Abstract
Introduction
Melioidosis causes sepsis and death in the Top End of Northern Australia during the monsoonal wet season. Dialysis-dependent adults suffer higher melioidosis rates compared to low rates among renal transplant patients who routinely receive trimethoprim+sulfamethoxazole prophylaxis.
Methods
We performed a prospective interventional study to determine the efficacy and safety of daily trimethoprim+sulfamethoxazole prophylaxis in hemodialysis patients during the wet season, from 1 November 2014 to 30 April 2015. Hemodialysis (for ≥ 3 months) patients ≥ 18 years of age were offered treatment. A total of 269 patients on hemodialysis were eligible. Eight of the 269 patients (3%) were excluded from the analysis for being on melioidosis treatment. In all, 169 of 261 patients (64.8%) received the prophylaxis, and 92 of 261 patients (35.2%) did not, because of allergy history (n = 10), remoteness and logistical reasons (n = 60), poor dialysis attendance (n = 11), and refusal (n = 11). We monitored for clinical side effects 3 times weekly and neutropenia, thrombocytopenia, and liver function monthly throughout treatment and for 2 months posttreatment.
Results
In all, 169 of 261 patients (64.8%) received the prophylaxis. There was no age (years) difference by group (prophylaxis vs. nonprophylaxis, 54.7 [11.3] vs. 54.3 [11.2] [P = 0.751]). Sixteen of 261 patients (6%) had melioidosis. The event frequency was 0% (0/169, prophylaxis, vs. 17.4% [16/92, nonprophylaxis], P < 0.001). Higher thrombocytopenia and neutropenia rates were noted in the prophylaxis group. These did not warrant treatment stoppage. There was no difference in liver function. Three patients (1.8%) withdrew from the treatment because of side effects.
Conclusion
Daily dosing was effective and safe. Posthemodialysis dosing in the subsequent seasons was effective and safer. We recommend this approach in melioidosis-prevalent regions.
Keywords: hemodialysis, melioidosis, northern Australia, sepsis, trimethoprim+sulfamethoxazole, wet season
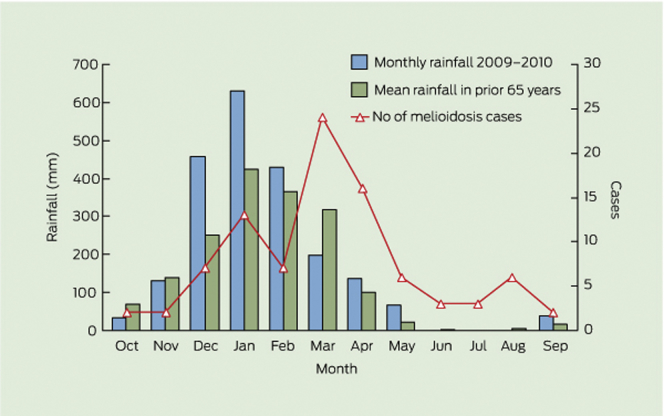
Melioidosis causes severe sepsis and death in the Top End of Northern Australia during the monsoonal wet season.1 The wet season (melioidosis season) is defined to capture the seasonal presentation in the tropical wet season (November to April),2 with average monthly rainfalls of 100 to 500 mm in the 6 months (Figures 1 and Supplementary Figure S1) and high humidity of > 80%.3 Melioidosis is caused by the saprophytic Gram-negative bacterium and Tier 1 select agent Burkholderia pseudomallei, which naturally occur in tropical soil and water.4 Burkholderia pseudomallei is widespread in Northern Australia and Southeast Asia and is increasingly recognized as being endemic in other tropical regions globally.1, 4, 5, 6 The Darwin Prospective Melioidosis Study (DPMS) is a long-running, large, prospective observational study started in October 1989 that aims to understand the clinical and microbiological aspects of melioidosis in the Top End of the Northern Territory (NT), and to use this information to lessen the burden of the disease through earlier diagnosis and improved treatment. The study has documented all cases of melioidosis in the Top End of the NT since 1 October 1989,7, 8 with around 85% of cases occurring during the tropical wet season (November to April)2 (Figure 1).
Figure 1.
Correlation between cases of melioidosis managed at Royal Darwin Hospital in 2009–2010 and rainfall at Darwin airport.
Reproduced with permission from Parameswaran U, Baird RW, Ward LM, et al. Melioidosis at Royal Darwin Hospital in the big 2009–2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196:345–348. Copyright © 2012 The Medical Journal of Australia.
