Abstract
Severe hyperkalemia is a medical emergency that can cause lethal arrhythmias. Successful management requires monitoring of the electrocardiogram and serum potassium concentrations, the prompt institution of therapies that work both synergistically and sequentially, and timely repeat dosing as necessary. It is of concern then that, based on questions about effectiveness and safety, many physicians no longer use 3 key modalities in the treatment of severe hyperkalemia: sodium bicarbonate, sodium polystyrene sulfonate (Kayexalate [Concordia Pharmaceuticals Inc., Oakville, ON, Canada], SPS [CMP Pharma, Farmville, NC]), and hemodialysis with low potassium dialysate. After reviewing older reports and newer information, I believe that these exclusions are ill advised. In this article, I briefly discuss the treatment of severe hyperkalemia and detail why these modalities are safe and effective and merit inclusion in the treatment of severe hyperkalemia.
Keywords: colonic necrosis, Kayexalate, low potassium dialysate, severe hyperkalemia, sodium bicarbonate, sodium polystyrene sulfonate
Severe hyperkalemia is a medical emergency that threatens patients with lethal arrhythmias. Successful management requires monitoring of the electrocardiogram and serum potassium concentrations, prompt administration of therapies that work both sequentially and synergistically (Figure 1), and timely repeat dosing as necessary. If monitoring and follow-up care are not done carefully or if any therapy is excluded, death may result. In 1 study of patients with severe hyperkalemia, the number of measures used to treat hyperkalemia correlated with achieving a serum potassium concentration of <5.5 mmol/l and with survival.1
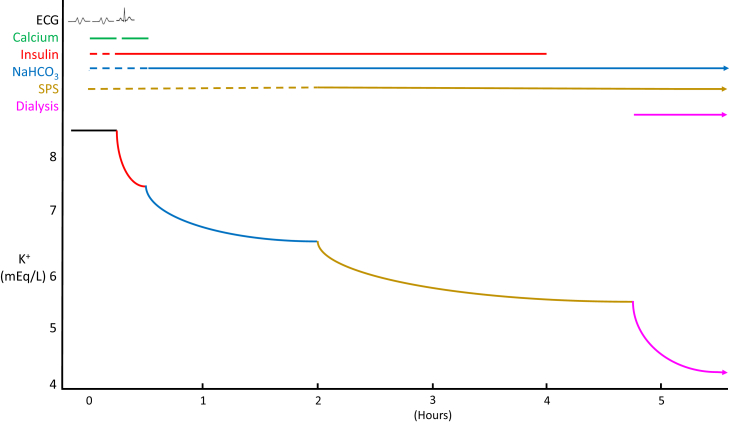
Figure 1.
Sequential response of hyperkalemia to 4 therapies administered as initial management to a virtual hemodialysis patient. This patient missed a week of dialysis and presented to the hospital with muscle weakness, bradycardia, a serum potassium concentration of 8.5 mmol/l, bicarbonate concentration of 16 mmol/l, and an electrocardiogram (ECG) showing loss of P waves and a QRS in a sine-wave pattern. The ECG reverted to normal sinus rhythm after a second dose of calcium before there was a significant change in serum potassium level. The arteriovenous fistula was clotted. By the time vascular access was re-established and dialysis was performed, the serum potassium fell to a safe level (5.2 mmol/l).
It is of concern then, that following published reports and opinions, many physicians no longer use 3 key modalities in the treatment of severe hyperkalemia: sodium bicarbonate, sodium polystyrene sulfonate (Kayexalate [Concordia Pharmaceuticals Inc., Oakville, ON], SPS [CMP Pharma, Farmville, NC]), and hemodialysis with low or no potassium dialysate. These exclusions, although well-meaning, are based on fallacies due to failure to consider all available information. The applicable literature is reviewed here to support this view.
As background, severe hyperkalemia is a serum potassium concentration of >6.0 or >5.5 mmol/l with an arrhythmia or hyperkalemic electrocardiographic changes. It is usually multifactorial, caused by various combinations of renal failure (often oliguric), potassium supplements, drugs that impair renal potassium excretion, and movement of potassium out of cells due to hyperglycemia or inorganic metabolic acidosis (Table 1).1, 2, 3, 4 In 1 study, severe hyperkalemia included approximately 0.6% of hospital admissions and was associated with high comorbidity (chronic kidney disease [70%], hypertension [46%], diabetes mellitus [41%], malignancy [32%], and multiorgan failure [25%]), and with high mortality of approximately 31%; the principal causes of death were septic shock (28%), respiratory (16%), and cardiac (15%).1 When serum potassium was ≥6.5 mmol/l, 50% of patients had hyperkalemia-induced electrocardiographic changes, and 47% were symptomatic with muscle weakness, arrhythmias, or cardiac arrest.
Table 1.
Principal causes of hyperkalemia
| Acute and/or chronic kidney failure (often oliguric): decreases urinary excretion of potassium | |
| Increased potassium intake | |
| Decreased tubular potassium secretion: decreases urinary excretion of potassium | |
| Decreased aldosterone level | Decreased aldosterone effect |
| Primary adrenal insufficiency | Mineralocorticoid receptor antagonists: spironolactone, eplerenone |
| Heparin: impairs aldosterone synthesis | Epithelial sodium channel inhibitors: amiloride, triamterene, trimethaprim |
| Low renin states: diabetic nephropathy, β-blockers, nonsteroidal anti-inflammatory drugs | Calcineurin inhibitors: cyclosporine, tacrolimus |
| Angiotensin-converting enzyme inhibitors | Tubular defects: lupus nephritis, obstructive uropathy, sickle cell disease |
| Angiotensin receptor blockers | |
| Movement of potassium out of cells (acute hyperkalemia) | |
| True hyperkalemia | Pseudohyperkalemia (false positive) |
| Metabolic acidosis-inorganica | Hemolysis in vitro |
| β-blockers | Platelets >500,000/mm3 |
| Insulin deficiency | White cells >120,000/mm3 |
| Hyperosmolality-hyperglycemia | |
| Cell breakdown-tumor, muscle, red cell | |
Drugs that impair renal potassium excretion are indicated in bold.
