Introduction
Human adenovirus (HAdV) is a linear, non-enveloped, double-stranded DNA virus that typically causes a mild respiratory, gastrointestinal, and conjunctival illnesses in healthy persons.1 However, HAdV may cause more severe infection in immunocompromised patients, especially those who have received organ transplants. In kidney transplant recipients, urinary tract infection is the most frequently reported manifestation at 4.8%, with rare dissemination.2, 3 In a surveillance study, 6.5% of kidney transplant recipients will have a positive polymerase chain reaction (PCR) viremia within the first year, without significant symptoms, that is self-limited.4 Disseminated HAdV, defined as symptomatic disease of multiple organ systems and associated viremia, is infrequently reported and is associated with transplant rejection, failure, and mortality.5, 6, 7, 8, 9, 10 A case of disseminated HAdV in a kidney transplant recipient is reported, including a review of management and outcomes in disseminated disease with the goal to guide clinical decision making.
Case Report
A 45-year-old man with a history of end-stage kidney disease, secondary to chronic reflux nephropathy, received a deceased donor kidney transplant. Induction immunosuppression comprised anti−thymocyte globulin and methylprednisolone. Maintenance therapy included tacrolimus, mycophenolate mofetil, and prednisone. His clinical course was complicated by delayed allograft function that required ongoing hemodialysis for 4 weeks.
At his 6-week posttransplantation follow-up visit, he reported a 2-day history of fever, fatigue, cough, and hematuria. He was admitted to the hospital with tachycardia, hypoxemia, and hypotension. He had lymphopenia, thrombocytopenia, and a serum creatinine level of 1.73 mg/dl, compared to his baseline of 1.58 mg/dl. Urinalysis had sterile pyuria and hematuria. Computed tomography of his chest showed an opacity consistent with pneumonia. Vancomycin, piperacillin−tazobactam, and levofloxacin were empirically administered.
Over the next 3 days, he had nightly fevers. His serum creatinine level increased to 2.4 mg/dl. Blood cultures were negative. Studies for serum fungal–(1,3)-B-D-glucan and Aspergillus galactomannan EIA, as well as serum viral PCR cytomegalovirus and human herpesvirus 6 infections, were negative. Urine BK viral particles were not detected by PCR. A nasal swab was positive for HAdV (PCR). Kidney ultrasound demonstrated a 12.6-cm allograft without evidence of renal artery stenosis and with high resistive indices of the upper pole. The 24-hour urine protein was 0.5 g/d at admission and increased to 3.3 g/d by hospital day 7.
A biopsy was performed on the kidney allograft. Paraffin sections showed an edematous parenchyma with a histiocyte-predominant nodular inflammatory infiltrate resembling granulomatous tubulointerstitial nephritis. In place of the epithelioid histiocytes characteristic of granulomas, the predominant cell type in the infiltrate was an atypical monocyte with an eccentrically located, elongated, curved, and crinkled nucleus. These cells infiltrated and distended the tubules, many of which contained epithelial cells with nuclear viral inclusions as well as apoptic cells (Figure 1). Inflammation with rupture of the tubule appeared to give rise to nodules that, in areas, coalesced into solid inflammatory infiltrate. Remnants of the ruptured tubular basement membranes could be found within the pseudogranulomas on periodic acid–Schiff staining. Strong nuclear staining for adenovirus antigen by cytopathic tubular epithelial cells confirmed adenovirus infection (Arkana Laboratories, Little Rock, AR). No histologic features of acute T-cell or antibody-mediated rejection were found, and the immunofluorescence stain for complement factor C4d was negative. Testing for serum and urine HAdV by PCR returned at 102,801 copies/ml and > 2,000,000 copies/ml, respectively.
Figure 1.
