Abstract
Introduction
Obstetric-related acute kidney injury (AKI) is associated with adverse outcomes for mother and fetus, particularly in low-income countries. However, laboratory-independent tools to facilitate diagnosis are lacking. We assessed the diagnostic performance of a salivary urea nitrogen (SUN) dipstick to detect obstetric-related acute kidney disease in Malawi.
Methods
Women at high risk for AKI admitted to an obstetric unit in Blantyre, Malawi, were recruited between 21 September and 11 December 2015. Patients underwent serum creatinine (SCr) testing alongside measurement of SUN using a dipstick on admission, and every 48 hours thereafter if evidence of kidney disease was found.
Results
A total of 301 patients were included (mean age 25.9 years, 11% HIV positive). Of the patients, 23 (7.6%) had AKI, stage 1 in 47.8%, most commonly due to preeclampsia/eclampsia. Mean presenting SCr values were 108.8 ± 21.8 μmol/l (1.23 ± 0.25 mg/dl), 118 ± 34.45 μmol/l (1.33 ± 0.39 mg/dl), and 136.1 ± 30.4 μmol/l (1.54 ± 0.34 mg/dl) in AKI stages 1 to 3 respectively. SUN > 14 mg/dl had a sensitivity of 12.82% and a specificity of 97.33% to detect acute kidney disease; the area under the receiver operating characteristic curve was 0.551. In patients with normal SUN on admission, perinatal mortality was 11.8%, and was 25.0% if SUN was > 14 mg/dl (P = 0.18).
Conclusion
The SUN dipstick was specific but insensitive when used to diagnose obstetric-related AKI. Limited biochemical derangement and low salivary urea concentrations due to physiological changes in pregnancy, as opposed to a technical limitation of the dipstick itself, are the likely reason for the lack of sensitivity in this study.
Keywords: acute kidney disease, acute kidney injury, dipstick, pre-eclampsia, salivary urea nitrogen, sub-Saharan Africa
Acute kidney injury (AKI) is a global health problem, which currently leads to preventable deaths in young persons in the poorest parts of the world.1, 2 This is particularly the case in sub-Saharan Africa, where AKI is common and severe, leading to adverse outcomes in many of those affected.3 In Malawi, a low-income country in southeast Africa, 21% of general medical admissions have kidney injury at presentation and in-hospital mortality is 44% in those with AKI, despite free access to tertiary nephrology services including renal replacement therapy.4
Although detailed data on the epidemiology of AKI in developing countries including its progression and subsequent outcomes are lacking, reports suggest that obstetric-related kidney disease contributes significantly to the overall burden of AKI in this part of the world.5 A lack of consistent antenatal care in sub-Saharan Africa means that obstetric-related disease, including AKI, may present late, with potentially disastrous outcomes for both mother and fetus.6, 7, 8
Early recognition of AKI is an essential step in allowing timely treatment in efforts to improve patient outcomes from this potentially reversible condition.9 Diagnosis of AKI is highly dependent on the measurement of serum creatinine (SCr), which is almost universally available in high-income countries; however it is often not available in less privileged health care settings. In sub-Saharan Africa, obstetric patients are managed largely in district hospitals and community health centers, where SCr tests are unavailable, creating a significant barrier to the management of AKI.
The salivary urea nitrogen (SUN) dipstick is a noninvasive and simple diagnostic tool that provides point-of-care estimation of renal function at very low cost.10, 11 It does not require electricity or refrigerated storage of reagents and therefore may be used in the remotest parts of the world. We have previously demonstrated, in both developed and resource-limited settings, that the SUN dipstick demonstrates good diagnostic performance to detect kidney injury in adult medical patients with acute and chronic kidney disease, albeit with less sensitivity at lower levels of SUN.10, 12, 13, 14 In this study, we assessed the performance of the SUN dipstick to detect obstetric-related AKI in Malawi.
Methods
Study Design and Setting
We conducted a prospective observational study at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi. This hospital acts as a district hospital for Blantyre and also provides tertiary obstetric and nephrology care to the southern region of Malawi. The majority of obstetric admissions are from Blantyre district, encompassing both urban and rural populations, and the obstetric unit delivers approximately 12,000 babies per year. Despite its being ranked as one of the poorest countries worldwide, health care in Malawi is government funded and provided to all free at the point of delivery. At QECH, this includes access to a high-dependency obstetric unit and hemodialysis for AKI.