Hyperkalemia is common with type 4 renal tubular acidosis and uremic acidosis (i.e. acidosis of renal failure); it is uncommon with the acidosis of diarrhea or types 1 (distal) or 2 (proximal) renal tubular acidosis.
The management of severe hyperkalemia involves 3 tasks, which should be carried out in close succession (Table 2)5:
-
i.
Antagonize any electrocardiographic changes caused by hyperkalemia with i.v. calcium chloride or gluconate. Repeat the dose if the changes do not resolve or recur.
-
ii.
Redistribute potassium into cells with insulin, albuterol, and/or sodium bicarbonate, if there is metabolic acidosis. Repeat as necessary. Monitor the blood glucose level hourly after insulin administration. If the blood glucose is <200 mg/dl, use i.v. glucose to prevent insulin-induced hypoglycemia.
-
iii.
Remove potassium from the body with oral (or rectal) sodium polystyrene sulfonate. This is usually suspended in 33% sorbitol to move the sodium polystyrene sulfonate through the gastrointestinal tract. Alternatively, remove potassium from the body with hemodialysis if the patient has a vascular access, and dialysis can be initiated in <4 hours (duration of insulin effect). Also, use hemodialysis if sodium polystyrene sulfonate cannot be given or is ineffective. Occasionally, rapid recovery of acute kidney injury produces urinary excretion of potassium, which augments and then may exceed the potassium removed by sodium polystyrene sulfonate. This can be seen after the repletion of a volume deficit in prerenal azotemia or after the relief of urinary obstruction. Unfortunately, the timing and amount of potassium excretion cannot be predicted.
Table 2.
Treatment of severe hyperkalemia
| Mechanism | Treatment (initial dose) | Onset (duration) of action | Redosing | Comments |
|---|---|---|---|---|
| Antagonize ECG changes | CaCl or Ca gluconate (1 g i.v. over 2–3 min) | 1 min (30–60 min) | If ECG changes do not resolve or recur | Does not affect serum K level |
| Redistribute K into cells | NaHCO3 (150 mEq i.v. over 3–4 h) | 2–3 h (permanent) | Not necessary if HCO3 normalized | Effective in metabolic acidosis (HCO3 ≤17) |
| Insulin (10 U i.v.) | 10–20 min (4–6 h) | If K rises >6 mEq/l | I.v. glucose for blood glucose <200 mg/dl | |
| Albuterol (10–20 mg in 4 ml saline over 10 min by nebulizer) | 30 min (2–6 h) | If K rises >6 mEq/l | Up to 40% ESRD patients are resistant | |
| Remove K from the body | Na polystyrene sulfonate (30–60 g in 33% sorbitol) | 1–2 h (permanent) | Every 2 h to achieve K<6 | Not used with ileus or bowel obstruction |
| Hemodialysis | Minutes (permanent) | If K rises >6 mEq/l | Give Na polystyrene sulfate first, if dialysis delayed >4 h |
Ca, calcium; CaCl, calcium chloride; ECG, electrocardiographic; ESRD, end-stage renal disease; K, potassium; NaHCO3, sodium bicarbonate.
The new potassium binding resin, patiromer, reduced serum potassium concentration by only 0.21 mmol/l in 7 hours in a recent study; therefore, it is only used for chronic hyperkalemia.6
The 4 Fallacies
Fallacy 1
Sodium bicarbonate fails to lower serum potassium acutely and has no role in the emergent treatment of hyperkalemia.5, 7, 8, 9, 10
This viewpoint is based on reports of hyperkalemic patients who had minimal (<1 mmol/l) or no decrease in serum potassium concentration after receiving i.v. sodium bicarbonate.11, 12, 13, 14, 15, 16 However, other case series observed satisfactory decreases in serum potassium concentration (1.5–3.0 mmol/l) in response to sodium bicarbonate.17, 18, 19, 20 The responsive patients had metabolic acidosis with pH < 7.35, significant hypobicarbonatemia (serum bicarbonate levels < 17 mmol/l), doses of sodium bicarbonate > 120 mEq, and mostly serum potassium concentrations > 6 mmol/l. In contrast, the unresponsive patients had few of these features. Also, the unresponsive cases were all hemodialysis patients, although it is unclear how this might diminish the response to sodium bicarbonate.
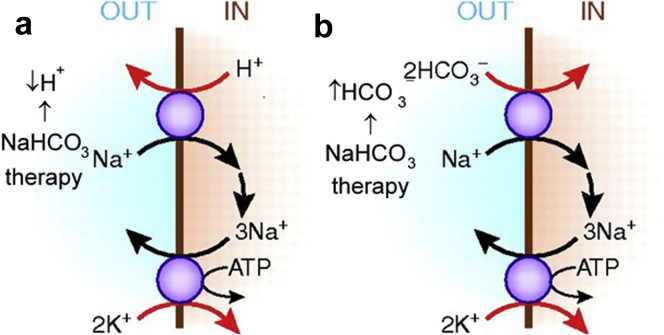
The greater efficacy of sodium bicarbonate in reducing serum potassium levels in patients with appreciable metabolic acidosis is ascribed to greater sodium entry into acidotic skeletal muscle. (Raising extracellular bicarbonate levels stimulates sodium bicarbonate co-transport into cells and falling extracellular hydrogen increases sodium cell entry via sodium−hydrogen exchange.) The higher intracellular sodium then increases sodium, potassium adenosine triphosphatase activity, and cellular potassium uptake (Figure 2).21 The response of hyperkalemia to sodium bicarbonate is observed even when an increase in partial pressure of carbon dioxide prevents any change in arterial pH; therefore, an increase in plasma bicarbonate independently appears to move potassium intracellularly.19, 22
Figure 2.
