Abstract
Introduction
Serum phosphate levels are insufficiently controlled in many patients with end-stage renal disease (ESRD), and novel therapeutic strategies are needed. Blocking intestinal phosphate absorption mediated by sodium-dependent phosphate co-transporter type 2b (NPT-IIb) holds promise; thus, we evaluated the efficacy, safety, tolerability, and pharmacokinetics of the novel and specific small molecule NPT-IIb inhibitor ASP3325 for the first time in humans.
Methods
We conducted a randomized, double-blind, placebo-controlled, phase 1a single (n = 88) and multiple (n = 36) ascending dose study in healthy subjects, and a randomized, open-label, uncontrolled, phase 1b study in hyperphosphatemic ESRD patients on hemodialysis (single oral dose, n = 5; multiple oral doses, n = 17). Primary efficacy measures were urinary phosphate and fecal phosphorous excretion (healthy subjects) and serum phosphate level (ESRD patients).
Results
No time- or dose-dependent changes in urinary phosphate or fecal phosphorous excretion were observed following single/multiple ASP3325 doses for 7 days in healthy subjects. In ESRD patients, ASP3325 administered 3 times daily for 2 weeks before or after a meal did not reduce serum phosphate levels. ASP3325 was safe and well tolerated in both populations.
Conclusion
NPT-IIb inhibition with ASP3325 was not effective in reducing serum phosphate levels in ESRD patients. The relevance of NPT-IIb in humans and feasibility of oral NPT-IIb inhibitors for treatment of hyperphosphatemia in ESRD remain uncertain.
Keywords: end-stage renal disease, hemodialysis, hyperphosphatemia, NPT-IIb inhibitor, pharmacokinetics, phase 1 trial
Chronic kidney disease affects approximately 10% to 13% of the adult population in developed countries1 and is characterized by impaired renal function (i.e., reduced glomerular filtration rate) and/or evidence of kidney damage. End-stage renal disease (ESRD) dictates the need for renal replacement therapy, including kidney transplantation or dialysis treatment, and is associated with myriad metabolic complications and dramatically increased morbidity and mortality.2
Hyperphosphatemia is a nearly inevitable complication of ESRD with prognostic implications for cardiovascular disease and mortality.3, 4, 5 Regular monitoring and prevention of hyperphosphatemia is therefore universally recommended in clinical guidelines,6, 7 although the current treatment paradigm with phosphate binders and dietary measures is insufficient to achieve recommended target concentrations of serum phosphate in many ESRD patients.8 Use of phosphate binders is also frequently accompanied by gastrointestinal side effects and poor patient compliance, which limits their use and underscores the need for additional therapeutic options.9
The mechanism of intestinal phosphate absorption in humans has not been fully elucidated. Conceptually, it entails 2 principal components: active transport (i.e., via specific ion channels) and inactive transport (i.e., paracellular). The sodium-dependent phosphate co-transporter type 2b (NPT-IIb; or SLC34A2) mediates active phosphate absorption in the small intestine, and in rodents it may account for up to 50% of the overall phosphate absorption.10, 11 This suggests that NPT-IIb may be a potential candidate for targeted hyperphosphatemia treatment.
ASP3325 is a novel and specific small-molecule NPT-IIb inhibitor that was found to be safe and effective in reducing systemic phosphate levels in nonclinical studies using a rat model of adenine-induced renal failure and in normal rats fed a high-phosphate diet.12 Consequently, ASP3325 was herein evaluated for the first time in a clinical setting. In addition to establishing its safety and pharmacokinetic profiles, the key objective was to determine its efficacy in reducing serum phosphate levels in ESRD patients as proof-of-concept for this therapeutic strategy.
Methods
Study Design
Two separate studies were performed with ASP3325. Study 1 (phase 1a) comprised both single ascending dose (SAD) and multiple ascending dose (MAD) experiments in healthy subjects, whereas study 2 was performed in ESRD patients on hemodialysis.
Study 1 was a randomized, double-blind, placebo-controlled dose escalation study. In the SAD part, ASP3325 was administered as a single dose in the dose range of 1 to 600 mg. There were a total of 12 cohorts, each with 6 subjects who received ASP3325 and 2 who received placebo. The study included healthy Japanese male and female subjects and 2 cohorts of Caucasian subjects receiving 10 or 100 mg ASP3325 or matching placebo. The study medication was administered under fasting conditions. Finally, 1 cohort received 100 mg ASP3325 or matching placebo under fed conditions. The study was completed with an end-of-study visit (follow-up examination) on day 7.