Participants
We included women admitted to QECH obstetric department who were > 20 weeks’ gestation or < 6 weeks postpartum presenting with conditions predetermined as being at risk of leading to AKI. These were admissions with a working diagnosis of the following: gestational hypertension, preeclampsia, eclampsia, antepartum hemorrhage, postpartum hemorrhage, sepsis, renal failure (diagnosed by admitting team prior to study recruitment), and heart failure (Supplementary Table S1).
All participants provided written informed consent. The University of Malawi, College of Medicine Research Ethics Committee, approved the study (ref. P.11/14/1660).
Data Collection
Patients were enrolled between 21 September and 11 December 2015. Those fulfilling the inclusion criteria were assessed within 48 hours of admission. Baseline clinical data were recorded, and screening for kidney disease (community-acquired) was undertaken with a SCr alongside simultaneous measurement of SUN using a dipstick (Integrated Biomedical Technology, Elkhart, IN).10, 12, 13, 14 Patients were excluded if they were unable to undergo concomitant measurement of both SCr and SUN.
A SCr of > 82μmol/l was considered to be elevated, predefined as 2 SDs above the mean in the third trimester of pregnancy.15 Patients with elevated SCr were managed jointly by the obstetric and nephrology teams, and the nature (as per definitions below) and cause of kidney injury were determined. The women underwent daily measurement of SCr and urine output, and further measurement of SUN dipstick and serum urea at 48 hours. The obstetric team alone managed patients without raised SCr on admission.
Patients and neonates were followed up until hospital discharge. Maternal and fetal outcomes (gestational age at delivery, birth weight, first Apgar score,16 in-hospital maternal and perinatal mortality) were recorded in the entire study population.
SUN Measurement
After a period of 15 minutes without eating or drinking, patients provided unstimulated saliva into a plastic cup, and a 50-μl quantity was used to moisten the test pad of a colorimetric SUN dipstick. The change in color of the test pad was assessed at 1 minute and compared to 6 reference pads corresponding to increasing SUN concentrations: 5 to 14 mg/dl (pad 1), 15 to 24 mg/dl (pad 2), 25 to 34 mg/dl (pad 3), 35 to 54 mg/dl (pad 4), 55 to 74 mg/dl (pad 5), and ≥ 75 mg/dl (pad 6) (Supplementary Figure S1). During analysis, SUN was transformed to a continuous variable by converting the test pad result to the midpoint for each range (e.g., a SUN result of 5−14 mg/dl was transformed to 9.5 mg/dl).
Serum Creatinine and Urea Measurement
SCr and urea were measured by the Jaffe and urease methods, respectively, using either a Flexor Junior Clinical Chemistry Analyzer (Vital Scientific, Dieren, Netherlands) or a Mindray Chemistry Analyzer BS-120 (Shenzen Mindray Bio-Medical Electronics Company, Shenzen, China) in a local laboratory. Analyzers were calibrated in accordance with the manufacturer’s instructions.
Definitions
AKI and CKD were defined and staged by Kidney Disease Improving Global Outcomes (KDIGO) criteria.17, 18 Patients with elevated SCr (>82 μmol/l) on admission that did not fulfill KDIGO criteria for AKI or CKD were defined, by our own definition, as having acute kidney disease (AKD) without AKI. Any acute kidney disease encompassed both AKI and AKD without AKI. Patients with normal SCr on admission were deemed as having no kidney disease (NKD) (Supplementary Table S2).
The nephrology study team determined the primary cause of AKI. Perinatal mortality was defined as fetal death after 20 weeks’ gestation (stillbirth or termination of pregnancy) or in-hospital neonatal death.
Outcome Measures
The primary outcome was the diagnostic performance of the SUN dipstick to detect obstetric-related acute kidney disease (AKI and AKD without AKI). Secondary outcomes were comparison of SUN concentrations in those with and without AKI, and assessment of maternal and perinatal in-hospital outcomes (gestational age, birth weight, first Apgar score, maternal and perinatal mortality) according to the presence of AKI and SUN results.
Statistical Analysis
Descriptive data are presented as mean ± SD and as median ± interquartile range (IQR), depending on the distribution of data and the standard parametric and nonparametric tests used. Diagnostic performance of SUN was determined by sensitivity and specificity, and by the area under the receiver operating characteristic (ROC) curve. The optimal threshold of SUN to diagnose kidney disease was calculated according to the Youden Index.