Muscle cell uptake of potassium during therapy with sodium bicarbonate (NaHCO3). Functional coupling between sodium−hydrogen (Na+–H+) exchange and Na+, potassium (K+) adenosine triphosphatase (ATPase) (a) leads to apparent K+–H+ exchange, and between Na+–HCO3– cotransport and Na+, K+–ATPase (b) leads to apparent K+–HCO3– cotransport.
Modified with permission from Figure 3 in Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981–1989.21 Copyright © American Society of Nephrology.
One concern about the efficacy of sodium bicarbonate is that it only works over several hours, which is not useful in severe hyperkalemia.7 This mostly reflects the usual practice of administering sodium bicarbonate over ≥4 hours. However, it is likely that rapid administration is also effective. In 1 patient, a dose of 144 mEq given over 135 minutes led to a fall of serum potassium concentration from 8.6 to 6.5 mmol/l and reversal of electrocardiographic changes caused by hyperkalemia.18 In 10 cases, 50 mEq given over 15 minutes produced a 0.5 mmol/l drop at 30 minutes.23
Therefore, when patients with severe hyperkalemia have significant metabolic acidosis, sodium bicarbonate should be part of the treatment. It has worked in patients on long-term hemodialysis. It provides additional potassium lowering when added to insulin or to insulin and albuterol.23 However, sodium bicarbonate should not be given to hypervolemic patients due to the risk of pulmonary edema or to patients with organic acidosis, such as lactic or ketoacidosis, in whom the acidosis does not contribute to the hyperkalemia.24
The amount of sodium bicarbonate needed is unpredictable.22, 24 One may start by giving 150 mEq in 1 liter 5% dextrose over 3 to 4 hours, and then look for a lowering of the serum potassium concentration and correction of acidosis. If necessary, additional sodium bicarbonate can be administered to completely correct the serum bicarbonate deficit (i.e., to bring the level to 24 mmol/l). The bicarbonate can be assumed to distribute through a virtual space equal to 50% of body weight, so the dose for complete correction would be body weight (in kilograms) × 0.5 × bicarbonate deficit per liter. With serum bicarbonate levels <10 mmol/l, the virtual space increases to 70% or even 100% of body weight, and dosing should be increased accordingly.25, 26
Parenteral sodium bicarbonate is available as a hypertonic solution of 44.6 or 50 mEq in 50-ml vials. It is usually given i.v. as an isotonic solution by adding 3 vials to 1 liter of 5% dextrose. Direct injection of the undiluted vials is discouraged because each vial would raise serum sodium concentration by approximately 1.3 mmol/l in a 70-kg patient, and this increase in osmolarity would partly counteract the effect of lowering potassium.22, 24 Nevertheless, decreases in serum potassium by 2.1 to 3.0 mmol/l have been seen using hypertonic sodium bicarbonate in severely acidotic patients (pH 7.10 to 7.18).18
Fallacy 2
Sodium polystyrene sulfonate is of uncertain efficacy,9, 10, 27, 28, 29, 30 and if effective, it is only after a delay of several hours, making its usefulness questionable for severe hyperkalemia.5, 8, 9, 28, 30, 31 Sodium polystyrene sulfonate is not recommended to treat life-threatening hyperkalemia,8, 9, 27, 29 but some would use it as a last resort.28
The questioning of the efficacy of sodium polystyrene sulfonate is based on 3 studies. In one, serum potassium concentrations fell by 1.4 mmol/l over 5 days in 5 hyperkalemic patients on 60-g sodium polystyrene sulfonate per day combined with sorbitol.32 This drop in potassium was <1.7 mmol/l seen in a control group on sorbitol alone. However, the 2 groups were not comparable because sorbitol alone produced voluminous diarrhea, which is known to cause potassium loss, and the sodium polystyrene sulfonate group experienced a 9 mmol/l increase in serum sodium, which is known to raise serum potassium concentration due to its effect on tonicity.22
In another study, serum potassium concentrations remained constant over 12 hours in 6 hemodialysis patients who were given 30-g sodium polystyrene sulfonate and sorbitol.33 However, serum potassium levels rose 0.4 mmol/l in a placebo group, perhaps due to absorption of dietary potassium and release of potassium from cells by catabolism. Therefore, rather than being ineffective, the sodium polystyrene sulfonate seemed to prevent this increase. Also, the patients were normokalemic; had they been hyperkalemic, stool potassium concentration would have been higher,34 and the sodium polystyrene sulfonate might have bound more potassium.
In a third study, 9 of 32 hyperkalemic patients given sodium polystyrene sulfonate had no or <0.5 mmol/l decrease in serum potassium concentrations; however, the other 23 patients had significant drops in serum potassium concentrations by 0.5 to 2.8 mmol/l.35 The authors believed that the failure of sodium polystyrene sulfonate to control hyperkalemia in these 9 cases was caused by tissue damage that released potassium and that higher sodium polystyrene sulfonate doses might have been more effective.