Chronic kidney disease (CKD) is an independent risk factor for melioidosis, and CKD is associated with a higher mortality rate whenever melioidosis occurs.9, 10 Other factors associated with high risk for melioidosis include diabetes mellitus, hazardous alcohol use, chronic lung disease, rheumatic heart disease and cardiac failure, and immune-suppressive medications, most notably the use of corticosteroids. Age and indigenous ethnicity are also independent predictors for melioidosis. These factors are also common among adult patients dependent on dialysis in this region.11 In our region, we have previously reported staggering higher incidence rates of melioidosis among adults dependent on dialysis than among those without dialysis-dependent CKD (988.8/100,000 vs. 24.0/100,000 patient-years), equating to a crude relative risk for melioidosis among adults dependent on dialysis of 38.4 (95% confidence interval [CI] = 25.7–57.5).11
As observed in some previous wet seasons, during the 2011 to 2012 wet season, we observed a higher frequency of melioidosis among the dialysis cohort.12 Rates of melioidosis are lower among our renal transplant cohort. Our routine practice to specifically mitigate wet season−associated melioidosis for the renal transplant and immunosuppressed cohort includes consideration of trimethoprim+sulfamethoxazole (TMP+SMX) prophylaxis treatment, at a dose higher than usually used for Pneumocystis jirovecii pneumonia (PJP) prophylaxis.13 Pharmacodynamic and pharmacokinetic studies of TMP+SMX for the treatment of melioidosis indicate that high doses of oral TMP+SMX are required for eradication after an initial intensive treatment with i.v. ceftazidime or meropenem.10, 14, 15, 16, 17, 18 There are no published data on the prophylactic use of TMP+SMX (or any other antibiotics) to reduce melioidosis in high-risk groups, although TMP+SMX has been used as postexposure prophylaxis for Burkholderia pseudomallei infection among laboratory staff.19 Therefore, following the increase in both the number of melioidosis cases observed in the 2011 to 2012 wet season, and the concomitant increase in the size of the prevalent dialysis patient cohort, we undertook a prospective open-label intervention by implementing a prophylaxis guideline for hemodialysis patients in the Top End of the Northern Territory over the wet season (1 November 2014 to 30 April 2015), using oral trimethoprim+sulfamethoxazole (TMP+SMX), 160/800 mg daily.
The aim of this study was to determine the efficacy and safety of prophylaxis with daily TMP+SMX for melioidosis in hemodialysis patients from the Top End of Northern Australia during the wet season from 1 November 2014 to 30 April 2015.
Materials and Methods
Study Design
The study was a prospective, open-label, interventional study carried out as part of the larger Darwin Prospective Melioidosis Study, which documents all cases of melioidosis and treatment in the Top End of the Northern Territory.20
Study Population
All patients ≥18 years of age who had been on maintenance hemodialysis for ≥3 months were offered the prophylactic treatment. All eligible hemodialysis patients throughout the Top End received daily TMP+SMX, excluding persons with known hypersensitivity to trimethoprim and/or sulfamethoxazole, lipoamides, or any other ingredients in the formulations of the tablets, severe hepatic failure, marked liver parenchymal damage or jaundice, or serious hematological disorders (thrombocytopenia < 80,000 platelets/μl, leukopoenia < 3.5 × 109/l (neutrophil count < 2.7 × 109), and porphyria, and any other contraindications to TMP+SMX. Those who declined the treatment were also excluded from the prophylaxis treatment. The cohort could therefore be described categorically as those who received the intervention and a control group of those who were ineligible for the intervention (or TMP+SMX-group [prophylaxis] vs. nonprophylaxis group).
All patients received the usual wet season advice on melioidosis prevention.14
Definitions
Patients’ ethnicity was entered as indigenous if they were Aboriginal and/or Torres Strait Islander in their demographical data entry in the clinical records. Abnormal liver function was defined by any rise in liver transaminases and bilirubin. Neutropenia was defined as a neutrophil count of <2.7 × 109/l and thrombocytopenia as a platelet count of <150,000 platelets/μl. For the purpose of assessing safety, we also defined categories of thrombocytopenia, based on the protocol that we have developed with platelet counts of <80,000, ≥80,000 to <150,000, and ≥150 000, and based on the conventional definition of platelet counts by severity of <50,000, ≥50,000 to <150,000, and ≥150 000.
Dosing of Trimethoprim+Sulfamethoxazole
There were no guidelines for dosing of TMP+SMX for prophylaxis of melioidosis in hemodialysis patients, so we initially used the standard dosage that has been safely used as eradication treatment for persons on a dialysis dose of 1 double-strength tablet of TMP+SMX 160 mg/800 mg once a day.21 On dialysis days, the patients received the drug after dialysis. All patients on the prophylaxis also received folic acid 5 mg once a day to avoid TMP+SMX−induced folate deficiency.