In the MAD part, healthy Japanese male and female subjects were administered ASP3325 or placebo as multiple doses, at doses of 10, 30, and 100 mg 3 times daily for 7 days. The study medication was administered 5 minutes after completion of a meal. In each cohort, 8 healthy subjects received ASP3325 and 4 subjects received placebo. The study was completed with an end-of-study visit on day 13 (follow-up examination) after final discharge. Details of the study design are provided in Supplementary Appendix S1. The inclusion and exclusion criteria are provided in Supplementary Appendix S2.
In both parts of study 1, the safety, tolerability, pharmacokinetics, and pharmacodynamics were evaluated. The study subjects consumed standardized meals with known phosphorus and calcium content during hospitalization. Urinary phosphate excretion was measured in both studies, whereas fecal phosphorus excretion was measured only in the MAD study. The study and data collection were conducted at Sumida Hospital, Tokyo, Japan.
Study 2 (phase 1b) was conducted in hyperphosphatemic ESRD patients undergoing hemodialysis and was a randomized, 2-arm, open-label study to evaluate the pharmacodynamics, pharmacokinetics, and safety of multiple oral dosing of ASP3325 100 mg 3 times daily before or just after each meal for 2 weeks. Twenty subjects with a serum phosphate level of ≥ 6.0 and < 10.0 mg/dl after washout with phosphate binders, and an increase of ≥ 1.5 mg/dl from the first day of the washout period, were randomized to the before-meal or after-meal group in a 1:1 ratio. The washout period lasted 1 week, and it was extended to a maximum of 2 weeks if not all phosphate-related inclusion criteria were met. Blood samples were collected at day 1, day 3, day 5, day 8, day 10, day 12, day 15, at the follow-up examination, and at the time of discontinuation. The study and data collection were conducted at Moriya Keiyu Hospital, Ibaraki, Japan; Ama Kyoritsu Clinic, Aichi, Japan; Hekikai Kyoritsu Clinic, Aichi, Japan; Sanaru Sun Clinic, Shizuoka, Japan; and Ibaraki Seinan Medical Center Hospital, Ibaraki, Japan. The inclusion and exclusion criteria are provided in Supplementary Appendix S2. Additional details are provided in Supplementary Appendix S3.
Outcomes
Study in Healthy Subjects
To evaluate the pharmacodynamics of ASP3325, the following parameters were evaluated: urinary phosphate excretion (SAD and MAD part) and fecal phosphorus excretion (MAD only). To evaluate the pharmacokinetics of ASP3325, the following parameters were evaluated: unchanged plasma ASP3325 concentration (SAD and MAD), urinary ASP3325 concentration (SAD and MAD), and fecal ASP3325 concentration (MAD only, ASP3325 100 mg 3 times daily). Safety evaluations included adverse events (AEs), vital signs, clinical laboratory tests, and standard 12-lead electrocardiograms.
Study in ESRD Patients
To evaluate the pharmacodynamics of ASP3325, the following parameters were measured: serum levels of phosphate, corrected calcium, intact parathyroid hormone, and FGF23. To evaluate the pharmacokinetics of ASP3325, trough plasma concentrations were measured up to day 15. Safety evaluations included AEs, vital signs, clinical laboratory tests, and standard 12-lead electrocardiograms.
Statistical Analysis
For study 1 in healthy subjects, no formal statistical sample size calculations were performed. The number of subjects was chosen based on practical grounds and previous experience with the first-in-human studies. For study 2 in ESRD patients, the sample size was determined as the sufficient number of subjects to perform a reasonable assessment of the pharmacodynamics and pharmacokinetics of ASP3325 based on previous experience and treatment effect similar to phosphate-binding studies.13, 14, 15 The pharmacodynamic variables were summarized by treatment group and visit and/or day. In addition, summaries are provided for the before-meal and after-meal groups.
Statistical analysis were performed using SAS Drug Development (version 3.4; SAS Institute, Cary, NC), PC-SAS (version 9.1.3; SAS Institute), and Phoenix WinNonlin (version 6.2; Pharsight Corporation, Mountain View, CA).