To dispel the doubt that these 3 studies cast on potassium lowering by sodium polystyrene sulfonate, all 7 of the most recent case series of sodium polystyrene sulfonate use confirmed its effectiveness in almost 800 patients; single 60- to 80-g doses were followed by average falls in serum potassium of 0.9 to 1.7 mmol/l.36, 37, 38, 39, 40, 41, 42 The average decreases in serum potassium increased significantly from 0.6 to 0.9 to 1.2 mmol/l as sodium polystyrene sulfonate doses increased from 15 to 30 to 60 g.36, 37, 38, 40, 42 Regarding onset of action, a significant fall of 0.6 mmol/l was noted in potassium concentrations measured from 0 to 4 hours after sodium polystyrene sulfonate administration.39
Fallacy 3
Intestinal necrosis, usually of the colon, is an infrequent, but often fatal complication of sodium polystyrene sulfonate.5, 28, 29, 30, 43, 44
The role of sodium polystyrene sulfonate in producing intestinal necrosis is based on 3 types of evidence. The first involves studies in rats. In 1 study, rats given 10-ml enemas with 70% sorbitol, which is 47 times the dose used in humans, developed colonic necrosis, whereas enemas of sodium polystyrene sulfonate at 23 times the human dose had no toxic effect.45 In contrast, in a more recent study, only 1 of 5 rats given enemas with 33% sorbitol had necrosis, but of the 8 rats given sodium polystyrene sulfonate enemas, 6 were dead or dying, and 3 had mucosal ulcerations, although the dose was only one-quarter the dose of the previous study.46 Both studies found that rats given sodium polystyrene sulfonate in sorbitol developed colonic necrosis, but the earlier study ascribed it to sorbitol, whereas the later study ascribed the necrosis to sodium polystyrene sulfonate. Although these results are concerning for intestinal toxicity of sodium polystyrene sulfonate and sorbitol, the use of doses many times the human dose and the contradictory findings between the 2 rat studies cast doubt on the clinical application of these reports.
Another observation that implicated sodium polystyrene sulfonate as the cause of intestinal necrosis was the finding of sodium polystyrene sulfonate crystals in histologic specimens of necrotic bowels of patients.47, 48 However, this only showed that patients received sodium polystyrene sulfonate because crystals may be seen with a normal bowel or with an unrelated pathology.47 Crystals are not always seen in the necrotic mucosa of patients reported to have sodium polystyrene sulfonate−induced necrosis.43
The third type of evidence is of the numerous patients who experienced intestinal necrosis after exposure to sodium polystyrene sulfonate.43, 49 Although of great concern, these reports do not prove causation. They support an etiologic role of sodium polystyrene sulfonate in intestinal necrosis if the patients had no other risk factors for it, such as old age, chronic kidney disease stages IV or V, congestive heart failure, coronary artery disease, diabetes mellitus, peripheral vascular disease, and chronic pulmonary disease.50, 51, 52 However, these factors were present in most cases of intestinal necrosis ascribed to sodium polystyrene sulfonate.43, 50, 53 Also, sodium polystyrene sulfonate would appear causative in cases of intestinal necrosis if this complication occurred more often in individuals exposed to sodium polystyrene sulfonate than in those who have not been exposed. However, in a retrospective cohort study of sodium polystyrene sulfonate and colonic necrosis in a tertiary medical center, 3 of 2194 individuals receiving sodium polystyrene sulfonate or 0.14% had colonic necrosis; this was not statistically different from the 79 of >121,000 individuals who were not given sodium polystyrene sulfonate or 0.07% who experienced colonic necrosis.49 Similarly, in a retrospective study of 11,409 hemodialysis patients, 20% were prescribed sodium polystyrene sulfonate. Colonic surgery, a marker for colonic necrosis, was no more common in patients who received sodium polystyrene sulfonate (0.6%) than in those not given sodium polystyrene sulfonate (1.0%).54 It is not clear if sodium polystyrene sulfonate causes intestinal necrosis; instead, it may just be a marker for risk factors for intestinal necrosis, such as chronic and end-stage renal disease. Even if sodium polystyrene sulfonate with or without sorbitol can rarely produce intestinal necrosis, it is so uncommon (much <1%) that physicians should not hesitate to prescribe it for severe hyperkalemia if it might be lifesaving.
Therefore, because of the effectiveness and relative safety of sodium polystyrene sulfonate, it could be concluded that unless hemodialysis is planned within 4 hours, it should be administered to patients with severe hyperkalemia after insulin, and after calcium, albuterol, and sodium bicarbonate, if they are used. Ileus and intestinal obstruction are contraindications.
The potassium-lowering response to sodium polystyrene sulfonate is unpredictable, although higher doses generally produce greater decreases than lower doses, and a 60-g dose decreases potassium by approximately 1.2 mmol/l (range: 0.9–1.7 mmol/l in different studies).36, 37, 38, 40, 42 A lesser response can be anticipated in patients who release potassium because of cell breakdown, as happens with rhabdomyolysis, and in larger patients, whose greater muscle mass contains a greater excess of total body potassium.24 The goal is to reduce the serum potassium to ≤5.5 mmol/l, so 60 g of sodium polystyrene sulfonate with 33% sorbitol should be given when potassium is >6.5 mmol/l. However, smaller or larger doses (e.g., 90 g) can be given initially depending on the size of the patient and degree of hyperkalemia. Serum potassium levels should be obtained every 2 hours, and doses of sodium polystyrene sulfonate should be repeated until the potassium level is at goal.
Fallacy 4
Although hemodialysis with low potassium dialysate (0 or 1 mmol/l) removes more potassium55, 56 and seems necessary to achieve normal serum potassium concentrations after the postdialysis rebound,57 low potassium dialysate may increase the risk of significant arrhythmias and sudden death and should not be used.5, 58, 59, 60
This viewpoint is based on 2 types of evidence. The first was the occurrence of frequent or complex ventricular premature contractions in some patients during and after hemodialysis,61, 62, 63, 64, 65 together with the observation that avoiding the rapid fall in serum potassium levels early in hemodialysis64, 65 or raising the dialysate concentration of potassium (to 3.0 or 3.5 mmol/l)61, 66 decreased the frequency of ventricular premature contractions. The rapid fall in serum potassium was avoided by starting with a higher than usual dialysate potassium concentration and reducing it during the dialysis to a lower than usual concentration, thus maintaining a constant concentration difference between the dialysate and the plasma (constant gradient dialysis). Unfortunately, the clinical significance of the reduced ventricular ectopy in these studies is clouded by contradictory reports. Constant gradient dialysis reduced ectopy only early in dialysis in 1 study,64 and only 14 hours after dialysis in another.65
The studies on low potassium dialysate are similarly difficult to interpret. One study found no increase in arrhythmias that occurred during potassium-free dialysate compared with standard potassium dialysate (2 mmol/l).62 When another study found increased arrhythmias with potassium-free dialysate, the significance was questionable because heart disease as measured by moderate or severe left ventricular hypertrophy was more common in patients with arrhythmias than in those without arrhythmias.67
A further challenge to the ventricular ectopy−low potassium association are studies that showed that the decrement in serum potassium during dialysis was no greater,62, 67 and the predialysis68 and postdialysis potassium concentrations were no lower61, 62 in patients with arrhythmias than in those without arrhythmias. A final weakness in the proposal that low potassium dialysate might cause sudden death through ventricular ectopy was found in a 4-year follow-up of 127 hemodialysis patients, including 97 with ventricular arrhythmias, in whom ectopy did not predict overall mortality.69 One should note that none of the previously described reports on ventricular ectopy during hemodialysis dealt with patients with severe hyperkalemia.