Treatment Rollout
All 261 patients who were eligible and were not receiving treatment for melioidosis were offered treatment. The medication rollout was undertaken from 1 November 2014, with the last patient receiving the first dose on 11 January 2015.
Clinical Safety and Laboratory Monitoring
The majority of our patients receive hemodialysis within satellite dialysis units at least 3 times a week, achieving a minimum of 12 hours of treatment per week,22 and have blood tests performed monthly (at the start of every month or whenever clinically indicated) as part of routine care. The renal pharmacist provided in-services to primary dialysis nurses across all dialysis units, who then routinely asked questions of each patient pertaining to any medication complications. Patients were asked to report signs of nausea, vomiting, and skin reactions at each dialysis session and whenever they attended a nephrologist’s clinic appointment. Patients concurrently were specifically monitored for the development of neutropenia, thrombocytopenia, and abnormal liver function at each monthly blood test throughout the treatment phase and for another 2 months after completion.
Statistical Analysis
A descriptive analysis was undertaken. Data are described as frequency and percentage for categorical variables, and continuous data were reported as mean (SD) with 95% CIs for normally distributed data and as median (interquartile range) for data that were not normally distributed. For comparisons, we used a 2-sample Student t test for continuous normally distributed data and the Mann−Whitney U test for non−normally distributed data. Comparisons between categorical data were performed using the χ2 and Fisher exact tests as appropriate. Data were analyzed using intention-to-treat analysis. Statistical significance was determined by a 2-tailed P value of < 0.05 and 95% CIs where appropriate.
Treatment Cessation
The treatment was stopped in > 95% of patients on 30 April 2015. In the rest of the patients, treatment was stopped within a few days after 30 April 2015.
Ethical Considerations
The Darwin Prospective Melioidosis Study is a prospective study approved by the Human Research Ethics Committee of the Northern Territory Department of Health and the Menzies School of Health Research (Approval # HREC 02/38).20
Results
Baseline Characteristics and Risks of Melioidosis
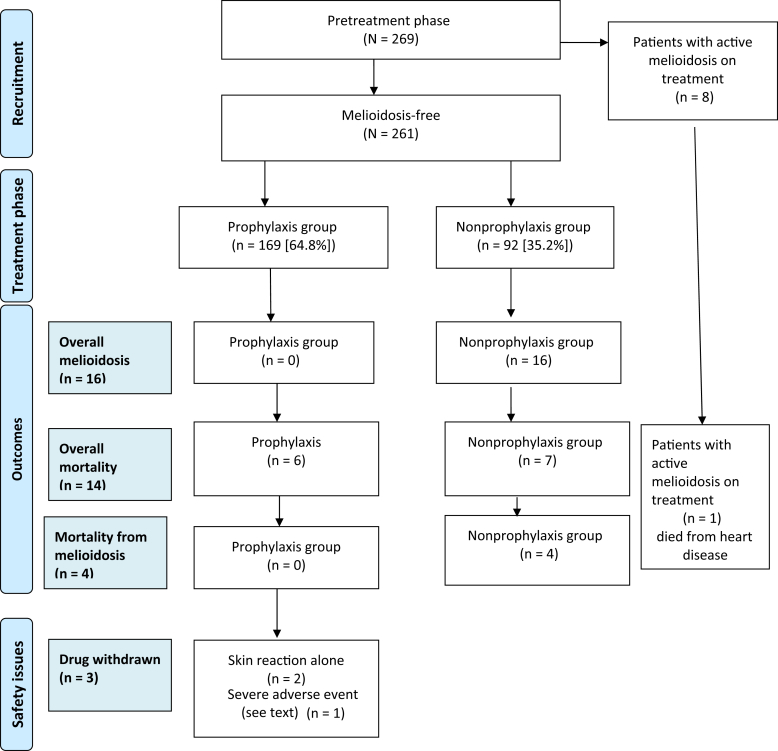
A total of 269 patients were receiving maintenance hemodialysis on 1 November 2014. Eight patients (3%) developed melioidosis and were already receiving appropriate therapy prior to the commencement of the prophylaxis intervention, and were thus excluded from this analysis. These 8 patients received the minimum 2-week intensive treatment with i.v. antibiotics (ceftazidime or meropenem), followed by the 3-month eradication therapy with TMP+SMX. They then continued on prophylaxis (dose similar to that of the eradication therapy) to the end of the wet season, and were not included in the overall analysis (Figure 2).
Figure 2.
Melioidosis intervention study flow diagram.