Study Approval
The 2 studies were approved by the institutional review board of each participating center. Each study was conducted in accordance with the principles of the Declaration of Helsinki. All subjects provided written informed consent prior to inclusion in each study. All studies were registered in ClinicalTrials.gov under the identifiers NCT02500953 and NCT02510274.
Results
Healthy Subjects
Disposition of Subjects
In the single ascending dose (SAD) study, 88 subjects were randomized to receive ASP3325 (n = 66) or placebo (n = 22). A total of 54 Japanese subjects (42 male, 12 female), and 12 Caucasian subjects (all male) received ASP3325. Eighteen Japanese subjects (14 male, 4 female) and 4 Caucasian subjects (all male) received placebo. In the multiple ascending dose (MAD) study, 36 Japanese subjects were randomized to receive ASP3325 (24 male, 12 female) or placebo (12 male, 6 female). All subjects who received the study drug completed the study and were eligible for inclusion in all analysis sets.
Demographic and Clinical Characteristics
In the SAD study, the mean age of each treatment group ranged from 22 to 36 years. The mean body weight of each treatment group among the Caucasian male subjects (range, 70–80 kg) was higher than that of the Japanese male (range, 56–68 kg) and female (range, 52–57 kg) subjects. In the MAD study, the mean age of each treatment group ranged from 27 to 28 years. No apparent differences in baseline characteristics were observed among the treatment groups.
Urinary and Fecal Phosphorus Excretion
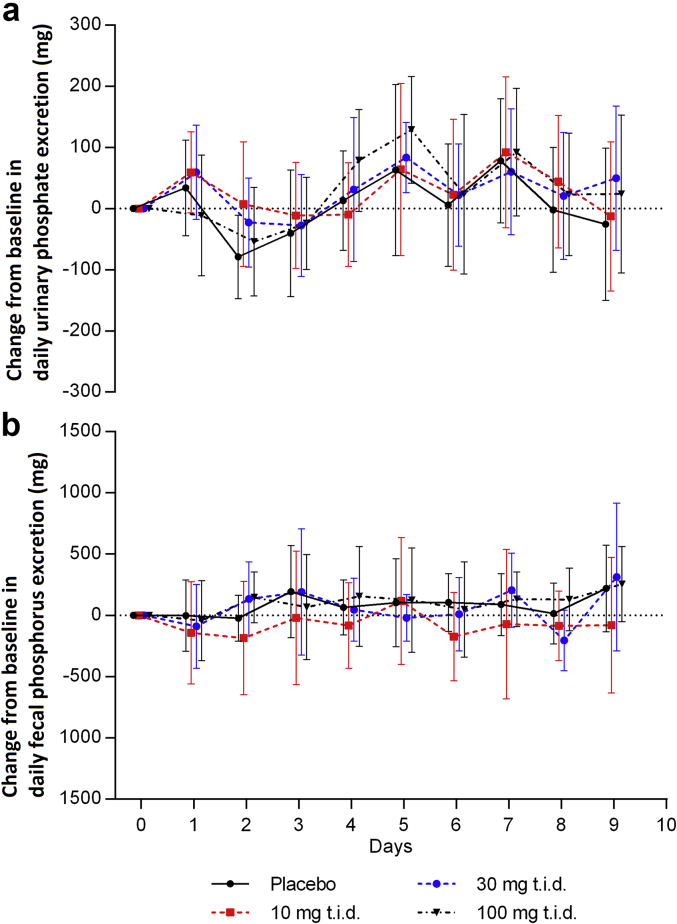
Figure 1a and b show the changes in urinary phosphate and fecal phosphorus excretion measured by 24-hour urine and fecal collections following multiple doses of ASP3325 (10, 30, or 100 mg) 3 times daily for 7 days in healthy subjects (MAD study). No apparent time- or dose-dependent changes were observed. Similarly, changes in these pharmacodynamic parameters were not observed in subjects who received a single dose of 3 to 600 mg of ASP3325 (SAD study, data not shown). Notably, there was a clear circadian variation in serum phosphate levels (data not shown) as described elsewhere.16
Figure 1.
Change from baseline in (a) daily urinary phosphate excretion; and (b) daily fecal phosphorous excretion in study 1 (multiple ascending dose; study drug dosed 3 times daily for 7 days) in healthy subjects with 3 dose levels of ASP3325 and placebo. No apparent time- or dose-dependent changes in these pharmacodynamic assessments were observed. Data are expressed as mean ± SD.
