Abstract
The devastating neurodegenerative disease multiple sclerosis (MS) could substantially benefit from an adeno-associated virus (AAV) immunotherapy designed to restore a robust and durable antigen-specific tolerance. However, developing a sufficiently potent and lasting immune-regulatory therapy that can intervene in ongoing disease is a major challenge and has thus been elusive. We addressed this problem by developing a highly effective and robust tolerance-inducing in vivo gene therapy. Using a pre-clinical animal model, we designed a liver-targeting gene transfer vector that expresses full-length myelin oligodendrocyte glycoprotein (MOG) in hepatocytes. We show that by harnessing the tolerogenic nature of the liver, this powerful gene immunotherapy restores immune tolerance by inducing functional MOG-specific regulatory T cells (Tregs) in vivo, independent of major histocompatibility complex (MHC) restrictions. We demonstrate that mice treated prophylactically are protected from developing disease and neurological deficits. More importantly, we demonstrate that when given to mice with preexisting disease, ranging from mild neurological deficits to severe paralysis, the gene immunotherapy abrogated CNS inflammation and significantly reversed clinical symptoms of disease. This specialized approach for inducing antigen-specific immune tolerance has significant therapeutic potential for treating MS and other autoimmune disorders.
Keywords: tolerance, regulatory T cell, autoimmunity, gene therapy, experimental autoimmune encephalomyelitis, adeno associated virus, viral vector, immunotherapy
Graphical Abstract

Multiple sclerosis is a neurodegenerative autoimmune disease with no known cure. Hoffman and colleagues present an AAV-based immunotherapy that induces antigen-specific tolerance to a neuropeptide that not only prevents disease onset, but is robust enough to alleviate preexisting neuro-inflammation and reverse disease in a model of MS.
Introduction
Multiple sclerosis (MS) is a complex T cell-driven autoimmune disease of the CNS for which there is no known cure. Although the exact etiology is unknown, the disease is thought to result from peripheral activation of myelin-reactive CD4+ effector T cells that have escaped immune-regulatory mechanisms.1, 2, 3, 4, 5 Active suppression by regulatory T cells (Tregs) plays a key role in the control of self-antigen-reactive T cells and the induction of peripheral tolerance in vivo.2 Unfortunately, abnormalities in the frequency or suppressive function of peripheral CD4+CD25+FOXP3+ Tregs have been observed in various autoimmune diseases, including MS.6, 7
An attractive therapeutic strategy for restoring self-tolerance and controlling disease is to selectively induce autoantigen-specific CD4+CD25+FOXP3+ Tregs. Numerous studies have demonstrated the power of Treg-based immunotherapies.6, 8, 9 For example, it has been shown that adoptive transfer of polyclonal CD4+CD25+ Tregs can temporarily prevent or reduce the neurological symptoms of experimental autoimmune encephalomyelitis (EAE), the murine model of MS.10 Recent clinical studies have reported that injection of CD4+CD25+ Tregs appears to be a safe and effective cellular treatment in patients with type 1 diabetes and graft-versus-host disease.11, 12 In an attempt to generate sufficient cells, several ex vivo approaches for expanding CD4+CD25+ Tregs or in vitro induction of Tregs have been explored.9 Polyclonal and in vitro antigen-specific Treg expansion are two well-known methods that have been used to generate an adequate amount of CD4+CD25+ Tregs. Unfortunately, there are several obstacles blocking the development of large-scale ex vivo or in vitro antigen-specific Treg expansion techniques.13
An alternative and efficient in vivo approach for inducing Ag-specific tolerance is through ectopic expression of an antigen in the liver.14, 15 Leveraging the tolerogenic nature of the liver, hepatic gene transfer has successfully been used to induce robust transgene tolerance in large- and small-animal disease models.16, 17 Viral vectors such as adeno-associated virus (AAV) have emerged as an effective vehicle for in vivo delivery of therapeutic genes to various tissues and are currently being used in multiple phase I/II clinical trials (ClinicalTrials.gov).
In this study, we demonstrate that hepatic gene therapy with an AAV vector containing the full DNA coding sequence for the neuroprotein, myelin oligodendrocyte glycoprotein (MOG), can prevent development of and reverse preexisting EAE. The vector therapy resulted in the induction/expansion of antigen-specific FOXP3+ Tregs. When vector is administered prophylactically, mice were protected from developing EAE disease. When administered to mice exhibiting mild-to-moderate neurological deficits, vector alone was effective at reversing both clinical and pathological signs of disease. When combined with a short course of immune suppression, the AAV immunotherapy can rescue mice from fatal end-stage EAE disease and restore mobility after exhibiting severe paralysis.
Results
Hepatic Gene Transfer with AAV8.MOG Induces Immunosuppressive MOG-Specific Tregs
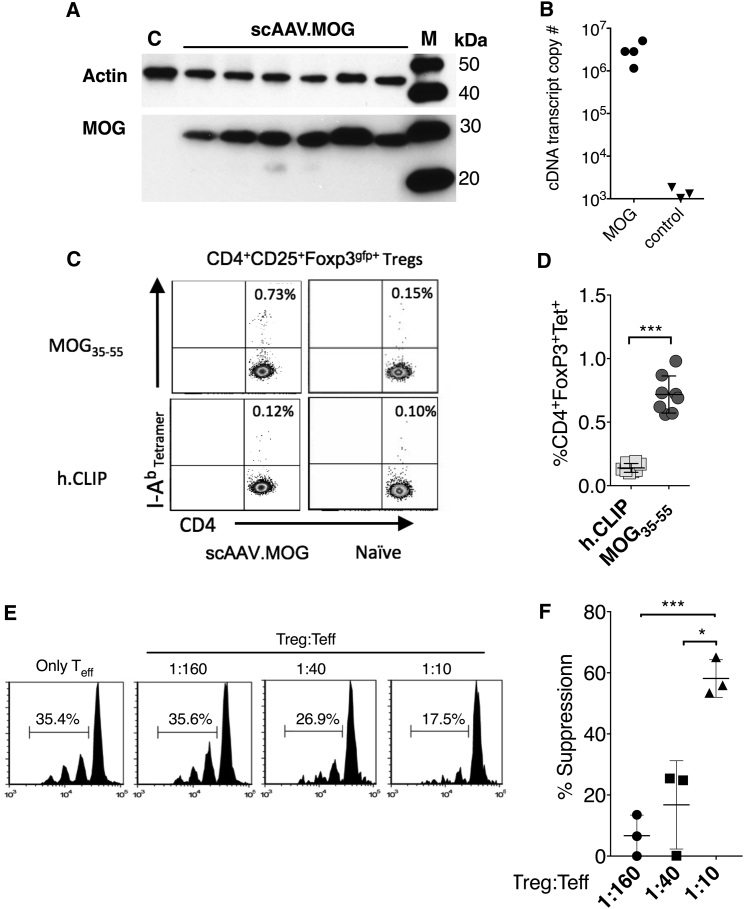
To study the ability of the liver to induce immune tolerance to a transgene protein, we engineered an AAV8 vector to contain the full coding sequence (CDS) of the neuroprotein MOG and placed it under control of a liver-specific promoter. To establish that AAV8.MOG can transduce hepatocytes and will stably express the non-secreted neuroprotein, C57BL/6 mice were systemically injected with a single dose of the vector (1011 vector genomes). Western blot and real-time qPCR analysis of liver lysates from tissue harvested 2 weeks later confirmed vector transduction and hepatocyte expression of MOG (Figures 1A and 1B).