The analysis describes the remaining 261 patients (97%), divided into the TMP+SMX prophylaxis group (who received prophylaxis, n = 169 [64.8%]) and the nonprophylaxis group (who did not receive prophylaxis, n = 92 [35.2%]). Of the 92 patients who did not receive the prophylaxis (92/262 [35.2%]), the reasons were history of allergy to the drug (n = 10), remoteness and other logistical reasons (n = 60), poor attendance for dialysis (n = 11), and refusal of treatment (n = 11). Overall, the main comorbidities included diabetes (66.7%), history of cardiovascular disease (70.5%; including cardiac arrhythmias, ischemic heart disease, congestive cardiac failure, and rheumatic heart disease), hypertension (66.7%), and chronic lung disease (36.8%). None of the 269 patients were on any immunosuppressive therapy.
There was no significant difference in the age of participants by group (TMP+SMX-prophylaxis group vs. nonprophylaxis group, 54.7 [11.3] years vs. 54.3 [11.2] years; P = 0.751). The proportion of indigenous patients in each group was also similar at ∼ 85% There was no statistically significant difference in the baseline platelet counts (180.8 [76.7], 95% CI = 156.0−205.7, vs. 178.4 [75.2], 95% CI = 154.0−202.8, P = 0.898) and neutrophil counts (5.0 [2.2], 95% CI = 4.2−5.7, vs. 5.9 [2.3], 95% CI = 5.1−6.7, P = 0.205) at the start of the study between the groups. Table 1 provides the details of the comparison in the baseline characteristics and the risks of melioidosis between the 2 groups.
Table 1.
Comparison of baseline characteristics and risk factors for melioidosis between the TMP+SMX prophylaxis group and the nonprophylaxis group
| Comorbidity | Prophylaxis (n = 169) n (%) |
Nonprophylaxis (n = 92) n (%) |
P value |
|---|---|---|---|
| Gender (male) | 94 (55.6) | 52 (56.5) | 0.897 |
| Ethnicity (indigenous) | 143 (84.6) | 78 (84.7) | 1.000 |
| Diabetes mellitus | 113 (66.9) | 61 (66.3) | 0.728 |
| Ischemic heart disease/heart failure | 119 (70.4) | 65 (70.7) | 0.840 |
| Chronic obstructive pulmonary disease | 62 (36.7) | 34 (37.0) | 0.635 |
| Chronic liver disease | 6 (3.6) | 4 (4.3) | 0.670 |
| Atrial fibrillation | 104 (61.5) | 56 (60.9) | 0.648 |
| Dyslipidemia | 118 (69.8) | 66 (71.7) | 0.216 |
| Hypertension | 114 (67.5) | 63 (68.5) | 0.917 |
| Thyroid disease | 7 (4.1) | 4 (4.3) | 1.000 |
| History of latent tuberculosis | 5 (3.0) | 3 (3.3) | 0.157 |
| Obesity | 8 (4.7) | 5 (5.4) | 0.517 |
| Pulmonary hypertension | 6 (3.6) | 4 (4.3) | 0.768 |
| Rheumatic heart disease | 11 (6.5) | 9 (9.7) | 0.547 |
| Systematic lupus erythematosus | 4 (2.4) | 2 (2.2) | 0.270 |
| Secondary hyperparathyroidism | 12 (7.1) | 8 (8.7) | 0.768 |
| Dialysis adequacy | |||
| KT/V > 1.4 | 150 (88.8) | 82 (89.1) | 0.675 |
| URR > 70 | 152 (90.0) | 81 (88.0) | 0.586 |
URR, urea reduction ratio.
Adherence to Treatment
Of the 169 patients who received the treatment, 159 patients (94%) received the full treatment through the study period. Of the remaining 10 patients (5.9%), 7 patients (4.1%) received treatment at least 2 times a week mainly due to missing some dialysis sessions, and 3 patients (1.8%) stopped the treatment due to adverse drug reactions.
Efficacy of TMP+SMX Prophylaxis and Cases of Melioidosis
During the period from 1 November 2014 to 30 April 2015, a total of 16 documented cases of culture-positive melioidosis (6%) were observed among the 261 hemodialysis patients eligible for this analysis. This corresponds to a melioidosis event frequency of 0% in the TMP+SMX prophylaxis group and 17.4%in the nonprophylaxis group (0/169 vs. 16/92, P < 0.001).
Mortality
Thirteen patients (5%) died during the 6-month period of the treatment. Six of 169 patients (3.6%) were from the TMP+SMX prophylaxis group, and 7 of 92 patients (7.6%) were from the nonprophylaxis group (P = 0.231). One patient among the 8 patients who were receiving treatment for melioidosis at the time of starting the study died of complications related to a myocardial infarction but had completely recovered from the infection at the time of death. The 6 patients in the TMP+SMX prophylaxis group died of complications related to cardiovascular disease.