Statistical analysis was performed using Graphpad Prism version 7 (www.graphpad.com). A P value of < 0.05 was considered statistically significant.
Results
Cohort Description
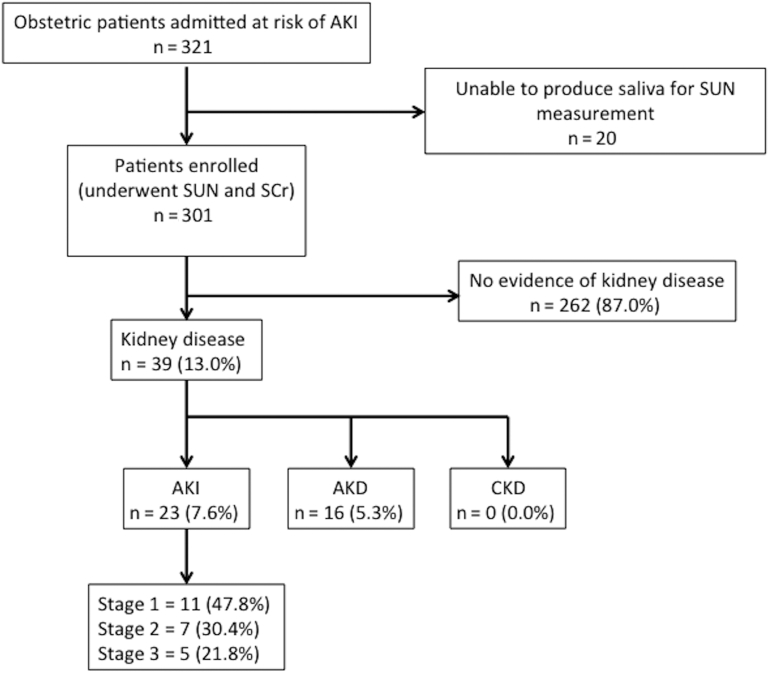
A total of 321 patients at risk for obstetric-related AKI were admitted, and 301 were enrolled (Figure 1).
Figure 1.
Cohort description. AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; SCr, serum creatinine; SUN, salivary urea nitrogen.
The mean age of the entire cohort was 25.9 ± 6.45 years. Of the patients, 33 (11.0%) were HIV positive, 30 of whom were on antiretroviral therapy. Eleven patients (3.7%) had known hypertension, but other comorbidities were rare. A total of 106 patients (35.2%) had used a nephrotoxin prior to admission, including nonsteroidal anti-inflammatory drugs (n = 65) and traditional remedies (n = 11). Presenting conditions leading to risk of AKI and inclusion in the study are outlined in Table 1.
Table 1.
Study inclusion diagnoses
| Inclusion diagnosis | Patients, n (%) |
|---|---|
| Hypertension in pregnancy | 57 (18.9%) |
| Preeclampsia | 80 (26.6%) |
| Eclampsia | 15 (5.0%) |
| Antepartum hemorrhage | 39 (13.0%) |
| Postpartum hemorrhage | 37 (12.3%) |
| Sepsisa | 47 (15.6%) |
| Renal failure | 0 (0.0%) |
| Cardiac failure | 0 (0.0%) |
| Multiple diagnoses aboveb | 26 (8.6%) |
Causes of sepsis: endometritis, n = 14; malaria, n = 11; gastroenteritis, n = 10; respiratory infection, n = 6; wound infection, n = 4; peritonitis, n = 1; chorioamnionitis, n = 1; unclear, n = 4.
Primary cause for inclusion in those with multiple reasons: hypertension in pregnancy, n = 4; preeclampsia, n = 9; eclampsia, n = 2; antepartum hemorrhage, n = 5; postpartum hemorrhage, n = 2; sepsis, n = 4.
Mean gravidity was 2.6 ± 1.7, there were 43 cases (14.3%) of multiple pregnancies. A total of 75 patients (24.9%) had a history of previous adverse fetal outcome (any of previous stillbirth, miscarriage, early or late neonatal death). The majority of patients (n = 293; 97.3%) had received some form of antenatal care, and 6 (3.0%) of the 199 patients who presented postpartum had delivered at home.