Figure 1.
Functional Ag-Specific Tregs Are Induced following AAV8.MOG Injection
C56Bl/6 mice were injected with 1011 vg of AAV8-MOG via tail vein. (A) Western blot analysis from liver lysates obtained from mice injected with AAV8.MOG 200 days after EAE or control (AAV8.GFP; lane C). Lane M is a molecular size marker in kDa. (B) Real-time qPCR analysis to estimate the transgene copy number from liver lysates (±SD) (n = 4). (C). Representative flow cytometry analysis of freshly isolated splenocytes from FOXP3gfp+ reporter mice tolerized with AAV8.MOG vector that were stained ex vivo with MOG/I-Ab or h.CLIP/I-Ab (control) tetramers. (D) Statistical comparison of I-Ab MOG and I-Ab h.CLIP (control) tetramer populations of CD4+CD25+FOXP3+ Tregs from mice that received AAV.MOG vector (n = 8; U = 0; p = 0.0002, two-tailed Mann-Whitney U test). (E and F) In vitro Treg suppression assay. (E) FOXP3gfp+ Tregs isolated from mice after being tolerized with AAV.MOG were co-cultured at indicated concentrations with MOG-specific 2D2 T cells in the presence of 1 μg/μL MOG35–55 peptide. (F) Mean % suppression of Tregs (n = 3; 1:160 versus 1:10: t = 9.753, df = 3.967, p = 0.0006; 1:40 versus 1:10: t = 4.565, df = 2.705, p = 0.0246, unpaired t test with Welch’s correction; experiment was repeated twice). Data are presented with mean values as indicated; error bars show ± SD. *p < 0.05; ***p < 0.001.
Although MOG protein accounts for only 0.05%–0.1% of total myelin proteins, it is reported to induce a more potent T cell response than other myelin antigens in patients with MS.18, 19 To complicate matters, a loss of immune tolerance because of deficits in either Treg numbers or their function has been observed in autoimmune and inflammatory diseases, including MS.5 Previously, in a model used for protein replacement therapy, we could only indirectly establish that hepatocyte expression induces transgene (Tg)-specific Tregs.20 Here, we developed an experimental system that allowed us to directly determine the frequency of MOG-specific FOXP3+ Tregs. Using a transgenic C57BL/6 Foxp3-EGFP reporter mouse that expresses EGFP under the control of the mouse Foxp3 promoter (FOXP3gfp+), in combination with a MOG38–49/I-Ab major histocompatibility complex (MHC) tetramer, allowed us to directly identify AAV8.MOG-induced Tg-specific CD4+ Tregs (Figures 1C and 1D). To do so, we chose to analyze freshly isolated splenocytes from mice that had previously received AAV8.MOG vector. This allowed us to determine the real-time frequency of tetramer-specific cells as opposed to values amplified by ex vivo restimulation.21 Multi-parametric flow cytometry revealed a significantly higher I-Ab MOG35–55 tetramer+ frequency from CD4+CD25+FOXP3gfp+ gated cells compared with the control (h.CLIP/I-Ab) tetramer (p < 0.0001) (Figures 1C and 1D; Figure S1). Similarly, age-matched naive reporter mice failed to bind either tetramer at levels above that of control mice, ruling out potential non-specific binding (Figure 1C). These findings provide direct and unambiguous evidence that liver-directed AAV induces transgene-specific Tregs in mice, further confirming that hepatic expression of a full-length transmembrane neuroprotein can indeed drive in vivo induction of antigen-specific Tregs.
Next, to test whether the vector-induced MOG-specific Tregs were functional, we compared the capacity of FAC-sorted CD4+FOXP3gfp+ Tregs harvested from the spleens of AAV8.MOG tolerized mice, or age-matched naive mice, to suppress the proliferation of MOG-specific effector T cells when co-cultured in the presence of the immunodominant MOG35–55 peptide. Indeed, at a 1:10 Treg/effector T cell (Teff) ratio, the vector-induced Tregs suppressed 58% of the effector cell proliferation. This was nearly three times more effective than naive polyclonal Tregs (Figures 1E and 1F). These results demonstrate that hepatocyte expression of a non-secreted transmembrane neuroprotein delivered by an AAV8 vector induces functionally suppressive MOG-specific Tregs in vivo.
Pre-treatment with AAV8.MOG Vector Prevents EAE Induction
Next, we asked whether given first, will AAV8.MOG induce transgene-specific immune tolerance and protect susceptible mice from developing EAE (Figure 2A). To answer this, we administered either AAV8.MOG or AAV8.GFP (irrelevant transgene control) vector to cohorts of mice. Two weeks later, mice were immunized with MOG35–55 emulsified in complete Freund’s adjuvant (CFA) to induce EAE. Mice were monitored for signs of neurological deficits using a five-point scale as described (Table 1). Beginning 10 days after EAE induction, mice receiving control vector developed severe neurological impairments (maximum mean clinical score [MCS]: 3.15 ± 0.2) (Figure 2B). Disease progression was also associated with increasing anti-MOG35–55 immunoglobulin (Ig) G1 and IgG2c antibody titers (Figures 2C and 2D). In contrast, mice that received AAV8.MOG were protected and failed to develop clinical signs of EAE or produce MOG35–55-specific antibody responses (Figures 2B–2D). Notably, neurological deficits in control mice continued to increase in terms of both maximum and cumulative EAE scores until they developed severe paralysis and needed to be humanely euthanized.
Figure 2.
Prophylactic Administration of AAV8.MOG Protects Mice from EAE
C57BL/6 mice (9 weeks old) were intravenously injected with 1011 vg/mouse via the tail vein with either AAV8.MOG or AAV8.GFP/control vector (day −14). Two weeks later (day 0), EAE was induced with MOG35–55/CFA. (A) Experimental scheme and initial timeline in days. (B) MCS (± SEM) of AAV8.MOG-treated mice and control mice (n = 5 per group; ****p < 0.0001, two-tailed t test, Mann-Whitney test). Experiments have been reproduced at least twice. (C) Anti-MOG35–55 IgG1and (D) IgG2c antibody titers were measured via ELISA (mean ± SEM) (n = 3 per group). (E) Frequency of CD4+CD25+FOXP3+ Tregs (mean ± SD) present in blood at 5 weeks after vector administration (n = 6 group; U = 4; p = 0.0260, two-tailed Mann-Whitney U test). (F) Plasma ALT levels (IU/L) from age-matched naive control mice and vector-treated mice at 105 days postinjection (n = 10 per group).
Table 1.