In the nonprophylaxis group, 4 of the 7 patients died of melioidosis, and the other 3 patients died of cardiovascular complications. The remaining 12 patients who had melioidosis (75%) were successfully treated as per protocol.16, 17
Safety of the Trimethoprim/Sulfamethoxazole Prophylaxis
There was a higher incidence rate of non−clinically significant thrombocytopenia noted in the TMP+SMX-prophylaxis group (Tables 2 and 3); however, the majority of cases were not severe enough to warrant the withdrawal of treatment. Three patients (1.8%) were withdrawn from the TMP+SMX-prophylaxis group due to significant side effects. These patients included 1 patient with drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) and thrombocytopenia and 2 patients with skin rash.
Table 2.
Comparison between the 2 groups of the proportion of patients in the categories with platelet count of 50,000/μl as the minimum during the study and 1 month after stopping treatment
| Montha | Platelet count categoryb | Group |
P valuec | |
|---|---|---|---|---|
| Prophylaxis n (%) | Nonprophylaxis n (%) | |||
| 1 | 1 | 2 (1.0) | 2 (2.2) | 0.613 |
| 2 | 72 (42.8) | 19 (20.6) | ||
| 3 | 95 (56.2) | 71 (77.2) | ||
| 2 | 1 | 2 (1.2) | 2 (2.2) | 0.613 |
| 2 | 69 (40.8) | 16 (17.4) | ||
| 3 | 98 (58.0) | 74 (80.4) | ||
| 3 | 1 | 4 (2.4) | 2 (2.2) | 1.000 |
| 2 | 68 (40.2) | 15 (16.3) | ||
| 3 | 97 (57.4) | 74 (81.5) | ||
| 4 | 1 | 3 (1.8) | 2 (2.2) | 1.000 |
| 2 | 67 (40.2) | 14 (16.3) | ||
| 3 | 97 (58.0) | 72 (81.5) | ||
| 5 | 1 | 3 (1.8) | 1 (1.1) | 1.000 |
| 2 | 54 (33.1) | 19 (21.7) | ||
| 3 | 107 (65.1) | 66 (77.2) | ||
| 6d | 1 | 4 (2.4) | 1 (1.1) | 0.660 |
| 2 | 49 (30.2) | 15 (17.4) | ||
| 3 | 110 (67.5) | 69 (81.5) | ||
There was no statistically significant difference in platelet counts at baseline between the 2 groups.
Month after start of treatment.
Platelet count categories (per μl): 1: ≤ 50,000; 2: 51,000−150,000; 3: ≥150,000.
Fisher exact test.
One month after stopping treatment.
Table 3.
Comparison between the 2 groups of the proportion of patients in categories with platelet count of 80,000/μl as the minimum during the study and 1 month after stopping treatment
| Montha | Platelet count categoryb | Group |
P valuec | |
|---|---|---|---|---|
| Prophylaxis n (%) | Nonprophylaxis n (%) | |||
| 1 | 1 | 10 (5.9) | 5 (5.4) | 1.000 |
| 2 | 64 (37.9) | 16 (17.2) | ||
| 3 | 95 (56.2) | 71 (77.2) | ||
| 2 | 1 | 14 (8.3) | 4 (4.4) | 0.309 |
| 2 | 57 (33.7) | 14 (15.2) | ||
| 3 | 98 (58.0) | 74 (80.4) | ||
| 3 | 1 | 14 (8.3) | 3 (3.3) | 0.187 |
| 2 | 58 (34.3) | 14 (15.2) | ||
| 3 | 97 (57.4) | 74 (81.5) | ||
| 4 | 1 | 13 (7.7) | 3 (3.3) | 0.185 |
| 2 | 57 (34.3) | 13 (15.2) | ||
| 3 | 97 (58.0) | 72 (81.5) | ||
| 5 | 1 | 13 (8.3) | 4 (4.3) | 0.309 |
| 2 | 44 (26.6) | 16 (18.5) | ||
| 3 | 107 (65.1) | 66 (77.2) | ||
| 6d | 1 | 10 (5.9) | 4 (4.4) | 0.776 |
| 2 | 43 (26.6) | 12 (14.1) | ||
| 3 | 110 (67.5) | 69 (81.5) | ||
There was no statistically significant difference in platelet counts at baseline between the 2 groups.
Month after start of treatment.
Platelet count categories (per μl): 1: ≤80,000; 2: 81,000−150 000; 3: ≥150,000.
Fisher exact test.
One month after stopping treatment.