The other evidence for increased risk of low potassium dialysate is the association between low potassium dialysate (0 or 1 mmol/l) and sudden death,70, 71, 72 which was noted in 3 observational studies of large hemodialysis populations. However, this association could be questioned in 1 study70 because it made no statistical adjustments for older age and the many comorbidities present in the individuals who died suddenly. The other 2 studies found that when the monthly predialysis serum potassium was in the severe hyperkalemic range, low potassium dialysate was not associated with increased sudden death.71, 72 When the monthly serum potassium concentration was >5.5 mmol/l, low potassium dialysate was associated with fewer sudden cardiac deaths in a third study73 and lower all-cause mortality in a fourth study.74
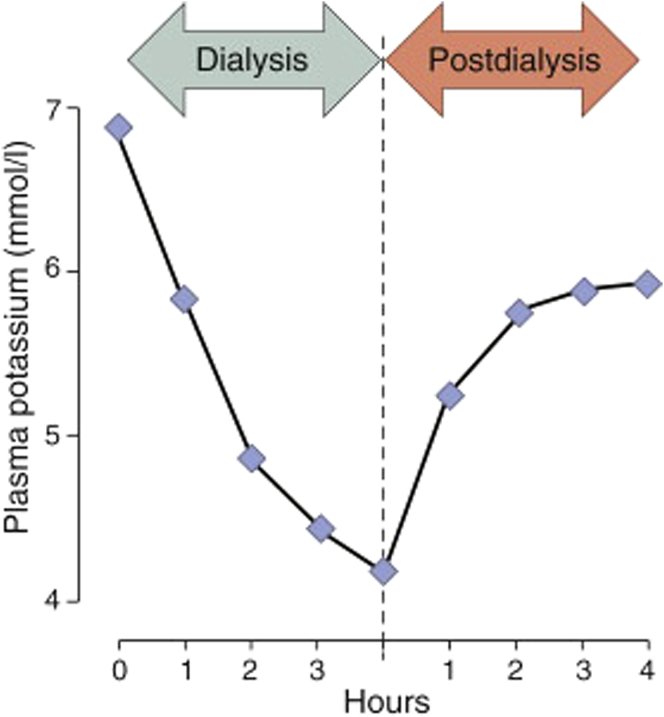
When hemodialysis is used to remove potassium in severe hyperkalemia, the aim is to safely remove enough potassium to bring the serum potassium concentration not only into the normal range, but bring it down low enough so that the dietary potassium intake of the patient will not lead to hyperkalemia by the next dialysis; however, only 1 study has shed light on how to do this.57 When 14 hyperkalemic patients were dialyzed against a 1 mmol/l dialysate, the average serum potassium fell from 5.7 to 3.6 mmol/l at the end of dialysis, but rose to 5.0 6 hours later as potassium reequilibrated from the cells to the extracellular fluid; 1 individual with a predialysis potassium level of 6.9 mmol/l equilibrated at 6.1 mmol/l (Figure 3). Therefore, not enough potassium was removed in any of the cases to bring the post-rebound serum potassium concentration comfortably within the normal range. A 0 mmol/l dialysate would have been better, as illustrated by a patient with a predialysis potassium concentration of 6.9 mmol/l who was dialyzed with a potassium-free dialysate; the postdialysis concentration was 3.3 mmol/l and the concentration 5 hours postdialysis was 4.4 mmol/l.75
Figure 3.
Plasma potassium concentration during and after dialysis.
Reproduced with permission from Blumberg A, Roser HW, Zehnder C, et al. Plasma potassium in patients with terminal renal failure during and after haemodialysis; relationship with dialytic potassium removal and total body potassium. Nephrol Dial Transplant. 1997;12:1629–1634. Copyright © Oxford University Press.
In summary, the original observation that ventricular ectopy during hemodialysis might be related to the rate of potassium removal or to low potassium dialysate has not been confirmed by subsequent studies, and the concern that ventricular ectopy presages sudden death or reduced survival was refuted by a study of 4-year mortality in such patients. Furthermore, rather than increasing risk in hyperkalemic patients, hemodialysis with low potassium dialysate was associated with less sudden death and mortality. Instead of avoiding the use of low potassium dialysate in patients with severe hyperkalemia, nephrologists should adopt it.