Pharmacokinetics
ASP3325 was systemically absorbed and showed a nearly dose-proportional increase in AUC up to 600 mg (single dose). Under fasting conditions, Cmax was reached within 1.4 to 6.7 hours. The plasma ASP3325 concentration decreased with a t1/2 of 15.5 to 21.8 hours. Only a small fraction of the dose was excreted in urine. After multiple dosing, the AUC24h and Cmax increased in an approximately dose-proportional manner. The t1/2 ranged from 17.8 to 18.3 hours. A steady state was attained within 7 days after multiple dosing, 3 times daily. Only a small fraction of the dose was excreted in urine, and approximately 74% of the dose was excreted in feces as unchanged drug following multiple oral dosing.
Safety
ASP3325 was safe and generally well tolerated in healthy subjects after single and multiple dosing. There were no deaths, serious treatment-emergent adverse events (TEAEs), or study discontinuations related to TEAEs. Following a single dose, the reported TEAEs included diarrhea (n = 1), nasopharyngitis (n = 1), an increase in total bile acids (n = 2), and an increase in blood creatine phosphokinase (n = 2). Following multiple dosing, enterocolitis (n = 1) was the only reported TEAE. All the TEAEs were mild in severity. Diarrhea (ASP3325 100-mg group, n = 1) and increase in total bile acids (ASP3325 10-mg group, n = 1) were the only events considered to be related to the study drug. No notable safety concerns were identified in vital regard to signs (i.e., blood pressure and pulse rate), clinical laboratory evaluations, or electrocardiograms.
ESRD Patients on Hemodialysis
Disposition of Subjects
Nineteen Japanese stable hemodialysis patients were randomized and received the study drug: 17 patients (89.5%) completed the study, and 2 patients (10.5%) discontinued. The primary reasons for discontinuation were AEs and subject withdrawal (n = 1, each) (Supplementary Appendix S4).
Demographic and Clinical Characteristics
Baseline demographic, clinical, and biochemical characteristics of the ESRD patients are shown in Table 1.
Table 1.
Baseline demographic, clinical, and biochemical characteristics of ESRD patients
| Parameter | Multiple dose (100 mg t.i.d.) |
||
|---|---|---|---|
| Before meal (n = 9) | After meal (n = 10) | Total (n = 19) | |
| Demographic and clinical characteristics | |||
| Age, yr | |||
| Mean ± SD | 60.7 ± 10.0 | 62.2 ± 8.9 | 61.5 ± 9.2 |
| Range | 47–73 | 44–73 | 44–73 |
| Sex, male, n (%) | 4 (44.4) | 5 (50.0) | 9 (47.4) |
| Primary CKD disease | |||
| Chronic glomerulonephritis | 4 (44.4) | 4 (40.0) | 8 (42.1) |
| Diabetic nephropathy | 2 (22.2) | 4 (40.0) | 6 (31.6) |
| Chronic pyelonephritis | 0 | 0 | 0 |
| Polycystic kidney disease | 2 (22.2) | 0 | 2 (10.5) |
| Nephrosclerosis | 0 | 1 (10.0) | 1 (5.3) |
| Other | 1 (11.1) | 1 (10.0) | 1 (10.5) |
| Before meal (n = 8) | After meal (n = 9) | Total (n = 17) | |
|---|---|---|---|
| Biochemical pharmacodynamic parameters | |||
| Serum phosphate at day 1 (mg/dl) | 8.33 ± 1.34 | 7.69 ± 1.27 | 7.99 ± 1.31 |
| Corrected serum Ca at day 1 (mg/dl) | 8.85 ± 0.84 | 8.72 ± 0.71 | 8.78 ± 0.75 |
| Serum intact PTH at day 1 (pg/ml) | 323.1±132.7 | 373.7 ± 205.1 | 349.9 ± 171.5 |
| Median | 304.0 | 441.0 | 321.0 |
| Serum FGF23 at day 1 (pg/ml) | 22,828 ± 29,562 | 15,180 ± 12,073 | 18,779 ± 21,695 |
| Median | 9870.00 | 17,200.00 | 12,100.00 |
Data are presented as n (%) unless otherwise indicated.
Ca, calcium; ESRD, end-stage renal disease; FGF23, fibroblast growth factor−23; PTH, parathyroid hormone; t.i.d., 3 times daily.