One patient had a severe skin reaction, which improved after stopping the TMP+SMX. The second patient in the TMP+SMX prophylaxis group who developed DRESS also had multiorganism culture−positive severe sepsis. She was on peritoneal dialysis (PD) and developed severe PD peritonitis requiring PD catheter removal. She was converted to hemodialysis as a bridging treatment pending complete recovery from the PD peritonitis. She had a short period dialysing via a temporary internal jugular catheter. Upon complete recovery from the infections, the catheter was converted to a tunneled catheter. She was then commenced on the TMP+SMX as prophylaxis for melioidosis. Four weeks later, she was admitted with a presumed infection of the right-sided internal jugular vein tunneled dialysis catheter and a skin rash. She was commenced on several antibiotics including vancomycin, meropenem, and fluconazole, as blood cultures were positive for multiple organisms which included Pseudomonas eruginosa, Staphylococcus capitis, Staphylococcus epidermidis, and Candida species. The TMP+SMX was immediately stopped on admission due to the rash, but the patient continued to deteriorate and subsequently died. The cause of death in this patient was attributed to a combination of sepsis with severe drug reaction. It was not possible to exclude other antibiotics as the cause of DRESS. Nevertheless, the temporal relationship between the initial appearance of the rash prior to commencement of the other antibiotics suggested that TMP+SMX was the most likely cause.
Neutropenia was more common in the TMP+SMX prophylaxis group than the nonprophylaxis group. However, this was generally not severe enough to cease the prophylaxis in any patient (Table 4).
Table 4.
Comparison between the 2 groups of the proportion of patients in neutrophil count categories during the study and 1 month after the study
| Montha | Neutrophil count categoryb | Group |
P valuec | |
|---|---|---|---|---|
| Prophylaxis proportion, n (%) | Nonprophylaxis proportion, n (%) | |||
| 1 | 1 | 15 (8.9) | 2 (2.2) | 0.038 |
| 2 | 154 (91.1) | 90 (97.8) | ||
| 2 | 1 | 14 (8.3) | 5 (5.4) | 0.464 |
| 2 | 155 (91.7) | 87 (94.6) | ||
| 3 | 1 | 15 (8.9) | 2 (2.2) | 0.038 |
| 2 | 154 (91.1) | 89 (97.8) | ||
| 4 | 1 | 15 (9.0) | 2 (2.2) | 0.039 |
| 2 | 152 (91.0) | 86 (97.8) | ||
| 5 | 1 | 18 (11.2) | 3 (3.3) | 0.009 |
| 2 | 146 (88.8) | 83 (96.7) | ||
| 6d | 1 | 13 (7.7) | 3 (3.3) | 0.185 |
| 2 | 150 (92.3) | 82 (96.7) | ||
Months from start of treatment.
Neutrophil count categories (× 109/l): 1: < 2.7; 2: ≥ 2.7.
Fisher exact test.
One month after stopping treatment.
There was no difference in abnormal liver function between the 2 groups. There was no impact on hemoglobin and requirements for erythropoiesis-stimulating agents. Thrombocytopenia and neutropenia had improved in all patients in the 2 months after ceasing the prophylaxis.
Figure 2 shows a flow diagram of how patients were evaluated, by TMP+SMX prophylaxis group and nonprophylaxis group, and presentation of selected events.
Discussion
We completed a prospective interventional study to determine the safety and efficacy of daily oral TMP+SMX prophylaxis to prevent melioidosis among a cohort of maintenance hemodialysis patients living in a region with endemic Burkholderia pseudomallei and melioidosis-related harms. The key findings are as follows: (i) daily oral TMP+SMX prophylaxis was probably efficacious against melioidosis in hemodialysis patients; (ii) daily dosing was relatively safe, although there was a tendency for increased episodes of nonsevere thrombocytopenia and neutropenia, without hepatic adverse events; (iii) logistical difficulties with administration of a daily dosing regimen and patient factors resulted in a number of patients being excluded from the prophylaxis; and (iv) the incidence rate of melioidosis of 17.4% in the nonprophylaxis group was higher than in our recent publication11; however, this is not unusual, as it has been shown in previous work in which rates as high as 29% have been recorded during some wet seasons12, 23 (Figure 1).
The predominant reason for some patients not receiving prophylaxis was remoteness and logistical reasons. Our dialysis patients from remote areas perform self-care dialysis and dialysis in remote satellite units. By selection, they tend to be the healthiest patients, who can manage self-care and require minimal support. They receive regular follow-up by their nephrologists. However, these logistical issues have been addressed in the subsequent wet seasons, and patients now receive the prophylaxis regardless of where they have dialysis.