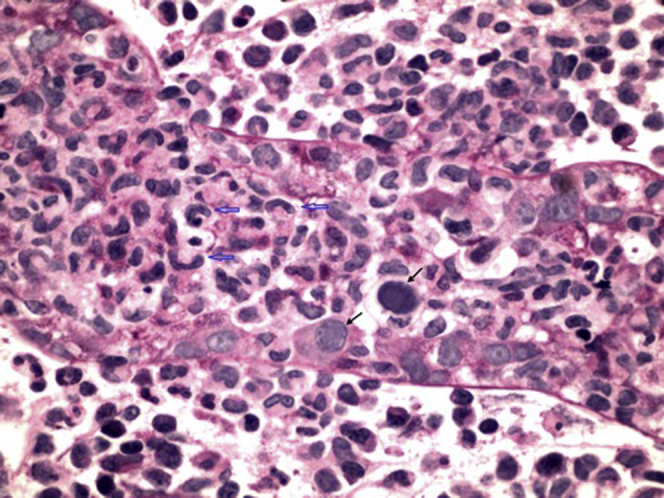
Kidney biopsy sample containing a virus-infected renal tubule. Most of the renal tubular epithelial cells are lost or damaged beyond recognition. Two of the residual epithelial cells contain “smudged” intranuclear viral inclusions typical of adenovirus, with nuclear enlargement and peripheral displacement of nuclear chromatin (black arrows). There is an associated intratubular and peritubular infiltrate consisting predominantly of distinct histiocytes with elongated, curved, and crinkled nuclei (open blue arrows). Periodic acid–Schiff stain, original magnification ×600.
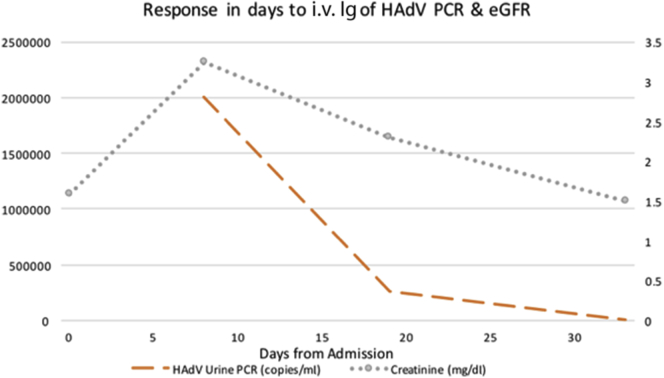
Initial treatment consisted of reduction of the patient’s immunosuppression by discontinuing mycophenolate mofetil and targeting a serum tacrolimus trough level of 3 to 7 ng/ml. The prednisone dose was increased from 5 mg to 10 mg daily. Because of the ongoing severity of his allograft dysfunction and associated respiratory illness, he was given i.v. Ig dosed at 0.5 g/kg for 2 days. Symptomatic improvement was reported by the second day after completion of i.v. Ig. Serum creatinine and urine PCR HAdV DNA levels declined steadily, with return to baseline and resolution, respectively (Figure 2). The transplant team was subsequently notified that the recipient of the donor’s other kidney also developed disseminated HAdV.
Figure 2.
Temporal trend of human adenovirus (HAdV) urine polymerase chain reaction (PCR), serum creatinine, and inception of immunotherapy. Discontinuation of mycophenolate mofetil and reduction of tacrolimus goal trough initiated at day 0, i.v. Ig 0.5 g/kg received on days 8 and 9.