Serum Phosphate and Other Markers of Mineral Metabolism
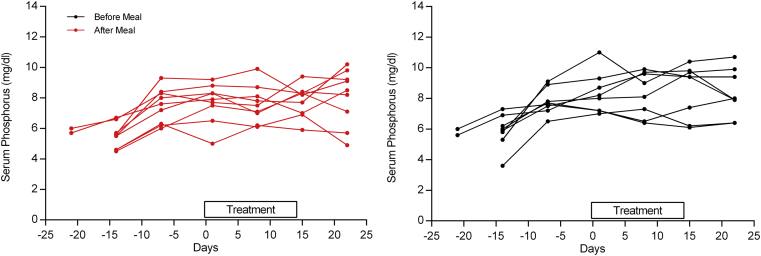
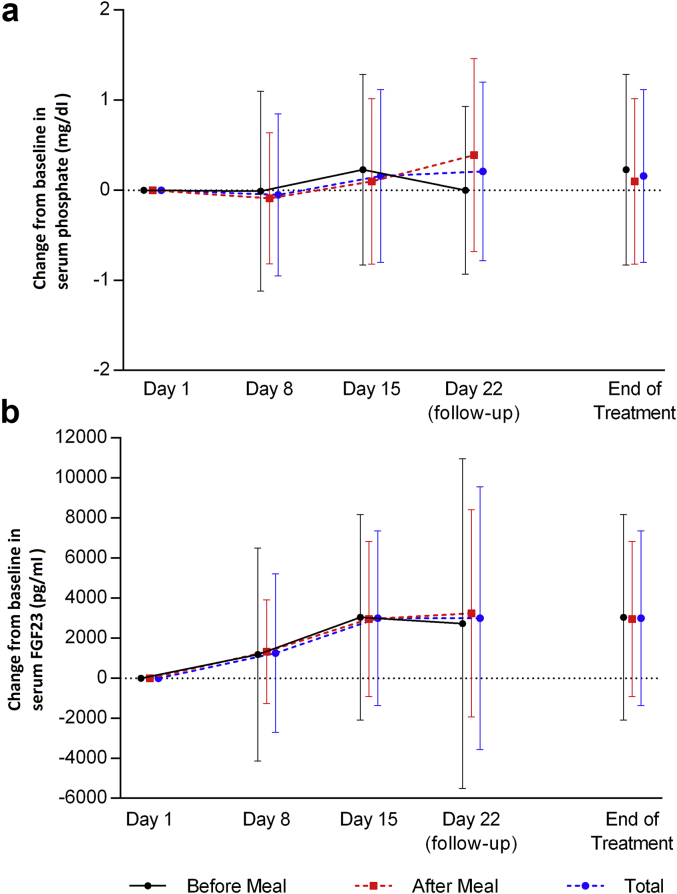
Prior to randomization, phosphate binders were washed out for 1 to 2 weeks (see inclusion and exclusion criteria in Supplementary Appendix S2). Eligible patients were required to have an increase in serum phosphate level of ≥ 1.5 mg/dl from the first day of the washout period; the increase in serum phosphate levels prior to randomization (driven by the study design) is shown in Figure 2. However, dosing with ASP3325 300 mg 3 times daily before or after a meal did not reduce serum phosphate levels in ESRD patients undergoing hemodialysis after 2 weeks of treatment (Figure 3a), and there were no apparent differences in serum phosphate levels between the before-meal and after-meal groups (Figure 2). The mean serum fibroblast growth factor−23 (FGF23) level increased somewhat during the study treatment, although the variability was high (Figure 3b). The mean corrected serum calcium level slightly decreased in the before-meal group and slightly increased in the after-meal group during the study treatment, whereas no consistent trend was observed in the median change from baseline in serum intact parathyroid hormone (data not shown).
Figure 2.
Changes in mean serum phosphate levels (n = 16) from screening to end of treatment. Note that serum phosphate levels increased after washout of phosphate-binding therapy up to the day of randomization (day 0); however, no reduction in serum phosphate levels occurred during the active treatment period with ASP3325 (days 0–14).
Figure 3.
Mean (± SD) change from baseline in (a) serum phosphate levels and (b) fibroblast growth factor−23 (FGF23) levels in end-stage renal disease patients undergoing hemodialysis (study 2). ASP3325 100 mg 3 times daily (administered either before or after meals) did not reduce serum phosphate or FGF23 levels after 2 weeks of treatment.