MCS
| Score | Clinical Presentation |
|---|---|
| 0.0 | no clinical signs |
| 0.5 | partial paralysis/limp tail |
| 1.0 | paralyzed tail |
| 1.5 | impaired coordination/balance |
| 2.0 | hind-limb paresis |
| 2.5 | one hind limb paralyzed |
| 3.0 | hind-limb paralysis (paraplegia) |
| 3.5 | hind limbs paralyzed and forelimb paresis |
| 4.0 | hind-limb and forelimb paralysis (quadriplegia) |
| 5.0 | moribund/deada |
Mice euthanized or found deceased were recorded as 5 for remainder of time.
Next, we evaluated the frequency of FOXP3+ Tregs in peripheral blood mononuclear cells. Flow cytometry analysis results showed that mice treated with AAV8.MOG had a small but significant increased frequency of CD4+CD25hiFOXP3+ Tregs in peripheral blood mononuclear cells (PBMCs) compared with control mice (Figure 2E), further supporting that AAV hepatic gene therapy administration selectively expands FOXP3+ Treg populations and induces tolerance to the encoded transgene antigen.16
Although various proteins have been safely expressed in the liver following AAV gene transfer,22 we wanted to evaluate the long-term stability of MOG expression in hepatocytes in the context of induced EAE. Elevations in serum/plasma alanine aminotransferase (ALT) level are routinely used in the clinic to screen for liver disease and cell-mediated immunity directed against AAV-transduced hepatocytes.23, 24 Analysis of plasma ALT enzyme activity in mice ∼4 months after receiving gene transfer revealed no significant difference between AAV8.MOG-treated and age-matched naive C57Bl6 mice, indicating that AAV8.MOG did not induce chronic liver disease (Figure 2F). Additionally, hepatocyte expression of MOG persisted in the mice that received AAV8.MOG until termination of experiment at 200 days after EAE induction (Figure 1A). Notably, throughout this protracted timeline, mice never developed any observable signs of neurological disability or general distress, suggesting that hepatocyte expression of MOG does not provoke any deleterious immune responses. Collectively, these data demonstrate that prophylactic administration of liver-directed AAV8.MOG produces long-term stable hepatocyte expression of MOG that has an immuno-suppressive effect capable of preventing the development of EAE.
The Immune Tolerance Induced by AAV8.MOG Is Robust
Next, we evaluated whether a single vector injection could provide long-term hepatic transgene expression and still induce immune tolerance. Two cohorts of mice were injected, intravenously, with either AAV8.MOG or PBS/sham (Figure 3A). EAE was induced in both cohorts of animals ∼200 days later with MOG35–55/CFA. Mice were then monitored daily, and collections of plasma and lymphocytes were obtained every 2 weeks for analysis and Treg staining. Even though vector was given over 7 months earlier, mice that received AAV8.MOG failed to develop any signs of EAE disease, whereas the age-matched control mice began exhibiting neurological deficits at day 14, which rapidly increased in severity (Figure 3B), and began to succumb to disease as early as 16 days after EAE induction (Figure 3C). These results demonstrate that vector-induced immune tolerance is stable and can be maintained long term.
Figure 3.
AAV8.MOG-Induced Immune Tolerance Is Robust
Age-matched C57BL/6 mice (9–10 weeks old) were intravenously injected with 1011 vg/mouse via the tail vein with either AAV8.MOG or PBS/control vector. EAE was induced with MOG35–55/CFA 200 days later and re-challenged after 84 more days. (A) Experimental scheme and timeline in days. (B) MCS (±SEM) of AAV8.MOG-treated mice and control mice (n = 9–10 per group; p < 0.0001, two-tailed t test, Mann-Whitney test). Right panel: blow-out-treated mice showing only 2 of 10 developed relapsing-remitting EAE. (C) Survival curve of mice (p > 0.0001, log rank [Mantel-Cox] test). (D) Plasma ALT levels (IU/L) from age-matched naive control mice and vector treated at various time points. Dashed line is time of re-challenge.
In EAE25, 26, 27, 28 and other models of protein replacement gene therapy,16, 29 long-term induction of tolerance is often confirmed by re-challenging the mice. To further demonstrate the robustness of our immunotherapy, we evaluated the ability of the AAV8.MOG treatment to maintain tolerance and prevent disease following a second attempt to induce MOG-specific EAE. Almost 3 months after the initial EAE induction (9.5 months from AAV8.MOG induction of tolerance), both groups of mice were re-challenged with MOG35–55 and monitored for development of or change in clinical signs. In the control mice, disease escalation occurred rapidly (Figure 3B). Within 15 days, half of the re-challenged control mice succumbed to disease, whereas 100% of the AAV8.MOG-treated mice survived (Figure 3C). However, at 16 and 37 days after secondary challenge, two mice developed a slow relapsing-remitting disease (Figure 3B, right). Nonetheless, 80% of the mice that received AAV8.MOG vector months earlier showed absolutely no signs of EAE or liver disease over the course of the experiment (Figures 3B–3D). The disease escalation in the control mice confirmed that vector-treated mice were indeed tolerized and not simply protected via a vaccination mechanism. Thus, these data clearly demonstrated that AAV8.MOG protection is indeed stable and robust.
AAV8.MOG Immunotherapy Reverses Established EAE Disease
The early symptoms of MS are often minor and overlooked. Diagnosis is usually made after the first clinically isolated syndrome (CIS), which is defined as an episode of neurological deficit that lasts at least 24 hr and is caused by inflammation or demyelination.30 In terms of rate and severity of disability, disease progression is highly variable and difficult to predict, which often results in a diagnosis well after disease has been established. Therefore, we investigated whether induction of Ag-specific tolerance following AVV8.MOG immunotherapy would be effective in diminishing or reversing disease in mice during progressive stages of neurological impairment. In this series of experiments, EAE was induced in age-matched mice before being treated with vector. As the mice developed signs of neurological impairment, they were divided in an alternating fashion into two different groups so that the baseline clinical scores would be comparable between the groups (referred to as rolling enrollment). As mice reached the target MCS, they were injected with either AAV8.MOG or PBS/sham vector (Figures 4A–4C). In the first cohort, mice received treatment early in the disease process as they began to lose tail tonality (Figure 4A). Both groups of mice continued to develop severe paralyzing EAE by day 7 (peak MCS: ∼3.5). Strikingly, beginning around day 8, all but one mouse that was treated with a single injection of AAV8.MOG began to exhibit a significant reversal of clinical symptoms (final MCS: 0.5 ± 0.3). In contrast, control mice proceeded to develop severe neurological disabilities (final MCS: 3.3 ± 0.4). In the next iteration, we evaluated the ability of AAV8.MOG immunotherapy to reverse moderate disease by withholding treatment until mice exhibited complete tail paralysis (MCS: ∼1). Like before, both groups of mice rapidly developed severe EAE with hind-leg paralysis (Figure 4B). After a brief remission, control mice relapsed and developed severe ascending paralysis (final MCS: 3.2 ± 0.4). In contrast, AAV8.MOG-treated mice went into a nearly complete remission and regained use of their hind legs (final MCS: 0.7 ± 0.2) (Figure 4B).
Figure 4.