Absent convincing contradictory information, the author recommends the traditional “rule of 7” to determine the dialysate potassium concentration to be used for a 3-hour hemodialysis (or a longer treatment in a very large or morbidly obese individual). It stipulates that the sum of the dialysate potassium concentration and the serum potassium levels of the patient equals 7, and thus, the midpoint between (or average of) the 2 values is 3.5 mmol/l. In this way, the amount of potassium removed increases as the potassium concentration increases, and the serum potassium concentration at the end of dialysis is close to 3.5 mmol/l. Accordingly, individuals with potassium concentrations of ≥7 mmol/l would be dialyzed with a zero potassium dialysate. Most studies that have reported pre- and postdialysis serum potassium concentrations showed that postdialysis concentrations are within 0.5 mmol/l of the average of the initial potassium concentration in the patient and the dialysate concentration.55, 56, 57, 64, 66, 75 The safety of using the rule of 7 is confirmed by an analysis of observational data from 1267 hemodialysis patients over 5 years. Individuals dialyzed by the rule of 7 had a similar risk of death compared with the rest of the patients.76
This review discussed the therapy of severe hyperkalemia with sodium bicarbonate, sodium polystyrene sulfonate, and hemodialysis with low potassium dialysate, measures that have been used since the 1940s.77 Concerns were raised about the effectiveness of sodium bicarbonate and sodium polystyrene sulfonate, and about the safety of sodium polystyrene sulfonate and low-potassium dialysate, with the result that these measures were used less often and that remissions of hyperkalemia and survival appeared to be affected.1 However, careful consideration of the older reports and of newer information supports the conclusion that these modalities are safe, effective, lifesaving, and merit inclusion again in treatment of severe hyperkalemia.
Disclosure
The author declared no competing interests.
References
- 1.An J.N., Lee J.P., Jeon H.J. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225–R237. doi: 10.1186/cc11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acker C.G., Johnson J.P., Palevsky P.M. Hyperkalemia in hospitalized patients. Arch Intern Med. 1998;158:917–924. doi: 10.1001/archinte.158.8.917. [DOI] [PubMed] [Google Scholar]
- 3.Muschart X., Boulouffe C., Jamart J. A determination of the current causes of hyperkalaemia and whether they have changed over the past 25 years. Acta Clinica Belgica. 2014;69:280–284. doi: 10.1179/0001551214Z.00000000077. [DOI] [PubMed] [Google Scholar]
- 4.Hagan A.E., Farrington C.A., Wall G.C. Sodium polystyrene sulfonate for the treatment of acute hyperkalemia: a retrospective study. Clin Nephrol. 2015;85:38–43. doi: 10.5414/CN108628. [DOI] [PubMed] [Google Scholar]
- 5.Mount D.B. Disorders of potassium balance. In: Skorecki K., Chertow G.M., Marsden P.A., Taal M.W., Wasser W.G., editors. Brenner and Rector’s The Kidney. 10th ed. Elsevier; Philadelphia: 2016. pp. 559–600. [Google Scholar]
- 6.Bushinsky D.A., Williams G.H., Pitt B. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015;88:1427–1433. doi: 10.1038/ki.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allon M. Hyperkalemia in end-stage renal disease: mechanisms and management. J Am Soc Nephrol. 1995;6:1134–1142. doi: 10.1681/ASN.V641134. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell A.P., Linden K., O’Donnell S. Management of hyperkalaemia. J R Coll Physicians Edinb. 2013;43:246–251. doi: 10.4997/JRCPE.2013.312. [DOI] [PubMed] [Google Scholar]
- 9.Medford-Davis L., Rafique Z. Derangements of potassium. Emerg Med Clin N Am. 2014;32:329–347. doi: 10.1016/j.emc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Alfonzo A, Soar J, MacTier R, et al. Clinical practice guidelines: treatment of acute hyperkalaemia in adults. UK Renal Association. March 2014. Available at: https://renal.org/wp-content/uploads/2017/06/hyperkalaemia-guideline-1.pdf. Accessed May 16, 2017.
- 11.Blumberg A., Weidmann P., Shaw S. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. Am J Med. 1988;85:507–512. doi: 10.1016/s0002-9343(88)80086-x. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez R., Schlessinger F., Oster J.R. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end-stage renal disease. Miner Electrolyte Metab. 1991;17:297–302. [PubMed] [Google Scholar]
- 13.Blumberg A., Weidmann P., Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int. 1992;41:369–374. doi: 10.1038/ki.1992.51. [DOI] [PubMed] [Google Scholar]
- 14.Allon M., Shanklin N. Effect of bicarbonate administration on plasma potassium in dialysis patients: interactions with insulin and albuterol. Am J Kid Dis. 1996;28:508–514. doi: 10.1016/s0272-6386(96)90460-6. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.-J. Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia in end-stage renal disease patients. Nephron. 1996;72:476–482. doi: 10.1159/000188917. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.-J. Acute therapy for hyperkalemia with the combined regimen of bicarbonate and beta2-adrenergic agonist (salbutamol) in chronic renal failure patients. J Korean Med Sci. 1997;12:111–116. doi: 10.3346/jkms.1997.12.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnell J.M., Villamil M.F., Uyeno B.T. The effect in humans of extracellular pH change on the relationship between serum potassium concentration and intracellular potassium. J Clin Invest. 1956;35:935–939. doi: 10.1172/JCI103352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz K.C., Cohen B.D., Lubash G.D. Severe acidosis and hyperpotassemia treated with sodium bicarbonate infusion. Circulation. 1959;19:215–220. doi: 10.1161/01.cir.19.2.215. [DOI] [PubMed] [Google Scholar]