Discussion
Kidney transplant recipients are at high risk over the first 6 months after transplantation for infectious complications, with bacterial cystitis as 1 of the most common culprits.11 HAdV is well recognized in the healthy population to be associated with self-limited respiratory, gastroenteritis, and conjunctivitis illness. However, in kidney transplant recipients, the spectrum of HAdV activity ranges from asymptomatic viremia to hemorrhagic cystitis to allograft loss and mortality.1, 4, 5, 6, 7, 8, 9, 10 In the largest case series to date following 349 kidney transplant recipients over a 3-year period, the incidence of HAdV urinary infection and disseminated disease was 4.8% and 3.1%, respectively. Onset of disease was within the first 3 months in 75% of the patients, and 97% were reported within 1 year.3, 12 A 10-year review of 170 kidney transplant recipients reported an incidence of 4.7% for hemorrhagic cystitis with median time to onset of 1 year.2 In comparison, recipients post−allogenic stem cell transplantation have a median time of diagnosis of adenovirus hemorrhagic cystitis and dissemination within the first few weeks and month, respectively.13 Asymptomatic HAdV viremia was reported as 6.5% from a surveillance study, over a 1-year period, of 92 kidney transplant recipients.4
HAdV in kidney transplant recipients may be secondary to reactivation of latent disease, but infection also has been reported to be de novo from environmental sources or from endogenous transmission through a donor organ.1, 5 The most frequent signs and symptoms at presentation include dysuria, fever, hematuria, sterile (bacterial) pyuriam and acute kidney injury.2, 3, 12, 14 Given the lack of specificity, a broad differential for the etiology of nephritis must be maintained.15 Common extrakidney manifestations among kidney transplant recipients with disseminated disease include orchitis, lymphopenia, gastroenteritis, and pneumonitis.3, 16, 17, 18 Confirmatory diagnostic testing is predominantly through HAdV PCR quantification and tissue biopsy histopathological assessments. Viral culture may be conducted in some cases.12 PCR is sensitive to all known serotypes of HAdV, and has the benefit of serial monitoring for response to treatment. PCR testing for HAdV prognostication has been reported in groups receiving hematopoietic stem cell therapy and solid organ transplants.9, 19, 20
In the present case, this patient had a classically defined presentation of disseminated HAdV. His kidney biopsy findings of inclusion-bearing proximal tubular cells and adjacent noncaseating granulomas in the tubulointerstitium were consistent with HAdV infection and confirmed by immunohistochemical staining.21 Given the severity of his acute kidney injury and progressive pneumonia, he was treated with i.v. Ig based on reports for hematopoietic stem cell transplant recipients.19, 22, 23, 24 Cidofovir was not used because of concerns about additional nephrotoxicity in the setting of severe acute kidney injury in the transplanted kidney.
Clinically symptomatic patients with HAdV viremia and 2 or more organ systems involved is considered disseminated disease. Little has been published about clinical management, and current recommendations for treatment are lacking.10, 25 Overall, 29 cases of disseminated HAdV disease have been reported, including the present case (Table 1). Adverse outcomes have been reported in 6 cases (20%), including 4 deaths and 2 kidney allograft losses. In all, 21 case patients (72%) received additional drug interventions along with reduction of their immunosuppressive regimen. Cidofovir, used in 14 of 20 cases, was the most commonly used antiviral agent, either alone or in combination with i.v. Ig.
Table 1.
Reported cases of disseminated adenovirus in patients post kidney transplantation
| Case no. | Author (reference) | Year | Transplant organ | Age (yr)/sex, onset post transplantation | Organ or disease condition | Diagnostic testing | Additional antiviral | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Myerowitz (34) | 1975 | Kidney | 61/F, 40 days | Nephritis, pneumonitis, leukopenia | Unavailable, histological confirmation | None | Dead |