AAV8.MOG Induces Clinical and Pathological Remission of EAE
EAE was induced in 9-week-old female C57BL/6 mice using MOG35–55 in CFA. (A–C) MCS (mean ± SEM) was recorded, and as mice developed increasing neurological symptoms, recorded as increasing MCS, mice were intravenously injected with either 1011 vector genomes (vg) AAV8.MOG or control via the tail vein in an alternating fashion. (A) MCS 0.3, loss of tail tonality (n = 5; final control versus final AAV8.MOG: q = 0.9342, degrees of freedom (D.F.) 12, p < 0.0001; peak AAV8.MOG versus final AAV8.MOG: q = 10.74, D.F. 12, p < 0.0001). (B) MCS 0.8, tail paralysis (n = 9–10; final control versus final AAV8.MOG: q = 9.042, D.F. 30, p < 0.0001; peak AAV8.MOG versus final AAV8.MOG: t = 8.627, D.F. 30, p < 0.0001). (C) MCS 1.3, tail paralysis with hind-leg paresis (n = 5; final control versus final AAV8.MOG: q = 4.358, D.F. 12, p = 0.0412; peak AAV8.MOG versus final AAV8.MOG: q = 6.9, D.F. 124, p = 0.0019). Dashed line indicates MCS at time of treatment. Statistical analysis was determined using two-way ANOVA Tukey’s multiple comparisons test. Gray symbols represent non-responder mice. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (D) Representative histological images of two different regions of spinal cord demonstrating multiple foci of inflammation in the white matter of control mice (H&E, top row) and serial section of spinal cord from the same mouse showing multifocal demyelination associated with the areas of inflammation (Luxol fast blue, bottom row). In contrast, despite having reached a higher peak clinical score, there was an absence of infiltrates in the CNS of AAV8.MOG-treated mice.
Lastly, we further probed the ability of AAV8.MOG to induce tolerance and abrogate disease in mice with even more advanced preexisting disease. Following induction of EAE, AAV8.MOG immunotherapy was withheld until disease advanced and mice presented with complete tail paralysis with hind-leg inhibition and loss of fine motor coordination that affected their gait and balance (combined MCS: 1.3 ± 0.2) (Figure 4C). Mice continued to develop severe EAE with hind-leg paralysis, which critically impeded their ability to freely move around the cage and obtain food (peak MCS: ≥3.3). By day 30, mice that received AAV8.MOG immunotherapy had a significantly greater reduction in clinical scores compared to control mice (p = 0.0412). Although not as robust as previously seen, these results have substantial clinical relevance. Notably, all the mice that responded to the gene immunotherapy regained the ability to freely ambulate, whereas control mice continued to have hind-leg paralysis.
Inflammation in the Spinal Cord of Treated Mice
In EAE, the spinal cord is the primary site of encephalitogenic effector cells and demyelination, and the degree of neurological impairment is related to the magnitude of inflammation during the early stages of the disease.31 To determine whether the amelioration of neurological deficits was associated with a reduction in encephalitogenic inflammation and/or demyelination, we compared serial sections from multiple regions of spinal cords from mice that received AAV8.MOG, to control mice for pathological differences 35 days after receiving vector. Histological examination showed that non-tolerized control mice had numerous foci of cellular infiltrates that were co-localized to areas of demyelination within the white matter (Figure 4D). In contrast, there was an absence of inflammatory lesions within the spinal cord of the mice treated with AAV8.MOG. These findings were consistent across the other treatment groups, as well as with previous literature.14, 32 These results suggest that AAV gene immunotherapy reverses the clinical symptoms associated with EAE disease through a mechanism that suppresses tissue-specific inflammation.
Transient Immune Suppression Enhances AAV8.MOG Immunotherapy in EAE
It has been shown in an EAE model that Tregs may accumulate in the target tissue but are non-suppressive.21 The failure to suppress the effector response is believed to be associated with the localized inflammation causing a Th1/Th17 microenvironment within the CNS. As suggested by the diminished impact of AAV8.MOG treatment in mice with delayed treatment seen in Figure 4C, this pro-inflammatory microenvironment may limit the effectiveness of the induced Tregs, especially at the height of inflammation. To overcome this limitation, we hypothesized that successful treatment may require adjunct immune suppression to modulate the pro-inflammatory environment within the CNS.33, 34 To address this, we investigated the immunosuppressive drug rapamycin. Rapamycin has been used to suppress graft refection in organ transplantation, and its safety and efficacy have been evaluated for use in humans with MS.35 In general terms, rapamycin has a potent anti-proliferative effect on antigen-stimulated effector T cells, while simultaneously allowing expansion of CD4+CD25+FOXP3+ Tregs, making it an ideal choice.36, 37
To test the hypothesis, we reestablished the experimental parameters that previously produced the smallest degree of disease reversal (Figure 4C). EAE was induced and AAV8.MOG treatment was withheld until mice developed complete tail paralysis with hind-leg paresis (MCS: 1.4 ± 0.1, combined). Immediately after being treated with either AAV8.MOG or PBS/control, all mice received an intraperitoneal injection of rapamycin (5 mg/kg). Subsequently, mice received two additional doses of rapamycin (5 mg/kg) 48 hr apart (Figure 5). As expected, EAE disease progressed quickly and both groups of mice developed severe neurological deficits and paralysis (peak MCS: 2.9–3.0) (Figure 5A). Within 72 hr of receiving the rapamycin, both AAV8.MOG-treated and control mice responded to the immunosuppression and displayed signs of remission (a sustained reduction in MCS ≥1).35 However, by day 10, 100% of the control mice had relapsed and rapidly developed end-stage EAE disease (final MCS: 3.5 ± 0.3). In contrast, neurological deficits in AAV8.MOG-treated mice continued to decrease, and all but one animal (90%) achieved complete remission (final MCS: 0.5 ± 0.3) (Figure 5A). Additionally, in a separate experiment, mice that received the AAV8.MOG vector/rapamycin combination remained symptom free (final MCS: 0.2 ± 0.1) until termination of the experiment at ∼100 days after EAE (Figure S2).
Figure 5.