In this high-risk population,11 the prophylaxis intervention was effective when provided in combination with wet season personal preventive advice,20 as no cases of melioidosis were observed in the TMP+SMX prophylaxis group. These measures include minimizing contact with the bacteria (covering open skin wounds, avoiding contact with soil and standing water, and wearing covered footwear) and using standard contact precautions (mask, gloves, and gown where appropriate) to help prevent infection.14
All cases of melioidosis occurred in patients who either developed the infection before the prophylaxis was introduced, or did not receive the prophylaxis, which supports the policy in our region of routinely providing melioidosis prophylaxis during the wet season. The nonprophylaxis group included patients who had nonmodifiable reasons (including TMP+SMX allergy, the frequency of which was similar to that in other reports of TMP+SMX),24 and reasons that may be modifiable. Potentially modifiable causes of ineligibility for the prophylaxis group included client preferences to decline a prophylactic treatment, and timely supply of the drug to home-based patients in regions remote from the treatment center.
Thrombocytopenia and neutropenia among participants in the TMP+SMX-group were observed (Table 2, Table 3, Table 4), but were not severe enough to warrant withdrawal of treatment. It is important to note that a number of patients in the sample had low platelet and neutrophil counts that were not related to the TMP+SMX, as evidenced by rates of thrombocytopenia and neutropenia in the nonprophylaxis group (Table 2, Table 3, Table 4). Withdrawal of TMP+SMX was required only in the event of a severe drug reaction.
Our data show the safe and efficacious use of TMP+SMX to mitigate melioidosis in our hemodialysis cohort, which has been developed into a comprehensive protocol for the next wet season (2015−2016) (Supplementary Material S1). To address logistic supply issues and pill burden and to improve medication adherence, we adapted the prophylaxis intervention, using TMP+SMX 1 double-strength tablet supervised post-dialysis 3 times a week in the wet season from 1 November 2015 to 30 April 2016. There were no identified cases of melioidosis during this period in those on the 3 times a week prophylaxis, and there were no significant adverse events. Following the success of this treatment, a comprehensive educational and awareness program led by the Northern Territory Centre for Disease Control has been undertaken to increase staff and patient education and awareness. We have produced a patient information video25 and are addressing medication prescribing and dispensing and monitoring issues. In the current incomplete wet season (1 November 2016 to 30 April 2017), there have been 3 cases of melioidosis in patients who had not started the prophylaxis due to various logistic issues. To date, we have not yet implemented a prophylaxis program for our peritoneal dialysis cohort, as antibiotic use can be linked to the development of fungal peritonitis.
A number of limitations of this study are acknowledged. It is a prospective observational study of an antibiotic intervention that required voluntary participation, which introduces the potential for bias. However, the study has clearly shown efficacy and safety of the administration of TMP+SMX in hemodialysis patients. There have been no events to date of TMP+SMX-resistant melioidosis in this cohort, but careful monitoring is required to ensure ongoing surveillance and drug stewardship. This prophylaxis program has been successful as part of a comprehensive multiagency strategy to mitigate harm in our patients. Patients within our hemodialysis cohort are regularly engaged in clinical care, which enables a greater responsiveness to side effects or adverse events. It is unknown whether this protocol may be effective in groups with less access to health care and monitoring, such as the wider diabetic population in melioidosis-endemic regions. Finally, potential confounding factors, especially the adherence to preventive measures for melioidosis, were not adjusted for in the study, although we have no reason to believe that this was different between the 2 groups.
In conclusion, the use of 1 double-strength tablet of TMP+SMX postdialysis in hemodialysis patients was shown to be effective and safe in the prevention of melioidosis. We recommend the use of this approach in tropical Northern Australia and other regions where melioidosis is prevalent.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We acknowledge our colleagues in the microbiology laboratory at Royal Darwin Hospital for their expertise in the laboratory diagnosis of melioidosis, and our clinical colleagues at Royal Darwin Hospital for management of the patients. JH was supported by NHMRC Fellowship 1092576 and a Jacquot Research Establishment Award. The Darwin Prospective Melioidosis study is supported by grants from the Australian National Health and Medical Research Council, including project grants 1098337 and 1131932 (the HOT NORTH initiative).
Footnotes
Supplementary Material S1. Prophylaxis guideline for melioidosis in hemodialysis patients.
Figure S1. Average monthly rainfall in millimeters (mm) at Darwin Airport for the years 1999 to 2015.
Figure S2. Consolidated Standards of Reporting Trials (CONSORT) flow diagram: melioidosis intervention study.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Prophylaxis guideline for melioidosis in hemodialysis patients.
Average monthly rainfall in millimeters (mm) at Darwin Airport for the years 1999 to 2015.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram: melioidosis intervention study.
References
- 1.Wiersinga W.J., Currie B.J., Peacock S.J. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 2.Stephens D.P., Thomas J.H., Ward L.M. Melioidosis causing critical illness: a review of 24 years of experience from the Royal Darwin Hospital ICU. Crit Care Med. 2016;44:1500–1505. doi: 10.1097/CCM.0000000000001668. [DOI] [PubMed] [Google Scholar]
- 3.Australian Bureau of Meteorology. Australian Bureau of Meteorology. (2017) Monthly Rainfall Climate Data, Darwin Airport. Australian Government [17 March 2017]. Available at: http://www.bom.gov.au/climate/data/. Accessed August 13, 2017.