| 2 | Ardehali (35) | 2001 | Kidney | 45/M, 6 yr | Nephritis, pneumonitis, gastritis, cardiac arrest | No PCR done, histological confirmation | None | Dead |
| 3 | Emovon (31) | 2003 | Kidney-Pancreas | 46/F, 540 days | HC, nephritis, septic shock | No PCR, histopathology confirmation | RBV + i.v. Ig | Alive, graft intact |
| 4 | Rosario (36) | 2006 | Kidney | 58/M, 22 days | Multiorgan failure, colitis | PCR: +blood, +stool | None; died prior to dx | Dead |
| 5 | Alsaad (37) | 2007 | Kidney | 19/M, 12 yr | HC, pneumonia | PCR: +urine/no serum testing | CDV | Alive, graft intact |
| 6 | Keswani (38) | 2007 | Kidney | 5/M, 65 days | HC, gastroenteritis | PCR: +urine, +serum | CDV | Alive, graft intact |
| 7 | Saquib (39) | 2009 | Kidney | 51/F, 6 yr | Pneumonitis, diarrhea, HA, no nephritis/HC | PCR: +urine, serum, stool, BAL | CDV + i.v. Ig | Alive, graft intact |
| 8 | Kidney | 62/M, 29 days | PNA, colitis | PCR: −urine, +serum, +stool | CDV + i.v. Ig | Alive, graft intact | ||
| 9 | Barraclough (26) | 2009 | Kidney | 68/M, 14 days | HC, nephritis, severe sepsis with respiratory compromise | PCR: +urine, +serum, −CSF | CDV + i.v. Ig | Alive, graft intact |
| 10 | Gaspert (6) | 2009 | Kidney | 64/M, 60 days | HC, nephritis, pneumonitis | PCR: +urine, +serum, +BAL | None | Alive, graft loss |
| 11 | Kozlowski (5) | 2010 | Kidney | 44/M, 20 days | HC, nephritis, pneumonitis | PCR: +BAL | GCV | Alive, graft loss |
| 12 | Watcharananan (3) | 2011 | Kidney | unkn | HC, nephritis | PCR: +serum | None | Alive, graft intact |
| 13 | Kidney | unkn | HC, orchitis, diarrhea | PCR: +serum | CDV + i.v. Ig | Alive, graft intact | ||
| 14 | Kidney | unkn | HC, pneumonitis, orchitis | PCR: +serum | CDV | Dead | ||
| 15 | Kidney | unkn | HC, URI, orchitis | PCR: +serum | CDV | Alive, graft intact | ||
| 16 | Kidney | unkn | HC, orchitis, graft rejection | PCR: +serum | CDV + i.v. Ig | Alive, graft intact | ||
| 17 | Kidney | unkn | HC, nephritis, enteritis | PCR: +serum | None | Alive, graft intact | ||
| 18 | Kidney | unkn | HC, nephritis enteritis | PCR: +serum | None | Alive, graft intact | ||
| 19 | Kidney | unkn | HC, leukopenia, enteritis | PCR: +serum | None | Alive, graft intact | ||
| 20 | Varma (20) | 2011 | Kidney-Pancrease | 57/M, 11 days | Fever, neutropenia/leukopenia, nephritis | PCR: +serum | i.v. Ig | Alive, graft intact |
| 21 | Sujeet (40) | 2011 | Kidney | 65/F, 3 yr | HC, nephritis, enteritis, respiratory symptoms | PCR: +serum | CDV | Alive, graft intact |
| 22 | Parasuraman (18) | 2013 | Kidney | 44/M, 22 days | HC, nephritis, pneumonitis, gastroenteritis | PCR: +urine, +serum | CDV, i.v. Ig | Alive, graft intact |
| 23 | Dawood (16) | 2014 | Kidney | 70/F, 810 days | HC, nephritis, gastroenteritis | PCR: +urine, +serum, +stool | CDV, i.v. Ig | Alive, graft intact |
| 24 | Kidney | 60/F, 28 days | HC, nephritis, gastroenteritis | PCR: +blood, +urine, +stool | CDV, i.v. Ig | Alive, graft intact | ||
| 25 | Lachiewicz (17) | 2014 | Kidney | 20/F, 2 yr | Nephritis, URI | PCR: +urine, +serum | CDV, i.v. Ig | Alive, graft intact |
| 26 | Rady (41) | 2014 | Kidney | 63/M, 42 days | HC, nephritis, gastroenteritis | PCR: +urine, serum not performed | GCV, i.v. Ig | Alive, graft intact |
| 27 | Park (42) | 2015 | Kidney | 32/F, 180 days | HC, nephritis, pneumonitis | PCR: +urine, +serum | RBV, i.v. Ig | Alive, graft intact |
| 28 | Index case | 2016 | Kidney | 45/M, 42 days | HC, nephritis, pneumonitis | PCR: +urine, +serum | i.v. Ig | Alive, graft intact |
| 29 | Veer (43) | 2017 | Kidney | 75/F, 365 days | HC, nephritis | PCR: +urine, +serum | i.v. Ig | Alive, graft intact |
BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; CDV, cidofovir; dx, diagnosis; F, female; GCV, ganciclovir; HA, headache; HC, hemorrhagic cystitis; M, male; PCR, polymerase chain reaction; PNA, pneumonia; RBV, ribavirin; unkn, unknown; URI, upper respiratory infection; +, positive; −, negative.