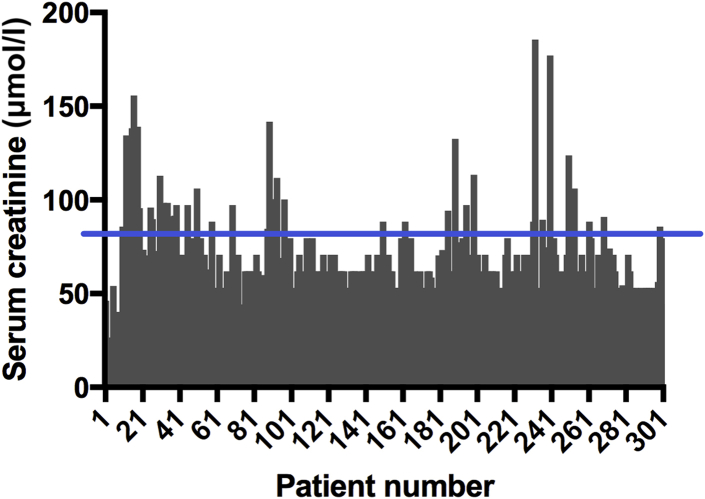
Of the total number of patients enrolled, 23 patients (7.6%) had AKI (stage 1, n = 11 [47.8%]; stage 2, n = 7 [30.4%]; stage 3, n = 5 [21.8%]. The primary causes of AKI were preeclampsia/eclampsia (n = 18 [78.3%]), antepartum hemorrhage (n = 3 [13.1%]), and sepsis (n = 2 [8.6%]). Mean presenting SCr values were 62.37 ± 23.27 μmol/l (0.71 ± 0.26 mg/dl) in all patients (Figure 2), and 108.8 ± 21.8 μmol/l (1.23 ± 0.25 mg/dl), 118 ± 34.45 μmol/l (1.33 ± 0.39 mg/dl), and 136.1 ± 30.4 μmol/l (1.54 ± 0.34 mg/dl) in AKI stages 1 to 3 respectively. No patient required renal replacement therapy.
Figure 2.
Presenting serum creatinine values in the entire study population. The blue line at 82 μmol/l represents upper limit of normal range.
SUN and Urea Measurement
In patients without evidence of kidney disease, SUN on admission was 5 to 14 mg/dl (testpad 1) in 255 patients (97.3%), 15 to 24 mg/dl (testpad 2) in 6 patients (2.3%), and 25 to 34 mg/dl (testpad 3) in 1 patient (0.4%). In patients with kidney disease, SUN on admission was 5 to 14 mg/dl (testpad 1) in 34 patients (87.2%), 15 to 24 mg/dl (testpad 2) in 3 patients (7.7%), and 25 to 34 mg/dl (testpad 3) in 2 patients (5.1%). The mean serum urea was 5.36 ± 3.89 mmol/l (blood urea nitrogen [BUN] = 15.08 ± 10.92 mg/dl) in patients with kidney disease who had it measured at 48 hours, and the mean SUN was 10.93 ± 3.78 mg/dl in these patients at this time point.
Diagnostic Performance of SUN
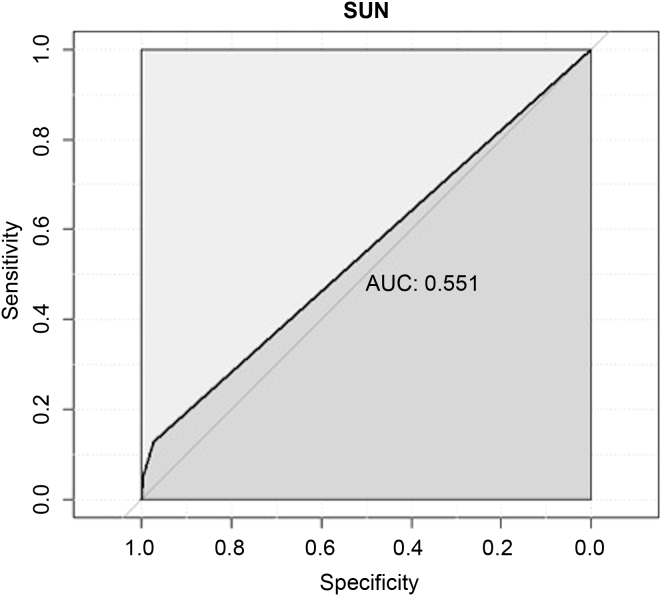
A SUN result of >14 mg/dl (i.e., greater than testpad 1) was the optimal threshold to diagnose kidney disease (Youden Index = 0.10); this had a sensitivity of 12.82% and a specificity of 97.33% to detect acute kidney disease (AKI or AKD without AKI). The area under the ROC curve was 0.551 (Figure 3).