Therapeutic Effects of Therapy Are Enhanced following Transient Rapamycin Immunosuppression
EAE was induced as in Figure 4. (A–C) Mice developed neurological symptoms: (A) MCS 1.4, tail paralysis with hind-leg paresis (n = 10; final control versus final AAV8.MOG: q = 12.03, D.F. 34, p < 0.0001; peak AAV8.MOG versus final AAV8.MOG: q = 9.95, D.F. 34, p < 0.0001). (B) MCS 3.0, hind-leg paralysis (n = 7–8; final control versus final AAV8.MOG: q = 11, D.F. 14, p < 0.0001; peak AAV8.MOG versus final AAV8.MOG: q = 8.085, D.F. 14, p = 0.0003). (C) MCS 3.5, hind-leg paralysis with forearm paresis (n = 5; final control versus final AAV8.MOG: q = 7.439, D.F. 12, p = 0.0010; peak AAV8.MOG versus final AAV8.MOG: q = 7.123, D.F. 12, p = 0.0014). Mice were intravenously injected with either AAV8.MOG and rapamycin (rapa) or rapamycin alone (control). Clinical scores (mean ± SEM) were recorded. Graphical representation of peak and endpoint MCS are shown above group statistics. Dashed lines indicate MCS at time of treatment. Arrows indicate time of vector and rapamycin injections. Data are representative of at least two repeat experiments. (D). Representative FACS analysis of CD25hiFOXP3+ Tregs in blood (isolated from mice in group A) after rapamycin treatment. (E and F) Percentage of (E) Tregs (mean ± SEM) (post-rapa control versus post-rapa AAV8.MOG: n = 3, t = 3.996, df = 4, p = 0.0162, unpaired two-tailed Student’s t test) or (F) activated CD44+ Tregs obtained from peripheral blood at the indicated times (post-rapa control versus post-rapa AAV8.mog: n = 3, q = 5.368, df = 8, p = 0.0219; pre-rapa AAV8.MOG versus post-rapa AAV8.MOG: n = 3, q = 7.698, df = 8, p = 0.0027, two-way ANOVA Tukey’s multiple comparisons test) (pre-EAE = naive mice; pre-Rapa = day 0; post-Rapa = day 10). (G and H) Plasma alanine aminotransferase (ALT) activity from AAV8.MOG-treated and control mice following rapamycin treatment with MCS 3.0 (G) and MCS 3.5 (H) (n = 10). Statistical analysis was determined for the responders by two-way ANOVA with Tukey’s multiple comparisons test. Muted colors in (A)–(C) indicate non-responder mice. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Next, we tested the effectiveness of the combined immunotherapy in late and end-stage EAE disease. In these cohorts, EAE disease was induced as before and allowed to develop until the mice began exhibiting complete tail and hind-limb paralysis (MCS: 3.0 ± 0.0) (Figure 5B) or borderline quadriplegia (hind-limb paralysis with forearm paresis that prevents the mouse from righting itself when placed on its back) (MCS: 3.5 ± 0.0) (Figure 5C) before AAV8.MOG/rapamycin treatment was administered. Remarkably, mice that received the AAV8.MOG/rapamycin immunotherapy, 71% (Figure 5B) and 80% (Figure 5C), respectively, responded to the treatment and went into near-complete remission (MCS: >1) by day ∼30. In contrast, after transiently responding to the rapamycin, the control mice relapsed into severe paralyzing or fatal EAE disease (Figures 5B and 5C). Notably, in both groups, a limited number of the animals failed to respond to rapamycin immunosuppression, suggesting the disease process was beyond the point of rescue.
To better illustrate the severity of EAE disease progression and the effect AAV8.MOG/rapamycin treatment has, a representative video visually documenting the reversal of clinical symptoms is available (Movie S1).
Hepatocyte Expression of MOG in Combination with Rapamycin Promotes Treg Expansion of Peripheral Tregs and Reverses EAE
Rapamycin blocks the activation of a serine/threonine protein kinase called mammalian target of rapamycin (mTOR), which has a potent anti-proliferative effect on antigen-stimulated effector T and B cells. This results in selective reduction of T helper (Th) 1, Th2, and Th17 cells while simultaneously allowing the expansion of Ag-specific Tregs.36 To determine whether rapamycin treatment enhanced the induction of tolerance and cellular responses during AAV8.MOG immunotherapy, we compared the frequency of Tregs from AAV8.MOG/rapamycin-treated mice with rapamycin-only control mice (Figures 5D–5F). Phenotypic analysis revealed no significant difference in the percentage of total CD4+CD25hiFOXP3+ Tregs obtained from peripheral blood of AAV8.MOG tolerized mice, compared with that of control mice before rapamycin treatment. In contrast, when analyzed after the final rapamycin dose on day 10, there was an ∼33% difference in total Tregs between control mice receiving rapamycin alone and AAV8.MOG-treated animals (Figures 5D and 5E).
CD44 is a cell-surface glycoprotein involved in cell-to-cell interactions that are important in activation, migration, and apoptosis. Its relative expression has been associated with FOXP3 expression and Treg function, and can be used to identify activated Tregs.38, 39 Similar to activated effector or memory CD4+ T cells, activated Tregs also express high levels CD44.39 Restricting the analysis to activated Tregs (CD4+CD44+ CD25hiFOXP3+) revealed a 58.9% increase in Tregs in mice that received rapamycin and AAV8.MOG immunotherapy (Figures 5D and 5F). In contrast, only a 10.5% increase was seen in rapamycin-only-treated mice.
Plasma ALT levels were also monitored as an indicator of liver damage and failure of therapy. As reported above, the level of ALT activity detected in AAV8.MOG-treated mice and control mice was unremarkable throughout the rapamycin treatment window (Figures 5G and 5H). However, at 35 days post-treatment the control mice had a significant increase in plasma ALT levels that corresponded with an increase in clinical score (MCS: 3.6 ± 0.5, final). Based on the profound level of neurological impairment the control mice were experiencing, the significant rise in ALT is indicative of liver toxicity associated with end-stage organ failure (Figures 5B and 5C).
Collectively, these findings demonstrate that transient immunosuppression with rapamycin has a synergistic effect on AAV8.MOG immunotherapy that selectively induces in vivo expansion of Tregs and restores tolerance in an antigen-dependent manner.36
Discussion
MS is a complex autoimmune disease that has no cure. Early diagnosis and aggressive treatment with immunomodulating agents can lower the relapse rate and slow progression. However, these treatments are generally non-specific and risk significant side effects with long-term use.40 Newer disease-modifying therapies that target specific immune responses or target specific CNS antigens have shown potential, but various experimental limitations have prevented clinical translation.8, 41, 42
Tregs are an essential component in preventing autoimmunity and controlling responses to alloantigens. A disruption in the homeostasis of tolerance in a variety of autoimmune diseases, including MS, may result from a substantial decrease in the number or functional impairment of Tregs.7, 43 Using the EAE model, studies have shown that adoptive transfer of polyclonal Tregs is able to attenuate the development of autoimmune diseases.10 In contrast, disease was exacerbated when CD4+CD25+ Tregs were depleted.44 Additionally, adoptive transfer of autologous ova-specific ex vivo-expanded Tregs has been evaluated in a clinical trial for Crohn’s disease.45 Although the treatment was well tolerated and showed efficacy, the results were only transient, lasting about 5 weeks, which is supported by in vivo and in vitro data suggesting that ova-Tregs have a limited survival capacity upon chronic activation.45 Other difficulties with ex vivo expansion of antigen-specific Tregs include proper identification of antigens, long culture times, and overall expense.
Although the mechanism is unclear, various in vivo techniques, such as transgenic expression, liver-targeting nanoparticles, and lentivirus (LV)-mediated gene transfer, have been shown to leverage the natural ability of the liver to induce specific tolerance to an ectopically expressed autoantigen.14, 15, 17, 20, 46, 47, 48 However, even though these and other studies have provided mechanistic insight, their clinical value is unclear. Rather, an approach is needed that is translatable to the clinic and achieves robust in vivo induction of a durable Treg response, capable of reversing established autoimmune disease.