The first step in standard-of-care for HAdV in transplant recipients is reduction of the immunosuppressive regimen. This intervention is often effective at resolving cases of hemorrhagic cystitis and nephritis.3, 14 The usual indication for additional therapies of antiviral or i.v. Ig is progressive disease despite immunosuppression reduction.3, 26 A PCR quantification of 1 log reduction within 2 to 3 weeks is considered a therapeutic response.19 Antiviral therapies in kidney transplant recipients include cidofovir, ganciclovir, ribavirin, and brincidofovir and i.v. Ig.27, 28 Cidofovir, the most commonly used antiviral agent, with activity against all serotypes of HAdV, is a cytosine purine analogue that leads to chain termination.27, 28 The primary adverse effect is hematological as well as kidney toxicity.29 Cidofovir is not considered a preferred therapy in kidney transplant recipients owing to clearance by the kidney and risk of nephrotoxicity. Brincidofovir (formally CMX101) is a new lipid derivative of cidofovir. The lipid formulation allows increased intracellular penetration that minimizes proximal tubular accumulation, and as such, may reduce the risk of nephrotoxicity.28 The AdVise Study (NCT02087306), a currently completed phase III trial, is predominantly a study of hematopoietic stem cell recipients with small subset of solid organ transplant recipients, evaluating brincidofovir for HAdV disease. Results are expected to be reported soon.28, 30 Ganciclovir, a 1-phoshorylated purine analog, has limited effectiveness in HAdV, but there are case reports of its use.2, 4, 28 Ribavirin, a nucleoside analogue of guanosine, is also not generally recommended due to in vitro activity against only subgroup C of HAdV. However, in the posttransplantation population, subgroup C is a common cause of disease.1, 27, 31 Intravenous Ig is proposed to promote antiviral activity or to provide passive immunotherapy.32, 33 Intravenous Ig has been used in combination with cidofovir or as a single agent.
In conclusion, in kidney transplant recipients, HAdV is an uncommonly recognized, but potentially serious, infectious complication. This case reaffirms the clinical context in which to consider such a viral source of infection in kidney transplant recipients. We report the successful management of disseminated HAdV with i.v. Ig as a single agent along with reduction of immunosuppressive therapy. Ongoing prospective studies of HAdV surveillance and antiviral strategies are needed to improve outcomes in this high-risk group.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are grateful to our patient to disclose this case for educational purposes and to the consultant physicians who helped to guide this patient’s care.
References
- 1.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanmoku K. Clinical characteristics and outcomes of adenovirus infection of the urinary tract after renal transplantation. Transpl Infect Dis. 2016;18:234–239. doi: 10.1111/tid.12519. [DOI] [PubMed] [Google Scholar]
- 3.Watcharananan S.P. Adenovirus disease after kidney transplantation: course of infection and outcome in relation to blood viral load and immune recovery. Am J Transplant. 2011;11:1308–1314. doi: 10.1111/j.1600-6143.2011.03479.x. [DOI] [PubMed] [Google Scholar]
- 4.Humar A., Kumar D., Mazzulli T. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005;5:2555–2559. doi: 10.1111/j.1600-6143.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 5.Kozlowski T., Nickeleit V., Andreoni K. Donor-transmitted adenovirus infection causing kidney allograft nephritis and graft loss. Transpl Infect Dis. 2011;13:168–173. doi: 10.1111/j.1399-3062.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- 6.Gaspert A., Lüthi B., Mueller N.J. Subacute allograft failure with dysuria and hematuria in a kidney transplant recipient. Am J Kidney Dis. 2009;54:154–158. doi: 10.1053/j.ajkd.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Komiya T., Goto N., Takeda A. A case of acute rejection with adenovirus infection after ABO-incompatible kidney. Clin Transplant. 2009;23(suppl 20):s27–s30. doi: 10.1111/j.1399-0012.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 8.Kolankiewicz L.M., Pullman J., Raffeld M. Adenovirus nephritis and obstructive uropathy in a renal transplant recipient: case report and literature review. Nephrol Dial Transplant Plus. 2010;3:388–392. doi: 10.1093/ndtplus/sfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florescu M.C., Miles C.D., Florescu D.F. What do we know about adenovirus in renal transplantation? Nephrol Dial Transplant. 2013;28:2003–2010. doi: 10.1093/ndt/gft036. [DOI] [PubMed] [Google Scholar]