Figure 3.
Receiver operating characteristic curve for salivary urea nitrogen (SUN) > 14 mg/dl (i.e., SUN dipstick greater than testpad 1) to detect acute kidney disease (acute kidney injury [AKI] and acute kidney disease without AKI). AUC, area under the curve.
Outcome Analyses
Maternal outcomes were determined in 252 patients; there was 1 maternal death. This was in a patient with NKD and SUN 15 to 24 mg/dl (testpad 2) on admission who presented at 24 weeks with eclampsia.
Fetal outcomes were determined in 266 cases. Mean gestational age at birth was 36.54 ± 3.9 weeks; fetal weight was 2.7 ± 1.2 kg; and first Apgar score was 6.4 ± 2.8. There was no significant difference in these parameters when assessed according to the presence of AKI or SUN levels.
Perinatal mortality was 12.2% in NKD and 13.0% in AKI (P > 0.99). In those mothers with SUN 5 to 14 mg/dl (testpad 1) on admission, perinatal mortality was 11.8%, and in mothers with SUN > 14 mg/dl (greater than testpad 1) it was 25.0% (P = 0.18).
Discussion
Obstetric-related acute kidney disease is common in low- and middle-income countries worldwide; however, simple, inexpensive, laboratory-independent tools for its diagnosis are currently lacking. In this study, we tested the performance of a SUN dipstick to detect obstetric-related acute kidney disease in a low-resource setting in southern Africa.
Main Findings
We screened 301 patients at high risk for obstetric AKI with a serum creatinine and SUN dipstick on admission to a tertiary hospital in Malawi over a 12-week period in 2015. In a cohort with predominantly mild AKI and limited biochemical derangement, a SUN result of >14 mg/dl (i.e., greater than testpad 1) had a sensitivity of 12.82% and a specificity of 97.33% to detect acute kidney disease (AKI or AKD without AKI). The perinatal mortality rate in mothers with an elevated SUN on admission was 25%.
Interpretation
Urea enters saliva through diffusion transport and, alongside bicarbonate, is responsible for the buffering capacity of this fluid. Its presence in saliva was first described in 1841 by Wright, and over the last 30 years there have been several reports of its correlation with serum urea and its potential use in the diagnosis of kidney disease (summarized in Supplementary Table S3). In studies in which salivary urea was measured in a laboratory, there has been a consistent positive correlation between serum and saliva urea, with correlation coefficients ranging from 0.51 to 0.98.19, 20, 21, 22, 23, 24, 25 In most cases, salivary urea concentrations are less than serum urea, with salivary urea (variety of cut-off concentrations) having a sensitivity of 80% to 100% and a specificity of 71% to 100% to detect kidney disease (using a variety of definitions, but including patients with both acute and chronic kidney disease).
In 2007, a dipstick was developed to measure SUN at the point of care. It has since been demonstrated that salivary urea measured by this method correlates with serum urea in controls and patients with acute and chronic kidney disease (r = 0.63−0.6910, 12) and performs well in diagnosing kidney disease and assessing its response to treatment in both developed and resource-poor settings13, 14 (Supplementary Table S4). Most recently, in a general medical cohort with predominantly severe AKI in Malawi, we demonstrated a SUN > 14 mg/dl (testpad > 1) had a sensitivity of 71% and a specificity of 87% to detect acute kidney disease.14 Specificity increased further if the dipstick result was combined with patient-reported oliguria. However, in all studies to date, there has been a lower accuracy of the SUN dipstick at lower levels of BUN (< 50 mg/dl), suggesting that the dipstick performs less well in patients with milder AKI and more limited biochemical derangement.
In the current study, measurement of SUN using the dipstick was highly specific but insensitive when used to diagnose obstetric-related acute kidney disease in a cohort of patients with relatively modest elevations of SCr and urea. The overall performance of the dipstick was poor, with an area under the ROC curve of 0.551 for a SUN > 14 mg/dl to detect kidney disease. Two possibilities could account for this: first, the dipstick may not have accurately measured salivary urea (i.e., a technical problem with the dipstick itself); and second, the dipstick may have accurately measured salivary urea, but salivary urea levels may not have been significantly raised in this obstetric cohort with acute kidney disease.