- 4.Limmathurotsakul D., Golding N., Dance D.A. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 5.Currie B.J., Kestli M. Epidemiology: a global picture of melioidosis. Nature. 2016;529:290–291. doi: 10.1038/529290a. [DOI] [PubMed] [Google Scholar]
- 6.Chewapreecha C., Holden M.T., Vehkala M. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat Microbiol. 2017;2:16263. doi: 10.1038/nmicrobiol.2016.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie B.J., Ward L., Cheng A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie B.J., Fisher D.A., Howard D.M. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981–986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 9.Currie B.J., Jacups S.P., Cheng A.C. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Health. 2004;9:1167–1174. doi: 10.1111/j.1365-3156.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 10.Jabbar Z., Currie B.J. Melioidosis and the kidney. Nephrology [Carlton] 2013;18:169–175. doi: 10.1111/nep.12024. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers R.M., Majoni S.W., Ward L. Melioidosis and end-stage renal disease in tropical northern Australia. Kidney Int. 2014;86:867–870. doi: 10.1038/ki.2014.228. [DOI] [PubMed] [Google Scholar]
- 12.Kestli M., Grist E.P.M., Ward L. The association of melioidosis with climatic factors in Darwin, Australia: a 23-year time-series analysis. J Infect. 2016;72:687–697. doi: 10.1016/j.jinf.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Davis J.S., Currie B.J., Fisher D.A. Prevention of opportunistic infections in immunosuppressed patients in the tropical top end of the Northern Territory. Commun Dis Intell Q Rep. 2003;27:526–532. [PubMed] [Google Scholar]
- 14.Currie B.J. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–125. doi: 10.1055/s-0034-1398389. [DOI] [PubMed] [Google Scholar]
- 15.Cheng A.C., Fisher D.A., Anstey N.M. Outcomes of patients with melioidosis treated with meropenem. Antimicrob Agents Chemother. 2004;48:1763–1765. doi: 10.1128/AAC.48.5.1763-1765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitman M.C., Luck T., Marshall C.S. Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm. PLoS Negl Trop Dis. 2015;9:e0003586. doi: 10.1371/journal.pntd.0003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarovich D.S., Ward L., Price E.P. Recurrent melioidosis in the Darwin Prospective Melioidosis Study: improving therapies mean that relapse cases are now rare. J Clin Microbiol. 2014;52:650–653. doi: 10.1128/JCM.02239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng A.C., McBryde E.S., Wuthiekanun V. Dosing regimens of cotrimoxazole (trimethoprim-sulfamethoxazole) for melioidosis. Antimicrob Agents Chemother. 2009;53:4193–4199. doi: 10.1128/AAC.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivalingam S.P., Sim S.H., Jasper L.C. Pre- and post-exposure prophylaxis of experimental Burkholderia pseudomallei infection with doxycycline, amoxicillin/clavulanic acid and co-trimoxazole. J Antimicrob Chemother. 2008;61:674–678. doi: 10.1093/jac/dkm527. [DOI] [PubMed] [Google Scholar]
- 20.Menzies School of Health Research. Darwin Prospective Melioidosis Study 2017. Available at: https://www.menzies.edu.au/page/Research/Projects/Melioidosis/Darwin_Prospective_Melioidosis_Study_DPMS/. Accessed August 13, 2017.
- 21.Brown G.R. Cotrimoxazole—optimal dosing in the critically ill. Ann Intensive Care. 2014;4:13. doi: 10.1186/2110-5820-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polkinghorne K. Hemodialysis. In: Clayton P., McDonald S., Hurst K., editors. ANZDATA Registry Report 2013. Australia and New Zealand Dialysis and Transplant Registry; Adelaide, South Australia: 2013. pp. 5–7. [Google Scholar]
- 23.Parameswaran U., Baird R.W., Ward L.M. Melioidosis at Royal Darwin Hospital in the big 2009–2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196:345–348. doi: 10.5694/mja11.11170. [DOI] [PubMed] [Google Scholar]
- 24.Macy E., Poon K.Y.T. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122:778. doi: 10.1016/j.amjmed.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JT. Melioidosis and hemodialysis. 2017. Available at: http://www.menzies.edu.au/page/Resources/Melioidosis_and_hemodialysis/. Accessed August 13, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prophylaxis guideline for melioidosis in hemodialysis patients.
Average monthly rainfall in millimeters (mm) at Darwin Airport for the years 1999 to 2015.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram: melioidosis intervention study.