Addressing these requirements, the liver-directed AAV immunotherapy procedure presented here is based on the clinically tested AAV gene therapy platform. Overall, it provides a less complex approach for inducing antigen-specific Tregs in vivo.16, 49 We have shown that a single dose of vector established a durable source of antigen needed for sustained induction and activation of autoreactive Tregs. Additionally, having engineered the vector to include the full coding sequence of MOG, it is likely to induce multiple immunodominant and sub-dominant antigen-specific Tregs, independent of MHC restrictions and without compromising long-term immune homeostasis. This is supported by our previous work in a hemophilia model where an AAV vector expressing clotting factor IX was used to induce tolerance to the same transgene in multiple strains of mice.29, 46, 50, 51
As explained above, Treg immunotherapy for MS has to be capable of reversing established disease in order to be clinically feasible. The data presented clearly demonstrate that AAV.MOG immunotherapy not only prevents induction of the autoimmune disease, but more importantly clearly reverses preexisting disease if administered during early onset. Albeit, AAV immunotherapy alone was not sufficient to fully reverse end-stage EAE disease. However, when augmented with transient immunosuppression, a potent synergistic effect was revealed that rescued mice with rapidly progressing paralysis. The use of rapamycin was specifically chosen because it induces de novo expression of FOXP3 and expands functional FOXP3+ Tregs from naive cells in vivo, while inhibiting the proliferation and trafficking of conventional CD4+ and CD8+ T cells.37, 52, 53, 54 Rapamycin has also been shown to be effective at modulating EAE. Esposito et al.35 demonstrated that continuous rapamycin monotherapy can effectively inhibit the induction and the progression of established disease; however, upon withdraw of the drug, mice rapidly developed a relapsing-remitting form of EAE. Clearly, mitigating the inflammation in the CNS was necessary for the AAV8.MOG immunotherapy to be maximally effective. Along similar lines, it is tempting to suggest that AAV immunotherapy alone (without the use of rapamycin immunosuppression) could reverse disease if given during a natural period of remission. However, because the EAE model used in this study does not undergo a relapsing-remitting cycle, direct evaluation was not possible.
In summary, we have developed a novel immunotherapy that reverses debilitating paralysis in an animal model of MS that is superior to the traditional non-specific immunosuppression therapies currently available. The application of this approach as a clinical therapy for treating MS and other human autoimmune diseases warrants further investigation.
Materials and Methods
Animal Strains
Female (9- to 12-week-old) inbred C57BL/6 and C57BL/6-Tg (Tcra2D2,Tcrb2D2), 1Kuch/J (MOGTCR 2D2), and B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr/J (FOXP3gfp+) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All procedures involving animals were carried out in accordance with the guidelines of the University of Florida Institutional Animal Care and Use Committee (IACUC).
Vector Production
A recombinant AAV8 vector expressing full-length MOG under a hepatocyte-specific promoter was produced by the method of transfection using anionic liposomes as a transfection reagent into human embryonic kidney (HEK293) cells, below passage 50. Two plasmid DNAs—recombinant construct flanked by the AAV inverted terminal repeats (iTRs), pAAV-Apolipoprotein E (ApoE)/hAAT-MOG, and a helper plasmid for AAV8 serotype (pDG8) mixed in equimolar amount—totaling 90 μg per 15 cm plate were added to each plate containing ∼1 × 107 cells. Virus was recovered from both cells and medium. Medium was collected on days 2 and 4 post-transfection, with consequent virus precipitation with 40% polyethylene glycol (PEG)8000/2.5 M NaCl solution. Cells were resuspended in 20 mM Tris/HCl (pH 8.5)/15 mM NaCl lysis buffer, 10 mL per 1–2 × 108 cells. Cells were lysed by one-time freeze/thaw cycle and three rounds, 1 min each, of sonication on ice. Virus pelleted by PEG/NaCl was processed similarly to the virus recovered from the cells and combined. Clarified lysates ran on a step iodixanol density gradient55 and dialyzed/concentrated on Apollo 20 spinning devices. The titer of each preparation was estimated using a dot-blot assay.
Induction of EAE
Mice were immunized by subcutaneous injection of MOG35–55 in CFA (Hooke Labs, Lawrence, MA, USA). Pertussis toxin (PT) 200 ng (Hooke Labs, Lawrence, MA, USA) was injected intraperitoneally (i.p.) 6 and 24 hr later. The clinical symptoms of EAE were checked daily and graded on a clinical score of 0–5: 0, no clinical signs; 0.5, partially limp tail; 1.0, paralyzed tail; 2.0, loss of coordinated movement and hind-limb paresis; 2.5, one hind limb paralyzed; 3.0, both hind limbs paralyzed; 3.5, hind limbs paralyzed and weakness in forelimbs; 4.0, forelimbs paralyzed (quadriplegia); and 5.0, moribund. Mice had to reach inclusion criteria of an MCS ≥2.0 to be included in the study group. Mice would be euthanized if an MCS ≥4.0 was maintained for 48 hr, as per IACUC policy.
Vector Administration
To examine the prophylactic effect of vector administration, we injected 7- to 9-week-old female C57BL/6 mice with 1011 vector genomes (vg) of AAV8.MOG or control vector. Two weeks later, EAE was induced. To evaluate the therapeutic effect of vector administration, we induced EAE first in 9- to 11-week-old mice before. As mice reached the targeted/indicated severity of disease they were injected with 1011 vg of AAV8.MOG or control vector (scAAV.GFP or sham/PBS) via the tail vein. Rapamycin (LC Laboratories, Woburn, MA, USA) was dissolved in a vehicle solution containing (0.2% w/v) carboxymethyl-cellulose sodium salt (C-5013) and (0.25% v/v) polysorbate-80 (P-8074) (Sigma, St. Louis, MO, USA) in distilled water and stored at 4°C protected from light according to the manufacturer’s instructions. Rapamycin (5 mg/kg) was given i.p. as indicated for a total of three and five doses beginning on the day of vector administration.
Gene Expression
Messenger RNA was isolated from 30 mg of liver samples harvested from mice that had received vector 2 weeks earlier using the RNeasy kit (QIAGEN, Valencia, CA, USA). Real-time qPCR was performed in duplicate using RT2 qPCR Primer Assay for Mouse MOG (QIAGEN) according to the manufacturer’s protocols. A MyIQ iCycler fluorescent detection system with iQ5 operating software Version 2.0 (Bio-Rad Laboratories, Hercules, CA, USA) was used to generate and analyze data. All gene expression was compared with that of glyceraldehyde-3-phosphate dehydrogenase.56
Flow Cytometry
Peripheral blood cells or splenocytes harvested from mice and processed to produce single-cell suspensions were stained with antibodies to CD3 (145-2C11), CD4 (RM4-5), CD25 (PC61), CD8 (53-6.7), B220 (RA3-6B2), CD44 (IM7), and CD62L (MEL14) (BD Biosciences, San Jose, CA, USA). Class II MHC tetramers included MOG38–49/I-Ab class II MHC (GWYRSPFSRVVH) and h.CLIP87–101 (PVSKMRMATPLLMQA), and were provided by the NIH Tetramer Core (Emory University, Atlanta, GA, USA). Red blood cell lysis was performed with VersaLyse (Beckman Coulter, Brea, CA, USA). Intracellular staining for FOXP3 was performed using the FOXP3 staining kit (eBioscience, San Diego, CA, USA). Samples were analyzed on an LSR-II flow cytometer (BD Biosciences) and post-analyzed using FCS Express 4 (Denovo Software, Los Angeles, CA, USA).