Pharmacokinetics
The pharmacokinetics of a single dose of ASP3325 (300 mg) was evaluated in 6 ESRD patients on hemodialysis and was comparable with the pharmacokinetics in healthy subjects (data not shown).
Following multiple oral dosing of ASP3325 100 mg 3 times daily before meals, the individual trough plasma concentration (Ctrough) appeared to reach a steady state on day 3 in most subjects. The mean and median Ctrough were generally higher in the after-meal group compared with the before-meal group.
Safety
ASP3325 was generally safe and well tolerated in ESRD patients on hemodialysis. No deaths or serious TEAEs were reported. TEAEs were reported in 6 of 19 subjects (31.6%), and drug-related TEAEs were reported in 3 (15.8%). Diarrhea and vomiting were TEAEs reported in ≥ 2 subjects (n = 2, each). Permanent discontinuation caused by TEAEs was reported in 2 subjects (10.5%). All of these events were mild or moderate in severity and were considered related to the study drug. No notable safety concerns were identified in vital signs, clinical laboratory evaluations, or electrocardiograms.
Discussion
The present study is the first to report on the efficacy and safety of a specific NPT-IIb inhibitor (ASP3325) for the treatment of hyperphosphatemia in ESRD patients. Although no concerns were raised in terms of safety and tolerability, ASP3325 failed to influence the primary efficacy assessments, namely, urinary phosphate and fecal phosphorous excretion in healthy subjects and serum phosphate levels in ESRD patients. ASP3325 also failed to reduce other biomarkers of disordered phosphate metabolism; for example, the mean FGF23 level increased rather than decreased, as would have been expected if intestinal phosphate absorption were reduced.17 Thus, it is unlikely that ASP3325 is able to effect downstream benefits of FGF23 lowering in ESRD patients, which were associated with improvements in cardiovascular outcomes as shown in a post hoc analysis of the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) study.18
The lack of efficacy of ASP3325 sharply contrasts with a preceding nonclinical study that demonstrated a robust effect in mitigating hyperphosphatemia in several rat models.12 Studies in mice harboring a deletion of the Npt2b gene indicate that NPT-IIb−mediated phosphate transport accounts for up to 50% of total intestinal phosphate reabsorption, and that Npt2b gene ablation mitigates hyperphosphatemia in mice with renal failure.11 Differences in intestinal phosphate transport among species could therefore possibly explain our negative clinical findings. Indeed, the relevance of NPT-IIb in human intestinal phosphate transport has not been proved, and data on its expression level and localization in the human small intestine are scarce. Insufficient drug exposure may be another reason, but is contradicted by pharmacokinetic simulations indicating intestinal drug exposure exceeding the inhibitor concentration above 90% (IC90, derived from in vitro studies) in the ESRD patient study. The lack of ASP3325 specificity should also be considered, especially because inhibition of the renal transporters NPT-IIa or NPT-IIc could offset a reduction of urinary phosphate excretion in healthy subjects because of the systemic absorption of ASP3325. However, the inhibition of these transporters would be expected to decrease serum phosphate levels in the healthy volunteers, and it cannot explain the null findings in ESRD patients in whom renal elimination of phosphate is typically negligible. Unpublished in vitro data also confirmed the specificity for NPT-IIb without relevant inhibitory effects on NPT-IIa or NPT-IIc. Finally, the lack of target engagement in the intestine remains a possibility; intestinal target engagement has not been proved and is critical for efficacy, because NPT-IIb expression in enterocytes is luminal, and its inhibition with ASP3325 does not warrant systemic exposure. Nevertheless, the physicochemical properties of ASP3325 did not indicate impaired intestinal solubility as a potential reason for insufficient target engagement.
Nicotinamide is a marketed drug with anti-inflammatory and cholesterol-lowering properties, which also reduces serum phosphate levels in hemodialysis patients.19, 20, 21 A postulated mechanism of action involves negative transcriptional regulation of NPT-IIb. Such a mechanism is inconsistent with our findings, which failed to demonstrate a role of NPT-IIb in the regulation of serum phosphate. The phosphate-lowering capacity of nicotinamide may therefore be attributed to other pleiotropic effects.22 Alternatively, the possibility remains that nicotinamide offers a superior target engagement as compared to ASP3325.
