [Google Scholar]
- 80.Sterns R.H., Riggs J.E., Schochet S.S., Jr Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314:1535–1542. doi: 10.1056/NEJM198606123142402. [DOI] [PubMed] [Google Scholar]
- 81.Sterns R.H. Severe symptomatic hyponatremia: treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107:656–664. doi: 10.7326/0003-4819-107-5-656. [DOI] [PubMed] [Google Scholar]
- 82.Sterns R.H. Neurological deterioration following treatment for hyponatremia. Am J Kidney Dis. 1989;13:434–437. doi: 10.1016/s0272-6386(89)80029-0. [DOI] [PubMed] [Google Scholar]
- 83.Gankam Kengne F. Astrocytes are an early target in osmotic demyelination syndrome. J Am Soc Nephrol. 2011;22:1834–1845. doi: 10.1681/ASN.2010111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gankam Kengne F., Couturier B.S., Soupart A., Decaux G. Urea minimizes brain complications following rapid correction of chronic hyponatremia compared with vasopressin antagonist or hypertonic saline. Kidney Int. 2015;87:323–331. doi: 10.1038/ki.2014.273. [DOI] [PubMed] [Google Scholar]
- 85.Gankam-Kengne F., Couturier B.S., Soupart A., Brion J.P., Decaux G. Osmotic stress-induced defective glial proteostasis contributes to brain demyelination after hyponatremia treatment. J Am Soc Nephrol. 2017;28:1802–1813. doi: 10.1681/ASN.2016050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugimura Y., Murase T., Takefuji S. Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol. 2005;192:178–183. doi: 10.1016/j.expneurol.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Takefuji S., Murase T., Sugimura Y. Role of microglia in the pathogenesis of osmotic-induced demyelination. Exp Neurol. 2007;204:88–94. doi: 10.1016/j.expneurol.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 88.Iwama S., Sugimura Y., Suzuki H. Time-dependent changes in proinflammatory and neurotrophic responses of microglia and astrocytes in a rat model of osmotic demyelination syndrome. GLIA. 2011;59:452–462. doi: 10.1002/glia.21114. [DOI] [PubMed] [Google Scholar]
- 89.Adler S., Verbalis J.G., Meyers S. Changes in cerebral blood flow and distribution associated with acute increases in plasma sodium and osmolality of chronic hyponatremic rats. Exp Neurol. 2000;163:63–71. doi: 10.1006/exnr.2000.7376. [DOI] [PubMed] [Google Scholar]
- 90.Baker E.A., Tian Y., Adler S., Verbalis J.G. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp Neurol. 2000;165:221–230. doi: 10.1006/exnr.2000.7474. [DOI] [PubMed] [Google Scholar]
- 91.Verbalis J.G., Martinez A.J. Neurological and neuropathological sequelae of correction of chronic hyponatremia. Kidney Int. 1991;39:1274–1282. doi: 10.1038/ki.1991.161. [DOI] [PubMed] [Google Scholar]
- 92.Shah S.O., Wang A., Mudambi L., Ghuznavi N., Fekete R. Asymptomatic central pontine myelinolysis: a case report. Case Rep Neurol. 2012;4:167–172. doi: 10.1159/000345225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Newell K.L., Kleinschmidt-DeMasters B.K. Central pontine myelinolysis at autopsy; a twelve year retrospective analysis. J Neurol Sci. 1996;142:134–139. doi: 10.1016/0022-510x(96)00175-x. [DOI] [PubMed] [Google Scholar]
- 94.Soupart A., Penninckx R., Stenuit A., Perier O., Decaux G. Treatment of chronic hyponatremia in rats by intravenous saline: comparison of rate versus magnitude of correction. Kidney Int. 1992;41:1662–1667. doi: 10.1038/ki.1992.239. [DOI] [PubMed] [Google Scholar]
- 95.Verbalis J.G., Martinez A.J. Determinants of brain myelinolysis following correction of chronic hyponatremia in rats. Vasopressin. 1991;208:539–547. [Google Scholar]
- 96.Martin R.J. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. Neurol Pract. 2004;75 Suppl 3 doi: 10.1136/jnnp.2004.045906. iii22−8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lohr J.W. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med. 1994;96:408–413. doi: 10.1016/0002-9343(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 98.Norenberg M.D., Papendick R.E. Chronicity of hyponatremia as a factor in experimental myelinolysis. Ann Neurol. 1984;15:544–547. doi: 10.1002/ana.410150606. [DOI] [PubMed] [Google Scholar]
- 99.Omari A., Kormas N., Field M. Delayed onset of central pontine myelinolysis despite appropriate correction of hyponatraemia. Intern Med J. 2002;32:273–274. doi: 10.1046/j.1445-5994.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- 100.Koul P.A., Khan U.H., Jan R.A. Osmotic demyelination syndrome following slow correction of hyponatremia: possible role of hypokalemia. Indian J Crit Care Med. 2013;17:231–233. doi: 10.4103/0972-5229.118433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sterns R.H., Hix J.K., Silver S. Treating profound hyponatremia: a strategy for controlled correction. Am J Kidney Dis. 2010;56:774–779. doi: 10.1053/j.ajkd.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 102.Soupart A., Penninckx R., Stenuit A., Perier O., Decaux G. Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol. 1996;55:594–601. doi: 10.1097/00005072-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Gankam Kengne F., Soupart A., Pochet R., Brion J.P., Decaux G. Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int. 2009;76:614–621. doi: 10.1038/ki.2009.254. [DOI] [PubMed] [Google Scholar]
- 104.Soupart A., Ngassa M., Decaux G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol. 1999;51:383–386. [PubMed] [Google Scholar]
- 105.Soupart A., Penninckx R., Crenier L. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45:193–200. doi: 10.1038/ki.1994.23. [DOI] [PubMed] [Google Scholar]
- 106.Perianayagam A., Sterns R.H., Silver S.M. DDAVP is effective in preventing and reversing inadvertent overcorrection of hyponatremia. Clin J Am Soc Nephrol. 2008;3:331–336. doi: 10.2215/CJN.03190807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sood L., Sterns R.H., Hix J.K., Silver S.M., Chen L. Hypertonic saline and desmopressin: a simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis. 2013;61:571–578. doi: 10.1053/j.ajkd.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 108.Berl T. Treating hyponatremia: damned if we do and damned if we don't. Kidney Int. 1990;37:1006–1018. doi: 10.1038/ki.1990.78. [DOI] [PubMed] [Google Scholar]