We believe that the latter hypothesis is more likely. Although we did not measure salivary urea by laboratory methods alongside the dipstick, we know that the dipstick has been tested against known urea concentrations in aqueous solution and, moreover, that it has demonstrated good correlation with serum urea and reasonable sensitivity to diagnose kidney disease in other cohorts in which it has been trialed.
A key difference in this study compared to previous work was the obstetric nature of the cohort. The physiological changes during pregnancy mean that obstetric patients represent a unique environment, with significant increases in glomerular filtration rate and circulating volume, resulting in reductions in both serum urea and SCr throughout pregnancy.15, 26 This results in difficulties in recognizing renal impairment, even when using laboratory-measured SCr and serum urea.26 Changes in salivary urea are not well characterized in pregnant women, but it would be reasonable to assume that reductions in serum urea are reflected by reductions in salivary urea concentration. The relatively mild nature of kidney disease, alongside the low-protein diet and muscle mass in young women in Malawi more generally, may have also contributed to low salivary urea concentrations in this obstetric cohort. Of note, mean BUN was 15.08 mg/dl when measured at 48 hours postadmission in a small number of patients with kidney disease; due to logistic and financial restrictions, we did not measure BUN on admission. Moreover, we know from previous work that SUN tends to underestimate BUN.
In addition to low salivary urea itself, other hormonal or biochemical changes in the composition of saliva in pregnancy may have affected the test, in particular pH. The test depends on urease within the test pad, which catalyses the conversion of urea to ammonia and hydroxyl ions, with subsequent detection of the resulting change in pH. Changes in salivary pH in pregnancy may affect this process,27 including in relation to alterations of oral bacterial flora,28, 29 albeit with the strip having a control pad for any gross abnormality in salivary pH at baseline.
Despite the diagnostic performance being worse than we had anticipated, the SUN dipstick did suggest in this study, as it has done in previous studies, that it may provide prognostic information. In general medical patients in Malawi, elevated SUN on admission was an independent predictor of in-hospital mortality.14 In this cohort, perinatal mortality was 25% in patients with elevated SUN (> 14 mg/dl) on admission, compared to 11.8% in patients with normal SUN (5−14 mg/dl), although, given the limited numbers of patients with elevated SUN in the cohort as a whole, firm conclusions are difficult to make.
Study Strengths and Limitations
To the best of our knowledge, this is the first study to assess the performance of a point-of-care, laboratory-independent tool to diagnose obstetric-related kidney disease in a resource-limited setting. It was undertaken prospectively in a large cohort of patients at risk for kidney disease, with outcome data in the majority. Through the inclusion of a novel cohort, it complements other studies investigating the use of the SUN dipstick across different settings in sub-Sahara Africa, and confirms the high specificity of the test.
Obstetric patients provide a unique challenge in diagnosing AKI. Few women had documented baseline SCr, and a lack of a “normal” glomerular filtration rate in pregnancy meant that imputed baselines assuming normal renal function were unable to be used like they can in the nonpregnant population. We were unable to determine those women with underlying CKD. Furthermore, local practical challenges such as a lack of urine measuring jugs, lack of catheter bags, and relatively low literacy rates meant that accurate estimation of urine output was difficult. Some cases of kidney disease may have been missed; AKI may have been misclassified as AKD; and severity may have been underestimated. We detected kidney disease that was present on admission, but the development of AKI in hospital was not assessed. This study also occurred outside of malaria season, anecdotally the most common cause of obstetric AKI requiring renal replacement therapy in our experience. Although it would have been possible, we did not use the SUN dipstick in patients with significantly reduced consciousness in this study, and hence we may have missed the most severe AKI.
Although we undertook this study in a truly resource-poor setting, its generalizability to the rest of Malawi and Africa need to be interpreted with caution. Malawi is unique within the region for providing health care free at the point of delivery, including, as was the case in this study, tertiary obstetric and nephrology care. Severity of AKI and outcomes may be worse in more rural settings within Malawi that lack this level of care, and in neighboring countries where financial restrictions may limit access to care. We are currently investigating the use of the dipstick in district and community settings in Malawi. We did not measure serum urea at recruitment or salivary urea by laboratory methods alongside the dipstick, and we aim to do this in upcoming studies.