- 19.Fraley D.S., Adler S. Correction of hyperkalemia by bicarbonate despite constant blood pH. Kidney Int. 1977;12:354–360. doi: 10.1038/ki.1977.122. [DOI] [PubMed] [Google Scholar]
- 20.Carvalhana V., Burry L., Lapinsky S.E. Management of severe hyperkalemia without hemodialysis: case report and literature review. J Crit Care. 2006;21:316–321. doi: 10.1016/j.jcrc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Aronson P.S., Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981–1989. doi: 10.1681/ASN.2011040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adrogue H.J., Madias N.E. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981;71:456–467. doi: 10.1016/0002-9343(81)90182-0. [DOI] [PubMed] [Google Scholar]
- 23.Ngugi N.N., McLigeyo S.O., Kayima J.K. Treatment of hyperkalaemia by altering the transcellular gradient in patients with renal failure: effect of various therapeutic approaches. East African Med J. 1997;73:503–509. [PubMed] [Google Scholar]
- 24.Sterns R.H., Cox M., Feig P.U. Internal potassium balance and the control of the plasma potassium concentration. Medicine. 1981;60:339–354. doi: 10.1097/00005792-198109000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Garella S., Dana C.L., Chazan J.A. Severity of metabolic acidosis as a determinant of bicarbonate requirements. N Engl J Med. 1973;289:121–126. doi: 10.1056/NEJM197307192890303. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez P.C., Cohen R.M., Feldman G.M. The concept of bicarbonate distribution space: the crucial role of body buffers. Kidney Int. 1989;36:747–752. doi: 10.1038/ki.1989.258. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed J., Weisberg L.S. Hyperkalemia in dialysis patients. Semin Dial. 2001;14:348–356. doi: 10.1046/j.1525-139x.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 28.Sterns R.H., Rojas M., Bernstein P. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 29.Kamel K.S., Schreiber M. Asking the question again: are cation exchange resins effective for the treatment of hyperkalemia? Nephrol Dial Transplant. 2012;27:4294–4297. doi: 10.1093/ndt/gfs293. [DOI] [PubMed] [Google Scholar]
- 30.Kovesdy C.P. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128:1281–1287. doi: 10.1016/j.amjmed.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 31.Ashurst J., Sergent S.R., Sergent B.R. Evidence-based management of potassium disorders in the emergency department. Emerg Med Pract. 2016;18:1–24. [PubMed] [Google Scholar]
- 32.Flinn R.B., Merrill J.P., Welzant W.R. Treatment of the oliguric patient with a new sodium-exchange resin and sorbitol. N Engl J Med. 1961;264:111–115. doi: 10.1056/NEJM196101192640302. [DOI] [PubMed] [Google Scholar]
- 33.Gruy-Kapral C., Emmett M., Santa Ana C.A. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol. 1998;9:1924–1930. doi: 10.1681/ASN.V9101924. [DOI] [PubMed] [Google Scholar]
- 34.Bastl C., Hayslett J.P., Binder H.J. Increased large intestinal secretion of potassium in renal insufficiency. Kidney Int. 1977;12:9–16. doi: 10.1038/ki.1977.73. [DOI] [PubMed] [Google Scholar]
- 35.Scherr L., Ogden D.A., Mead A.W. Management of hyperkalemia with a cation-exchange resin. N Engl J Med. 1961;264:115–119. doi: 10.1056/NEJM196101192640303. [DOI] [PubMed] [Google Scholar]
- 36.Mikrut M., Brockmiller-Sell H. Sodium polystyrene sulfonate dosing guidelines for the treatment of adult hyperkalemia. Hosp Pharm. 2004;39:765–771. [Google Scholar]
- 37.Joshi P., Beaulieu J., Shemin D. The effect of a single dose of sodium polystyrene sulfonate (SPS) and sorbitol in hyperkalemic patients with kidney disease. J Am Soc Nephrol. 2008;19:355A. [Google Scholar]
- 38.Kessler C., Ng J., Valdez K. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med. 2011;6:136–140. doi: 10.1002/jhm.834. [DOI] [PubMed] [Google Scholar]
- 39.Sandal S., Karachiwala H., Noviasky J. To bind or to let loose: effectiveness of sodium polystyrene sulfonate in decreasing serum potassium. Int J Nephrol. 2012;2012:940320. doi: 10.1155/2012/940320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fordjour K.N., Walton T., Doran J.J. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347:93–100. doi: 10.1097/MAJ.0b013e318279b105. [DOI] [PubMed] [Google Scholar]
- 41.Batterink J. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68:296–303. doi: 10.4212/cjhp.v68i4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mistry M., Shea A., Giguere P. Evaluation of sodium polystyrene sulfonate dosing strategies in the inpatient management of hyperkalemia. Ann Pharmacother. 2016;50:455–462. doi: 10.1177/1060028016641427. [DOI] [PubMed] [Google Scholar]
- 43.Harel Z., Harel S., Shah P.S. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126:264e9–264e24. doi: 10.1016/j.amjmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Mount DB. Potassium disorders. In: Kasper D, Fauci A, Hauser S, et al., eds. Harrison’s Principles of Internal Medicine. New York, NY: McGraw Hill Education; 2015:295–312.