Importantly, inactivating mutations of the NPT-IIb gene in humans cause a syndrome termed pulmonary alveolar microlithiasis that is predominantly characterized by interstitial lung calcifications.23 These patients have normal serum phosphate levels, presumably due to compensatory renal mechanisms. To date, there are no reported cases of pulmonary alveolar microlithiasis with severe impairments in renal function to support the assumption that NPT-IIb becomes progressively more important when normal homeostatic mechanisms are no longer operational (e.g., in renal failure). Our results indicate that NPT-IIb plays a small or negligible role in humans under such circumstances.
Currently marketed phosphate binders have several disadvantages, such as a large pill burden and low drug adherence,24 gastrointestinal side effects,25, 26 and risk of metal accumulation.27, 28, 29, 30 Clinical experience further suggests a maximum achievable reduction in serum phosphate level of approximately 2.0 mg/dl, which invariably leads to persistent hyperphosphatemia in many ESRD patients. The fundamental premise of any NPT-IIb inhibitor is to overcome these limitations and ultimately to improve hyperphosphatemia management. The current results cast a shadow on the future prospects of NPT-IIb inhibitors.
The present study has some limitations inherent to phase 1 studies, including a relatively small sample size and short treatment duration. The sample size and 2-week treatment duration in ESRD patients should nevertheless be sufficient to detect a clinically relevant pharmacodynamic signal based on the dynamics of phosphate transport, and as evidenced in previous clinical studies with phosphate binders.13, 14, 15 Finally, the study involving ESRD patients was limited by its open-label design and lack of a control arm. The strengths of the present study include a thorough evaluation of the total body phosphorus balance in healthy subjects (i.e., standardized food intake with known phosphorous and calcium content, urine excretion, fecal excretion, and serum level) under standardized conditions in a residential setting.
In conclusion, the role of orally administered NPT-IIb inhibitors for the treatment of hyperphosphatemia is uncertain and merits further investigation. It will be critical to establish whether our findings are compound specific or can be generalized to other development candidates.
Disclosure
TEL and RAS are employees of Astellas Pharma Europe BV. CK, IN, YT, and SY are employees of Astellas Pharma Inc (Japan). TA has consultancy agreements with Astellas Pharma Inc, Kyowa Hakko Kirin Co Ltd, Bayer HealthCare Pharmaceuticals, JT Pharmaceuticals Corporate, Nipro Medical Corporation, Fuso Pharmaceutical Industries Ltd, and Ono Pharmaceutical Co Ltd, and is a member of the Astellas advisory panel. TA is also a scientific advisor of Bayer HealthCare Pharmaceuticals, Kyowa Hakko Kirin Co Ltd, Chugai Pharmaceutical Co Ltd, and Torii Pharmaceutical. TEL is affiliated with the Department of Clinical Science, Intervention and Technology, Renal Unit, Karolinska Institutet, Stockholm, Sweden.
Acknowledgments
The authors would like to thank all study participants. The authors wish to thank Michelle Belanger, MD, and Keyra Martinez Dunn, MD, of Edanz Medical Writing, on behalf of Springer Healthcare, for providing medical writing assistance.
This study was sponsored by Astellas Pharma Inc, the manufacturer of ASP3325. Medical writing assistance was provided by Michelle Belanger, MD, and Keyra Martinez Dunn, MD of Edanz Medical Writing, and was funded by Astellas. Medical writing services complied with international guidelines for Good Publication Practice. Astellas Pharma Inc was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Acknowledgments
Author contributions
All authors participated in the study design and interpretation of the data. In addition, SY was involved in the statistical analysis.
Footnotes
Appendix S1. Additional details of the study design (study 1; single ascending dose and multiple ascending dose).
Appendix S2. Inclusion and exclusion criteria.
Appendix S3. Additional details of the study design (study 2).
Appendix S4. Disposition of subjects (study 2).
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Additional details of the study design (study 1; single ascending dose and multiple ascending dose).
Inclusion and exclusion criteria.
Additional details of the study design (study 2).
Disposition of subjects (study 2).