In conclusion, in this study, measuring SUN using a dipstick was specific but insensitive when used to diagnose obstetric-related acute kidney disease in Malawi. Low salivary urea concentrations in this cohort, in combination with the reduced accuracy of the dipstick at lower levels of BUN, are the likely main drivers for the lack of sensitivity seen in the study. The development of laboratory-independent diagnostic tools remains essential in the management of kidney disease across sub-Saharan Africa and other resource-poor settings worldwide. A modified SUN dipstick with increased sensitivity at the lower ranges of SUN is currently under development, with plans to test this in both pregnant and nonpregnant patients with kidney disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was funded by the International Society of Nephrology (Research and Prevention Program) and the Royal Society of Tropical Medicine and Hygiene.
Footnotes
Figure S1. Reference pads (1−6) of the salivary urea nitrogen (SUN) dipstick, with increasing green color corresponding to increased urea nitrogen range. The change in color of the test pad is compared to these reference pads.
Table S1. Study inclusion criteria and definitions.
Table S2. Definitions of kidney disease.
Table S3. Selected studies of laboratory-measured salivary urea, its correlation with serum urea, and its performance in the diagnosis of kidney disease.
Table S4. Studies of dipstick-measured salivary urea, its correlation with serum urea, and its performance in the diagnosis of kidney disease.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Reference pads (1−6) of the salivary urea nitrogen (SUN) dipstick, with increasing green color corresponding to increased urea nitrogen range. The change in color of the test pad is compared to these reference pads.
Study inclusion criteria and definitions.
Definitions of kidney disease.
Selected studies of laboratory-measured salivary urea, its correlation with serum urea, and its performance in the diagnosis of kidney disease.
Studies of dipstick-measured salivary urea, its correlation with serum urea, and its performance in the diagnosis of kidney disease.
References
- 1.Mehta R.L., Cerdá J., Burdmann E.A. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 2.Mehta R.L., Burdmann E.A., Cerdá J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 3.Olowu W.A., Niang A., Osafo C. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016;4:e242–e250. doi: 10.1016/S2214-109X(15)00322-8. [DOI] [PubMed] [Google Scholar]
- 4.Evans R.D.R., Hemmilä U., Craik A. Incidence, aetiology and outcome of community-acquired acute kidney injury in medical admissions in Malawi. BMC Nephrol. 2017;18:21. doi: 10.1186/s12882-017-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lameire N.H., Bagga A., Cruz D. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 6.Randeree I.G., Czarnocki A., Moodley J. Acute renal failure in pregnancy in South Africa. Ren Fail. 1995;17:147–153. doi: 10.3109/08860229509026251. [DOI] [PubMed] [Google Scholar]
- 7.Drakeley A.J., Le Roux P.A., Anthony J. Acute renal failure complicating severe preeclampsia requiring admission to an obstetric intensive care unit. Am J Obstet Gynecol. 2002;186:253–256. doi: 10.1067/mob.2002.120279. [DOI] [PubMed] [Google Scholar]
- 8.Kaze F.F., Njukeng F.A., Kengne A.-P. Post-partum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in sub-Saharan Africa: a 6-months cohort study. BMC Pregnancy Childbirth. 2014;14:134. doi: 10.1186/1471-2393-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerdá J., Mohan S., Garcia-Garcia G. Acute kidney injury recognition in low- and middle-income countries. Kidney Int Rep. 2017;2:530–543. doi: 10.1016/j.ekir.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calice-Silva V., Vieira M.A., Raimann J.G. Saliva urea nitrogen dipstick—a novel bedside diagnostic tool for acute kidney injury. Clin Nephrol. 2014;82:358–366. doi: 10.5414/CN108370. [DOI] [PubMed] [Google Scholar]
- 11.Raimann JG, Riella MC, Levin NW. International Society of Nephrology’s 0by25 initiative (zero preventable deaths from acute kidney injury by 2025): focus on diagnosis of acute kidney injury in low-income countries. Clin Kidney J. https://doi.org/10.1093/ckj/sfw134. [DOI] [PMC free article] [PubMed]
- 12.Raimann J.G., Kirisits W., Gebetsroither E. Saliva urea dipstick test: application in chronic kidney disease. Clin Nephrol. 2011;76:23–28. doi: 10.5414/cn106826. [DOI] [PubMed] [Google Scholar]
- 13.Raimann J.G., Calice-Silva V., Thijssen S. Saliva urea nitrogen continuously reflects blood urea nitrogen after acute kidney injury diagnosis and management: longitudinal observational data from a collaborative, international, prospective, multicenter study. Blood Purif. 2016;42:64–72. doi: 10.1159/000445041. [DOI] [PubMed] [Google Scholar]
- 14.Evans R., Calice-Silva V., Raimann J.G. Diagnostic performance of a saliva urea nitrogen dipstick to detect kidney disease in Malawi. Kidney Int Rep. 2017;2:219–227. doi: 10.1016/j.ekir.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams D., Davison J. Chronic kidney disease in pregnancy. BMJ. 2008;336:211–215. doi: 10.1136/bmj.39406.652986.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ACOG. The Apgar score. Available at: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/The-Apgar-Score. Accessed 6July 6, 2017.