In Vitro Suppression Assay
Spleens from FOXP3gfp+ mice that received vector no less than 2 weeks earlier were homogenized and enriched for CD4+ T cells by magnetic depletion of non-target cells over an LS column (Miltenyi Biotec, San Diego, CA, USA). GFP+ cells, representing the Treg population of CD4+ T cells (∼10%), were further isolated using the FACSAria II cell sorter (BD Biosciences). Splenocytes isolated from 2D2-MOGTCR mice were labeled with CellTrace Violet (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. CD4+FOXP3GFP+ Tregs and CellTrace Violet-labeled responder splenocytes were seeded at the indicated effector/responder ratios in complete 5% RPMI media containing 1 μg/mL MOG35–55 peptide for 72 hr at 37°C. Cells were resuspended and stained with anti-CD4 antibody to assess proliferation of responder CD4+ T cells. GFP was used to discriminate between responder cells and Tregs. Proliferation was determined by quantitating CellTrace Violet fluorescence intensity relative to the parent population of unstimulated responder cells (0% proliferation) and stimulated cells incubated without Tregs (100% proliferation). Percentage of CD4+ responder T cell proliferation was determined using FCS Express 4.
Analysis of Plasma Samples
Plasma was analyzed for anti-MOG IgG1 and IgG2c by ELISA as previously described.46
Western Blot
Protein was extracted from liver tissue using T-PER Tissue Extraction Reagent (Thermo) in the presence of Halt Protease Inhibitor (Thermo). Total protein concentration was measured using the bicinchoninic acid protein assay (Pierce). Samples was separated on 4%–20% Mini-PROTEAN TGX gels (Bio-Rad) and transferred to polyvinylidene fluoride (PVDF) membrane following standard protocols. After blocking, the membrane was incubated for 1 hr at room temperature with antibody against MOG or β-actin in 1% fat-free dry milk in 1× tris-buffered saline with tween (TBST). HRP-conjugated secondary antibody was used for signal detection with the ECL 2 Western Blotting Substrate (Pierce).
Histopathology
For histopathological analysis of the spinal cord, formalin-fixed, paraffin-embedded, 5 μm sections were stained with Luxol Fast Blue or H&E by standard procedures.
Statistical Analysis
Results are reported in figure legends as mean ± SEM, unless otherwise stated. Statistical significance was determined using GraphPad Prism software (La Jolla, CA, USA). The p values are reported as indicated.
Study Approval
All studies were in accordance with protocols approved by IACUC at the University of Florida, Gainesville.
Author Contributions
B.E.H. designed and performed experiments, collected and analyzed data, wrote the manuscript, supervised the work, and provided funding. G.D.K. and S.K. conducted experiments and collected and analyzed data. E.L.S., B.P., and N.T.J. monitored animals and acquired data. D.M.M. helped design experiments and edited the manuscript.
Conflicts of Interest
B.E.H. is an inventor on a pending patent related to this work.
Acknowledgments
We acknowledge and thank the National Multiple Sclerosis Society (grants PP2157 and RG 5315-A-1) andthe NIH-NIAID (grant R01AI128074) for providing funding to B.E.H., and the Children’s Miracle Network for providing funding to B.E.H. and G.D.K., the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of (MHC, CD1d, etc.) tetramers, and the University of Florida ICBR Flow cytometry core for providing resources.
Footnotes
Supplemental Information includes two figures and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.09.001.
Supplemental Information
References
- 1.Gonsette R.E. Self-tolerance in multiple sclerosis. Acta Neurol. Belg. 2012;112:133–140. doi: 10.1007/s13760-012-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zozulya A.L., Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat. Clin. Pract. Neurol. 2008;4:384–398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- 4.Paust S., Cantor H. Regulatory T cells and autoimmune disease. Immunol. Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalla Libera D., Di Mitri D., Bergami A., Centonze D., Gasperini C., Grasso M.G., Galgani S., Martinelli V., Comi G., Avolio C. T regulatory cells are markers of disease activity in multiple sclerosis patients. PLoS ONE. 2011;6:e21386. doi: 10.1371/journal.pone.0021386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone F., De Rosa V., Carrieri P.B., Montella S., Bruzzese D., Porcellini A., Procaccini C., La Cava A., Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 7.Vila J., Isaacs J.D., Anderson A.E. Regulatory T cells and autoimmunity. Curr. Opin. Hematol. 2009;16:274–279. doi: 10.1097/MOH.0b013e32832a9a01. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald K.G., Hoeppli R.E., Huang Q., Gillies J., Luciani D.S., Orban P.C., Broady R., Levings M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossetti M., Spreafico R., Saidin S., Chua C., Moshref M., Leong J.Y., Tan Y.K., Thumboo J., van Loosdregt J., Albani S. Ex vivo-expanded but not in vitro-induced human regulatory T cells are candidates for cell therapy in autoimmune diseases thanks to stable demethylation of the FOXP3 regulatory T cell-specific demethylated region. J. Immunol. 2015;194:113–124. doi: 10.4049/jimmunol.1401145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyersdorf N., Gaupp S., Balbach K., Schmidt J., Toyka K.V., Lin C.H., Hanke T., Hünig T., Kerkau T., Gold R. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J. Exp. Med. 2005;202:445–455. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas H.R., Gitelman S.E. Altering the course of type 1 diabetes: an update on prevention and new-onset clinical trials. Pediatr. Diabetes. 2013;14:311–321. doi: 10.1111/pedi.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trzonkowski P., Bieniaszewska M., Juścińska J., Dobyszuk A., Krzystyniak A., Marek N., Myśliwska J., Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin. Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Safinia N., Sagoo P., Lechler R., Lombardi G. Adoptive regulatory T cell therapy: challenges in clinical transplantation. Curr. Opin. Organ Transplant. 2010;15:427–434. doi: 10.1097/MOT.0b013e32833bfadc. [DOI] [PubMed] [Google Scholar]
- 14.Lüth S., Huber S., Schramm C., Buch T., Zander S., Stadelmann C., Brück W., Wraith D.C., Herkel J., Lohse A.W. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J. Clin. Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbarpour M., Goudy K.S., Cantore A., Russo F., Sanvito F., Naldini L., Annoni A., Roncarolo M.G. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl. Med. 2015;7:289ra81. doi: 10.1126/scitranslmed.aaa3032. [DOI] [PubMed] [Google Scholar]