References
- 1.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268:456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Yang J., Ackerson L.M. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 3.Tentori F., Blayney M.J., Albert J.M. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Wald R., Sarnak M.J., Tighiouart H. Disordered mineral metabolism in hemodialysis patients: an analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis. 2008;52:531–540. doi: 10.1053/j.ajkd.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Block G.A., Klassen P.S., Lazarus J.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 8.Shaman A.M., Kowalski S.R. Hyperphosphatemia management in patients with chronic kidney disease. Saudi Pharm J. 2016;24:494–505. doi: 10.1016/j.jsps.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fissell R.B., Karaboyas A., Bieber B.A. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial Int. 2016;20:38–49. doi: 10.1111/hdi.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabbagh Y., Giral H., Caldas Y. Intestinal phosphate transport. Adv Chronic Kidney Dis. 2011;18:85–90. doi: 10.1053/j.ackd.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbagh Y., O’Brien S.P., Song W. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol. 2009;20:2348–2358. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniguchi K., Terai K., Terada Y. Novel NaPi-IIb inhibitor ASP3325 inhibits phosphate absorption in intestine and reduces plasma phosphorus level in rats with renal failure. J Am Soc Nephrol. 2015;(suppl):582A. FR-PO936. [Google Scholar]
- 13.Gulati A., Sridhar V., Bose T. Short-term efficacy of sevelamer versus calcium acetate in patients with chronic kidney disease stage 3-4. Int Urol Nephrol. 2010;42:1055–1062. doi: 10.1007/s11255-009-9688-9. [DOI] [PubMed] [Google Scholar]
- 14.Al-Baaj F., Speake M., Hutchison A.J. Control of serum phosphate by oral lanthanum carbonate in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short-term, placebo-controlled study. Nephrol Dial Transplant. 2005;20:775–782. doi: 10.1093/ndt/gfh693. [DOI] [PubMed] [Google Scholar]
- 15.Block G.A., Fishbane S., Rodriguez M. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3-5. Am J Kidney Dis. 2015;65:728–736. doi: 10.1053/j.ajkd.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Portale A.A., Halloran B.P., Morris R.C., Jr. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80:1147–1154. doi: 10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakova T., Barchi-Chung A., Enfield G. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol. 2013;8:1009–1018. doi: 10.2215/CJN.09250912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moe S.M., Chertow G.M., Parfrey P.S. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation. 2015;132:27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y., Tanaka A., Nakamura T. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 20.Shahbazian H., Zafar Mohtashami A., Ghorbani A. Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: a double-blind randomized clinical trial. Nefrologia. 2011;31:58–65. doi: 10.3265/Nefrologia.pre2010.Nov.10734. [DOI] [PubMed] [Google Scholar]
- 21.Vasantha J., Soundararajan P., Vanitharani N. Safety and efficacy of nicotinamide in the management of hyperphosphatemia in patients on hemodialysis. Indian J Nephrol. 2011;21:245–249. doi: 10.4103/0971-4065.83735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shils M.E., Olson J.A., Shike M. 9th ed. Williams & Wilkins; Baltimore, MD: 1999. Modern Nutrition in Health and Disease. [Google Scholar]
- 23.Corut A., Senyigit A., Ugur S.A. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu Y.W., Teitelbaum I., Misra M. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akizawa T., Origasa H., Kameoka C. Randomized controlled trial of bixalomer versus sevelamer hydrochloride in hemodialysis patients with hyperphosphatemia. Ther Apher Dial. 2014;18:122–131. doi: 10.1111/1744-9987.12068. [DOI] [PubMed] [Google Scholar]
- 26.Xu J., Zhang Y.X., Yu X.Q. Lanthanum carbonate for the treatment of hyperphosphatemia in CKD 5D: multicenter, double blind, randomized, controlled trial in mainland China. BMC Nephrol. 2013;14:29. doi: 10.1186/1471-2369-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama K., Hirakata H., Akiba T. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36:478–487. doi: 10.1159/000344008. [DOI] [PubMed] [Google Scholar]
- 28.Hutchison A.J., Laville M. SPD405-313 Lanthanum Study Group. Switching to lanthanum carbonate monotherapy provides effective phosphate control with a low tablet burden. Nephrol Dial Transplant. 2008;23:3677–3684. doi: 10.1093/ndt/gfn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drüeke T.B. Lanthanum carbonate as a first-line phosphate binder: the “cons”. Semin Dial. 2007;20:329–332. doi: 10.1111/j.1525-139X.2007.00299.x. [DOI] [PubMed] [Google Scholar]
- 30.Tonelli M., Pannu N., Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010;362:1312–1324. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional details of the study design (study 1; single ascending dose and multiple ascending dose).
Inclusion and exclusion criteria.
Additional details of the study design (study 2).
Disposition of subjects (study 2).