Abstract
Exposure to replication-competent lentivirus (RCL) is a theoretical safety concern for individuals treated with lentiviral gene therapy. For certain ex vivo gene therapy applications, including cancer immunotherapy trials, RCL detection assays are used to screen the vector product as well as the vector-transduced cells. In this study, we reviewed T cell products screened for RCL using methodology developed in the National Gene Vector Biorepository. All trials utilized third-generation lentiviral vectors produced by transient transfection. Samples from 26 clinical trials totaling 460 transduced cell products from 375 subjects were evaluated. All cell products were negative for RCL. A total of 296 of the clinical trial participants were screened for RCL at least 1 month after infusion of the cell product. No research subject has shown evidence of RCL infection. These findings provide further evidence attesting to the safety of third-generation lentiviral vectors and that testing T cell products for RCL does not provide added value to screening the lentiviral vector product.
Keywords: immunotherapy, lentivirus, replication-competent virus, clinical gene therapy, safety
The safety of lentiviral vectors is a factor in their acceptance as clinical therapies. In this issue of Molecular Therapy, Cornetta et al. (2017) screened 460 cell products for replication-competent lentivirus (RCL); none were positive. The low risk of RCL suggests that revisions to U.S. FDA testing guidelines are warranted.
Introduction
Adoptive therapy with genetically modified T cells using lentiviral vectors is in advanced stages of clinical development for cancer indications by academic investigators and several companies.1, 2, 3, 4 Commercial approval by the US Food and Drug Administration (FDA) of CTL019, a CD19 chimeric antigen receptor (CAR) T cell for the therapy of relapsed leukemia, is expected in 2017. In addition, several centers are testing engineered hematopoietic stem cells and other targets using gene transfer with lentiviral vector technology.5, 6, 7, 8, 9 Thus, detection of replication-competent lentivirus (RCL) is emerging as a major issue, given the widespread use of lentiviral vector technology.
Detecting RCL in lentiviral vector products is a key release test to ensure that patients are not inadvertently exposed to replicating virus. The most likely source of RCL would be recombination between vector sequences and the viral genes expressed during vector manufacture.10, 11, 12 Detection of a vector-associated RCL is challenging, given that this virus is still theoretical; therefore, the components of the virus are unknown. Replicating viruses have been described in the manufacture of vectors based on murine leukemia viruses (MLVs). Most commonly, these MLV-derived viruses arose through the recombination of vector and packaging sequences, and decreasing homology between vector and packaging sequences has been shown to decrease virus formation.13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Some recombinant retroviruses have also been shown to contain vector and packaging sequences and cellular-derived genes.23, 24 This raises the possibility that an RCL could contain packaging sequences along with endogenous human retroviral25 or other cellular components. This experience with MLV-based vectors has shaped FDA recommendations for recombinant virus testing, including recommendations for RCL assays.26
In the United States, a lentiviral vector lot must be screened for RCL prior to clinical use.27 Research subjects are also continuously monitored after treatment for the presence of RCL. A third assessment is also required for any cell product cultured ex vivo for more than 4 days, since a putative RCL that was not detected in the vector release assay may be amplified in cell culture and, thus, become detectable. As the majority of T cell receptor (TCR) and CAR vector trials use cell expansion, RCL screening of the infused T cell product is required for most cancer immunotherapy trials. This requirement presents challenges to the clinical development of T cell applications due to the number of cells that must be tested (1% of the cell product or 108 cells, whichever is less),27, 28 the complexity of assessing RCL in high titer vector,29 and the associated expense of screening this large number of cells.
RCL detection is also complicated by the similarity between vector and viral particles. Many components of an RCL will be similar to those of a vector particle (capsid, integrase, and reverse transcriptase), so most protein detection methods will not be fruitful. Similarly, an assay for reverse transcriptase activity30, 31 cannot distinguish RCL from vector particles. While vector genomes lack genes used in viral replication, these genes must be expressed in vector-producing cells, and any carryover of cellular or plasmid DNA into the vector product can lead to false-positive molecular assays. Moreover, all non-culture assays, to date, lack the sensitivity of culture-based assays where, theoretically, one infectious unit can be amplified to large numbers.11 A number of RCL culture assays have been described, including syncytia formation assays capable of detecting a fully competent lentivirus, but the sensitivity of this approach in detecting an attenuated virus has not been extensively studied.32 Marker rescue assays have also been described for HIV-1, but whether a RCL arising from vector production will mobilize the marker is unknown.33, 34 To date, the most common assays for screening gene therapy products are assays that combine an amplification phase, using a cell line capable of expanding attenuated viruses to high titer, with subsequent detection of virus using ELISA or molecular assays.29, 35, 36, 37, 38, 39
Since RCLs arising during vector production are still theoretical, their growth rate is unknown, but it is likely to be significantly attenuated, compared to wild-type lentiviruses, due to the absence of accessory genes.33 Therefore, regulators have required biologic assays to utilize an extended culture period of approximately 3 weeks (a minimum of 5 passages)27 to amplify any slow-growing viruses. Using this stringent screening method, RCL has not been reported in any research or clinical lentiviral vector preparations. The lack of RCL provides support for the overall safety of lentiviral vectors and suggests the multiple safety features incorporated in vector design are effective in limiting RCL development.40
Since the advent of lentiviral vectors for clinical application in 2003, a conservative RCL testing approach has been implemented to allow for an optimal risk:benefit analysis for patients, while the field gathered experience with this new vector system. It is unknown whether the testing of infused products adds additional value to the testing of the vector product. In this paper, we review the experience of the National Gene Vector Biorepository (NGVB), an NHLBI (National Heart, Lung, and Blood Institute)-funded resource to assist investigators in meeting FDA requirements for gene therapy (www.NGVBCC.org). The NGVB has assisted investigators in testing T cell products for RCL from a variety of clinical trials. To date, none of the products tested were found to contain RCL. The data suggest that RCL testing of infused products does not provide additional assurances of safety and that screening vector products is a sufficient release test for third-generation lentiviral vectors.