- 10.Echavarria M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21:704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green M. Introduction: infections in solid organ transplantation. Am J Transplant. 2013;13(suppl 4):3–8. doi: 10.1111/ajt.12093. [DOI] [PubMed] [Google Scholar]
- 12.Hofland C.A., Eron L.J., Washecka R.M. Hemorrhagic adenovirus cystitis after renal transplantation. Transplant Proc. 2004;36:3025–3027. doi: 10.1016/j.transproceed.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi K., Yoshihara S., Tamaki H. Incidence and treatment strategy for disseminated adenovirus disease after haploidentical stem cell transplantation. Ann Hematol. 2012;91:1305–1312. doi: 10.1007/s00277-012-1440-3. [DOI] [PubMed] [Google Scholar]
- 14.Hensley J.L., Sifri C.D., Cathro H.P. Adenoviral graft-nephritis: case report and review of the literature. Transplant Int. 2009;22:672–677. doi: 10.1111/j.1432-2277.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- 15.Raghavan R., Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149–162. doi: 10.5414/CN108386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawood U.S., Nelson A., Wu D. Disseminated adenovirus infection in kidney transplant recipient. Nephrology. 2014;19:10–13. doi: 10.1111/nep.12192. [DOI] [PubMed] [Google Scholar]
- 17.Lachiewicz A.M., Cianciolo R., Miller M.B., Derebail V.K. Adenovirus causing fever, upper respiratory infection, and allograft nephritis complicated by persistent asymptomatic viremia. Transpl Infect Dis. 2014;16:648–652. doi: 10.1111/tid.12248. [DOI] [PubMed] [Google Scholar]
- 18.Parasuraman R, Zhang PL, Samarapungavan D, et al. Severe necrotizing adenovirus tubulointerstitial nephritis in a kidney transplant recipient. Case Rep Transplant. 2013; article ID 969186, http://dx.doi.org/10.1155/2013/969186. [DOI] [PMC free article] [PubMed]
- 19.Leruez-Ville M. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis. 2004;38:45–52. doi: 10.1086/380450. [DOI] [PubMed] [Google Scholar]
- 20.Varma M.C., Kushner Y.B., Ko D.S. Early onset adenovirus infection after simultaneous kidney-pancreas transplant. Am J Transplant. 2011;11:623–627. doi: 10.1111/j.1600-6143.2010.03408.x. [DOI] [PubMed] [Google Scholar]
- 21.Asim M., Chong-Lopez A., Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41:696–701. doi: 10.1053/ajkd.2003.50133. [DOI] [PubMed] [Google Scholar]
- 22.Echavarria M., Forman M., van Tol M.J.D. Prediction of severe disseminated adenovirus infection by serum PCR. Lancet. 2001;358:384–385. doi: 10.1016/S0140-6736(01)05580-5. [DOI] [PubMed] [Google Scholar]
- 23.Lankester A.C., van Tol M.J., Claas E.C. Quantification of adenovirus DNA in plasma for management of infection in stem cell graft recipients. Clin Infect Dis. 2002;34:864–867. doi: 10.1086/339073. [DOI] [PubMed] [Google Scholar]
- 24.Schilham M.W., Claas E.C., van Zaane W. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin Infect Dis. 2002;35:526–532. doi: 10.1086/341770. [DOI] [PubMed] [Google Scholar]
- 25.Ison M.G., Green M. AST Infectious Diseases Community of Practice: adenovirus in solid organ transplant recipients. Am J Transplant. 2009;9:S161–S165. doi: 10.1111/j.1600-6143.2009.02907.x. [DOI] [PubMed] [Google Scholar]
- 26.Barraclough K., Oliver K., Playford E.G. Life-threatening adenovirus infection in a kidney transplant recipient. Nephrol Dial Transplant Plus. 2009;2:250–253. doi: 10.1093/ndtplus/sfp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ison M.G. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43:331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 28.Wold W.S., Toth K. New drug on the horizon for treating adenovirus. Expert Opinion Pharmacother. 2015;16:2095–2099. doi: 10.1517/14656566.2015.1083975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman P., Ribaud P., Eyrich M. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31:481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 30.Clinicaltrials.gov. Phase III, open-labeled, multicenter study of the safety and efficacy of Brincidofovir (CMX001) in the treatment of early versus late adenovirus infection (CMX001 Adv). Available at: https://clinicaltrials.gov/ct2/show/study/NCT02087306?term=brincidofovir&rank=1. Accessed May 20, 2017.