- 17.KDIGO. Acute kidney injury (AKI). Available at: http://kdigo.org/guidelines/acute-kidney-injury/. Accessed August 17, 2017.
- 18.KDIGO. CKD evaluation and management. Available at: http://kdigo.org/guidelines/ckd-evaluation-and-management/. Accessed June 17, 2017.
- 19.Yajamanam N., Vinapamula K.S., Sivakumar V. Utility of saliva as a sample to assess renal function and estimated glomerular filtration rate. Saudi J. Kidney Dis Transplant. 2016;27:312–319. doi: 10.4103/1319-2442.178549. [DOI] [PubMed] [Google Scholar]
- 20.Pandya D., Nagrajappa A.K., Ravi K.S. Assessment and correlation of urea and creatinine levels in saliva and serum of patients with chronic kidney disease, diabetes and hypertension—a research study. J Clin Diagn Res. 2016;10:ZC58–ZC62. doi: 10.7860/JCDR/2016/20294.8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasisi T.J., Raji Y.R., Salako B.L. Salivary creatinine and urea analysis in patients with chronic kidney disease: a case control study. BMC Nephrol. 2016;17:10. doi: 10.1186/s12882-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seethalakshmi C., Koteeswaran D., Chiranjeevi V. Correlation of serum and salivary biochemical parameters in end stage renal disease patients undergoing hemodialysis in pre and post-dialysis state. J Clin Diagn Res. 2014;8:CC12–CC14. doi: 10.7860/JCDR/2014/10404.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zúñiga M.E., Estremadoyro L.O., León C.P. Validation of the salivary urea test as a method to diagnose chronic kidney disease. J Nephrol. 2012;25:431–436. doi: 10.5301/jn.5000022. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso E.M.L., Arregger A.L., Tumilasci O.R. Assessment of salivary urea as a less invasive alternative to serum determinations. Scand J Clin Lab Invest. 2009;69:330–334. doi: 10.1080/00365510802588076. [DOI] [PubMed] [Google Scholar]
- 25.Sein K.T., Arumainayagam G. Correlation between serum urea and salivary urea. Clin Chem. 1987;33:2303–2304. [PubMed] [Google Scholar]
- 26.Acharya A., Santos J., Linde B. Acute kidney injury in pregnancy—current status. Adv Chron Kidney Dis. 2013;20:215–222. doi: 10.1053/j.ackd.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Rockenbach M.I., Marinho S.A., Veeck E.B. Salivary flow rate, pH, and concentrations of calcium, phosphate, and sIgA in Brazilian pregnant and non-pregnant women. Head Face Med. 2006;2:44. doi: 10.1186/1746-160X-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basavaraju A., Durga S.V., Vanitha B. Variations in the oral anaerobic microbial flora in relation to pregnancy. J Clin Diagn Res. 2012;6:1489–1491. doi: 10.7860/JCDR/2012/4609.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y.L., Nascimento M., Burne R.A. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4:135–140. doi: 10.1038/ijos.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reference pads (1−6) of the salivary urea nitrogen (SUN) dipstick, with increasing green color corresponding to increased urea nitrogen range. The change in color of the test pad is compared to these reference pads.
Study inclusion criteria and definitions.
Definitions of kidney disease.
Selected studies of laboratory-measured salivary urea, its correlation with serum urea, and its performance in the diagnosis of kidney disease.
Studies of dipstick-measured salivary urea, its correlation with serum urea, and its performance in the diagnosis of kidney disease.