- 45.Lillemoe K.D., Romolo J.L., Hamilton S.R. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101:267–272. [PubMed] [Google Scholar]
- 46.Ayoub I., Oh M.S., Gupta R. Colon necrosis due to sodium polystyrene sulfonate with and without sorbitol: an experimental study in rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rashid A., Hamilton S. Necrosis of the gastrointestinal tract in uremic patients as a result of sodium polystyrene sulfonate (Kayexalate) in sorbitol: an underrecognized condition. Am J Surg Pathol. 1997;21:60–69. doi: 10.1097/00000478-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Abraham S.C., Bhagavan B.S., Lee L.A. Upper gastrointestinal tract injury in patients receiving kayexalate (sodium polystyrene sulfonate) in sorbitol. Am J Surg Pathol. 2001;25:637–644. doi: 10.1097/00000478-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Watson M.A., Baker T.P., Nguyen A. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60:409–416. doi: 10.1053/j.ajkd.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 50.Li S.-Y., Chen Y.-T., Chen T.-J. Mesenteric ischemia in patients with end-stage renal disease: a nationwide longitudinal study. Am J Nephrol. 2012;35:491–497. doi: 10.1159/000338451. [DOI] [PubMed] [Google Scholar]
- 51.Diamond S.M., Emmett M., Henrich W.L. Bowel infarction as a cause of death in dialysis patients. JAMA. 1986;256:2545–2547. [PubMed] [Google Scholar]
- 52.Bassilios N., Menoyo V., Berger A. Mesenteric ischaemia in haemodialysis patients: a case/control study. Nephrol Dial Transplant. 2003;18:911–917. doi: 10.1093/ndt/gfg004. [DOI] [PubMed] [Google Scholar]
- 53.McGowan C.E., Saha S., Chu G. Intestinal necrosis due to sodium polystyrene sulfonate (kayexalate) in sorbitol. South Med J. 2009;102:493–497. doi: 10.1097/SMJ.0b013e31819e8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jadoul M., Karaboyas A., Goodkin D.A. Potassium-binding resins: associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol. 2014;39:252–259. doi: 10.1159/000360094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolson G.M., Ellis K.J., Bernardo M.V. Acute decreases in serum potassium augment blood pressure. Am J Kid Dis. 1995;26:321–326. doi: 10.1016/0272-6386(95)90652-5. [DOI] [PubMed] [Google Scholar]
- 56.Zehnder C., Gutzwiller J.-P., Huber A. Low-potassium and glucose-free dialysis maintains urea but enhances potassium removal. Nephrol Dial Transplant. 2001;16:78–84. doi: 10.1093/ndt/16.1.78. [DOI] [PubMed] [Google Scholar]
- 57.Blumberg A., Roser H.W., Zehnder C. Plasma potassium in patients with terminal renal failure during and after haemodialysis; relationship with dialytic potassium removal and total body potassium. Nephrol Dial Transplant. 1997;12:1629–1634. doi: 10.1093/ndt/12.8.1629. [DOI] [PubMed] [Google Scholar]
- 58.Weisberg L.S., Rachoin J.-S. The safety of low-potassium dialysis. Semin Dial. 2010;23:556–560. doi: 10.1111/j.1525-139X.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 59.Labriola L., Jadoul M. Sailing between Scylla and Charybdis: the high serum K-low dialysate K quandary. Semin Dial. 2014;27:463–471. doi: 10.1111/sdi.12252. [DOI] [PubMed] [Google Scholar]
- 60.Golper TA. Acute hemodialysis prescription. September 2016. Available at: https://www.uptodate.com/contents/acute-hemodialysis-prescription?source=search_result&search=acute%20prescription&selectedTitle=1∼150. Accessed May 16, 2017.
- 61.Morrison G., Michelson E.L., Brown S. Mechanism and prevention of cardiac arrhythmias in chronic hemodialysis patients. Kidney Int. 1980;17:811–819. doi: 10.1038/ki.1980.93. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez G., Brueggemeyer C.D., Newton J.L. Cardiac arrhythmias on hemodialysis in chronic renal failure patients. Nephron. 1984;36:212–218. doi: 10.1159/000183156. [DOI] [PubMed] [Google Scholar]
- 63.Ebel H., Saure B., Laage Ch. Influence of computer-modulated profile haemodialysis on cardiac arrhythmias. Nephrol Dial Transplant. 1990;S1:165–166. doi: 10.1093/ndt/5.suppl_1.165. [DOI] [PubMed] [Google Scholar]
- 64.Santoro A., Mancini E., London G. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant. 2008;23:1415–1421. doi: 10.1093/ndt/gfm730. [DOI] [PubMed] [Google Scholar]
- 65.Redaelli B., Locatelli F., Limido D. Effect of a new model of hemodialysis potassium removal on the control of ventricular arrhythmias. Kidney Int. 1996;50:609–617. doi: 10.1038/ki.1996.356. [DOI] [PubMed] [Google Scholar]
- 66.Hou S., McElroy P.A., Nootens J. Safety and efficacy of low-potassium dialysate. Am J Kid Dis. 1989;13:137–143. doi: 10.1016/s0272-6386(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 67.Saragoca M.A., Canziani E., Cassiolato J.L. Left ventricular hypertrophy as a risk factor for arrhythmias in hemodialysis patients. J Cardiovasc Pharmacol. 1991;17(S2):S136–S138. doi: 10.1097/00005344-199117002-00032. [DOI] [PubMed] [Google Scholar]
- 68.Gruppo emodialisi e patologie cardiovascolari Multicentre, cross-sectional study of ventricular arrhythmias in chronically haemodialysed patients. Lancet. 1988;332:305–309. [PubMed] [Google Scholar]
- 69.Sforzini S., Latini R., Mingardi G. Ventricular arrhythmias and four-year mortality in haemodialysis patients. Gruppo emodialisi e patologie cardiovascolari. Lancet. 1992;339:212–213. doi: 10.1016/0140-6736(92)90008-q. [DOI] [PubMed] [Google Scholar]
- 70.Karnik J.A., Young B.S., Lew N.L. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60:350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 71.Pun P.H., Lehrich R.W., Honeycutt E.F. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 72.Jadoul M., Thumma J., Fuller D.S. Modifiable practices associated with sudden death among hemodialysis patients in the dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol. 2012;7:765–774. doi: 10.2215/CJN.08850811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang C.-W., Lee M.-J., Lee P.-T. Low potassium dialysate as a protective factor of sudden cardiac death in hemodialysis patients with hyperkalemia. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0139886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovesdy C.P., Regidor D.L., Mehrotra R. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 75.Feig P.U., Shook A., Sterns R.H. Effect of removal during hemodialysis on the plasma potassium concentration. Nephron. 1981;27:25–30. doi: 10.1159/000182015. [DOI] [PubMed] [Google Scholar]
- 76.Al-Ghamdi G., Hemmelgarn B., Klarenbach S., for the Alberta Kidney Disease Network Dialysate potassium and risk of death in chronic hemodialysis patients. J Nephrol. 2010;23:33–40. [PubMed] [Google Scholar]
- 77.Merrill J.P., Levine H.D., Somerville W. Clinical recognition and treatment of acute potassium intoxication. Ann Intern Med. 1950;33:797–830. doi: 10.7326/0003-4819-33-4-797. [DOI] [PubMed] [Google Scholar]