- 16.LoDuca P.A., Hoffman B.E., Herzog R.W. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr. Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sack B.K., Herzog R.W., Terhorst C., Markusic D.M. Development of gene transfer for induction of antigen-specific tolerance. Mol. Ther. Methods Clin. Dev. 2014;1:14013. doi: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerlero de Rosbo N., Milo R., Lees M.B., Burger D., Bernard C.C., Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J. Clin. Invest. 1993;92:2602–2608. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varrin-Doyer M., Shetty A., Spencer C.M., Schulze-Topphoff U., Weber M.S., Bernard C.C., Forsthuber T., Cree B.A., Slavin A.J., Zamvil S.S. MOG transmembrane and cytoplasmic domains contain highly stimulatory T-cell epitopes in MS. Neurol. Neuroimmunol. Neuroinflamm. 2014;1:e20. doi: 10.1212/NXI.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman B., Dobrzynski E., Wang L., Hirao L., Mingozzi F., Cao O., Herzog R.W. Muscle as a target for supplementary factor IX gene transfer. Hum. Gene Ther. 2007;18:603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- 21.Korn T., Reddy J., Gao W., Bettelli E., Awasthi A., Petersen T.R., Bäckström B.T., Sobel R.A., Wucherpfennig K.W., Strom T.B. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao O., Dobrzynski E., Wang L., Nayak S., Mingle B., Terhorst C., Herzog R.W. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim W.R., Flamm S.L., Di Bisceglie A.M., Bodenheimer H.C., Public Policy Committee of the American Association for the Study of Liver Disease Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 25.Pedotti R., Mitchell D., Wedemeyer J., Karpuj M., Chabas D., Hattab E.M., Tsai M., Galli S.J., Steinman L. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat. Immunol. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Guo Y., Xia C.Q. Infusion of sulfosuccinimidyl-4-[N-maleimidomethyl]cyclohexane-1-carboxylate-conjugated MOG35-55-coupled spleen cells effectively prevents and reverses experimental autoimmune encephalomyelitis in mice. J. Immunol. Res. 2015;2015:129682. doi: 10.1155/2015/129682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse H.Y., Li J., Zhao X., Chen F., Ho P.P., Shaw M.K. Lessons learned from studies of natural resistance in murine experimental autoimmune encephalomyelitis. Curr. Trends Immunol. 2012;13:1–12. [PMC free article] [PubMed] [Google Scholar]
- 28.Raine C.S., Traugott U., Stone S.H. Applications of chronic relapsing experimental allergic encephalomyelitis to the study of multiple sclerosis. In: Bauer H.J., Poser S., Ritter G., editors. Progress in Multiple Sclerosis Research. Springer Berlin Heidelberg; 1980. pp. 3–10. [Google Scholar]
- 29.Markusic D.M., Hoffman B.E., Perrin G.Q., Nayak S., Wang X., LoDuca P.A., High K.A., Herzog R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus J.F., Waubant E.L. Updates on clinically isolated syndrome and diagnostic criteria for multiple sclerosis. Neurohospitalist. 2013;3:65–80. doi: 10.1177/1941874412457183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohm A.P., Carpentier P.A., Anger H.A., Miller S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 32.Berard J.L., Wolak K., Fournier S., David S. Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia. 2010;58:434–445. doi: 10.1002/glia.20935. [DOI] [PubMed] [Google Scholar]
- 33.Bluestone J.A., Bour-Jordan H., Cheng M., Anderson M. T cells in the control of organ-specific autoimmunity. J. Clin. Invest. 2015;125:2250–2260. doi: 10.1172/JCI78089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie H., Zheng Y., Li R., Guo T.B., He D., Fang L., Liu X., Xiao L., Chen X., Wan B. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 35.Esposito M., Ruffini F., Bellone M., Gagliani N., Battaglia M., Martino G., Furlan R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J. Neuroimmunol. 2010;220:52–63. doi: 10.1016/j.jneuroim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Biswas M., Sarkar D., Kumar S.R., Nayak S., Rogers G.L., Markusic D.M., Liao G., Terhorst C., Herzog R.W. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood. 2015;125:2937–2947. doi: 10.1182/blood-2014-09-599266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battaglia M., Stabilini A., Roncarolo M.G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 38.Liu T., Soong L., Liu G., König R., Chopra A.K. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+ Treg cells. Biol. Direct. 2009;4:40. doi: 10.1186/1745-6150-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M.O., Rudensky A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lallana E.C., Fadul C.E. Toxicities of immunosuppressive treatment of autoimmune neurologic diseases. Curr. Neuropharmacol. 2011;9:468–477. doi: 10.2174/157015911796557939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregori S., Passerini L., Roncarolo M.G. Clinical outlook for type-1 and FOXP3(+) T regulatory cell-based therapy. Front. Immunol. 2015;6:593. doi: 10.3389/fimmu.2015.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunstein C.G., Blazar B.R., Miller J.S., Cao Q., Hippen K.L., McKenna D.H., Curtsinger J., McGlave P.B., Wagner J.E. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol. Blood Marrow Transplant. 2013;19:1271–1273. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGeachy M.J., Stephens L.A., Anderton S.M. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J. Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 45.Desreumaux P., Foussat A., Allez M., Beaugerie L., Hébuterne X., Bouhnik Y., Nachury M., Brun V., Bastian H., Belmonte N. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207–1217. doi: 10.1053/j.gastro.2012.07.116. e1–e2. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman B.E., Martino A.T., Sack B.K., Cao O., Liao G., Terhorst C., Herzog R.W. Nonredundant roles of IL-10 and TGF-β in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol. Ther. 2011;19:1263–1272. doi: 10.1038/mt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X., Oppenheim J.J., Winkler-Pickett R.T., Ortaldo J.R., Howard O.M. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006;36:2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor R.A., Anderton S.M. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J. Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman B.E., Ertl H.C., Terhorst C., High K.A., Herzog R.W. Gene therapy research at the frontiers of viral immunology. Front. Microbiol. 2012;3:182. doi: 10.3389/fmicb.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao O., Hoffman B.E., Moghimi B., Nayak S., Cooper M., Zhou S., Ertl H.C., High K.A., Herzog R.W. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol. Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper M., Nayak S., Hoffman B.E., Terhorst C., Cao O., Herzog R.W. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum. Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman N.M., Chi H. mTOR signaling, Tregs and immune modulation. Immunotherapy. 2014;6:1295–1311. doi: 10.2217/imt.14.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Battaglia M., Stabilini A., Migliavacca B., Horejs-Hoeck J., Kaupper T., Roncarolo M.G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 54.Moghimi B., Sack B.K., Nayak S., Markusic D.M., Mah C.S., Herzog R.W. Induction of tolerance to factor VIII by transient co-administration with rapamycin. J. Thromb. Haemost. 2011;9:1524–1533. doi: 10.1111/j.1538-7836.2011.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zolotukhin S., Potter M., Zolotukhin I., Sakai Y., Loiler S., Fraites T.J., Jr., Chiodo V.A., Phillipsberg T., Muzyczka N., Hauswirth W.W. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 56.Martino A.T., Suzuki M., Markusic D.M., Zolotukhin I., Ryals R.C., Moghimi B., Ertl H.C., Muruve D.A., Lee B., Herzog R.W. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.