- 31.Emovon O.E., Lin A., Howell D.N. Refractory adenovirus infection after simultaneous kidney-pancreas transplantation: successful treatment with intravenous ribavirin and pooled human intravenous immunoglobulin. Nephrol Dial Transplant. 2003;18:2436–2438. doi: 10.1093/ndt/gfg365. [DOI] [PubMed] [Google Scholar]
- 32.Sener A., House A.A., Jevnikar A.M. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81:117–120. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 33.Broeders E.N., Wissing K.M., Hazzan M. Evolution of immunoglobulin and mannose binding protein levels after renal transplantation: association with infectious complications. Transpl Int. 2008;21:57–64. doi: 10.1111/j.1432-2277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 34.Myerowitz R.L., Stalder H., Oxman M.N. Fatal disseminated adenovirus infection in a renal transplant recipient. Am J Med. 1975;59:591–598. doi: 10.1016/0002-9343(75)90267-3. [DOI] [PubMed] [Google Scholar]
- 35.Ardehali H., Volmar K., Roberts C. Fatal disseminated adenoviral infection in a renal transplant patient. Transplantation. 2001;71:998–999. doi: 10.1097/00007890-200104150-00029. [DOI] [PubMed] [Google Scholar]
- 36.Rosario R.F., Kimbrough R.C., Van Buren D.H., Laski M.E. Fatal adenovirus serotype-5 in a deceased-donor renal transplant recipient. Transpl Infect Dis. 2006;8:54–57. doi: 10.1111/j.1399-3062.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- 37.Alsaad K.O., Tobar A., Belanger E. Late-onset acute haemorrhagic necrotizing granulomatous adenovirus tubulointerstitial nephritis in a renal allograft. Nephrol Dial Transplant. 2007;22:1257–1260. doi: 10.1093/ndt/gfl843. [DOI] [PubMed] [Google Scholar]
- 38.Keswani M., Moudgil A. Adenovirus-associated hemorrhagic cystitis in a pediatric renal transplant recipient. Pediatr Transplant. 2007;11:568–571. doi: 10.1111/j.1399-3046.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- 39.Saquib R., Melton L.B., Chandrakantan A. Disseminated adenovirus infection in renal transplant recipients: the role of cidofovir and intravenous immunoglobulin. Transpl Infect Dis. 2010;12:77–83. doi: 10.1111/j.1399-3062.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 40.Sujeet K., Vasudev B., Desai P. Acute kidney injury requiring dialysis secondary to adenovirus nephritis in renal transplant recipient. Transpl Infect Dis. 2011;13:174–177. doi: 10.1111/j.1399-3062.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 41.Rady K., Walters G., Brown M., Talaulikar G. Allograft adenovirus nephritis. Clin Kidney J. 2014;7:289–292. doi: 10.1093/ckj/sfu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park U.J., Hyun S.K., Kim H.T. Successful treatment of disseminated adenovirus infection with ribavirin and intravenous immunoglobulin in an adult renal transplant recipient: a case report. Transplant Proc. 2015;47:791–793. doi: 10.1016/j.transproceed.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 43.Veer M., Abdulmassih R., Como J. Adenoviral nephritis in a renal transplant recipient: case report and literature review. Transpl Infect Dis. 2017:e12716. doi: 10.1111/tid.12716. [DOI] [PubMed] [Google Scholar]