Results
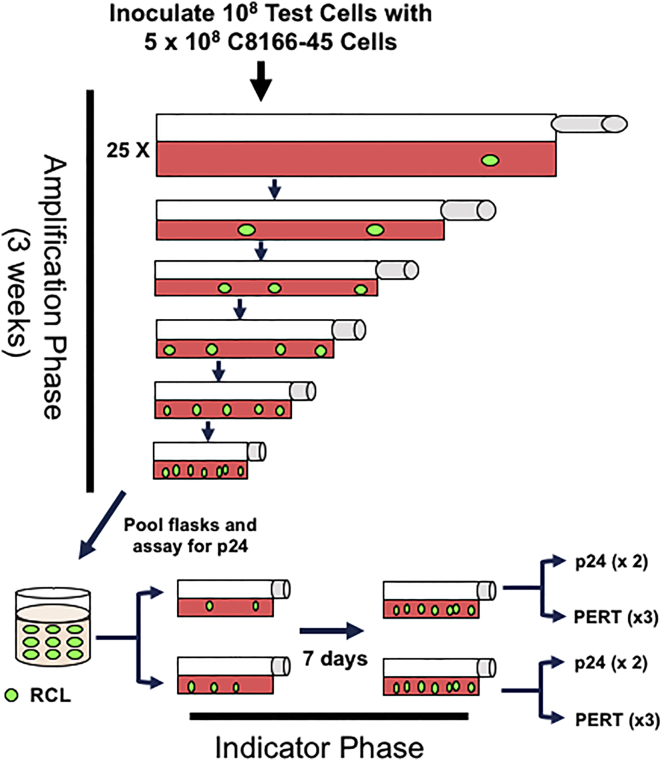
Lentiviral vectors have much in common with native lentiviruses, making it difficult to distinguish vector particles from RCLs. Biologic assays, which expose permissive cell lines to vector preparations, have been shown to be the preferred method of RCL detection.11, 35, 36 In 2011, we reported our assay’s methodology and performance in testing clinical vector products.29 The general components of the RCL assay used in this study are shown in Figure 1. Test articles and parallel positive and negative controls are added to C8166-45 T cells, and the cultures are maintained for 2 weeks (amplification phase). C8166-45 T cells have been shown to generate HIV-1 to high titer and also show high transduction with VSV-G (vesicular stomatitis virus G protein) pseudotyped vector.11, 35 Culture supernatant is then used to inoculate naive C8166-45 cells, which are cultured for an additional week. Cultures are then screened for RCL using two methods: (1) p24 ELISA for HIV capsid protein and (2) a molecular-based assay (either psi-gag PCR11 or product-enhanced reverse transcriptase30 [PERT]). As the true nature of a RCL remains theoretical, we chose two methods of virus detection to improve the chance of identifying an unusual recombinant.
Figure 1.
Schematic Representation of the RCL Assay
T cell products are incubated with C8166-45 cells at a ratio of 1:5. Over half of test articles submitted contained approximately 1 × 108 T cells and were divided into twenty-five 300-cm2 flasks. Cultures were passed a minimum of 5 passages using increasingly smaller vessels. After 3 weeks, cells were placed in fresh media, and conditioned media were harvested after 24 hr. Media from all amplification cultures are pooled, and two aliquots are then incubated with naive C8166-45 cells. After 7 days, culture media were analyzed for p24 antigen by ELISA and either the psi-gag PCR assay or PERT. The figure depicts amplification of a low-titer RCL present in the test article.
T Cell Product Analysis
This analysis reviewed RCL testing of cellular products from June 2011 until August 2016. All investigators who submitted more than 10 test articles to the NGVB were invited to participate; all agreed to submit data. The test articles represent 97.9% of the T cell products tested within this time period. Table 1 summarizes the general characteristics of the 26 research studies. All trials utilized a third-generation lentiviral vector41; 4 studies involved TCR vector, while 17 utilized CAR vectors. The trials utilized vectors to transduce T cell populations or T cell subset populations. All vectors utilized in clinical trials were determined to be RCL negative by the manufacturer.
Table 1.
Clinical Protocols Submitting Transduced T Cell Products for Replication-Competent Lentivirus Testing
| Study No. | Clinical Trial No. | Principal Investigator | Initial Assay Initiated | Final Assay Completed | Vector Generation | Transgene Class | Transduced Cell Type |
|---|---|---|---|---|---|---|---|
| 11-2 | NCT01350401 | C.H.J. | 8/8/11 | 10/28/13 | third | TCR | T cell |
| 11-3 | NCT01352286 | C.H.J. | 6/27/11 | 4/15/13 | third | TCR | T cell |
| 11-4 | NCT01343043 | C.H.J. | 12/6/12 | 2/13/13 | third | TCR | T cell |
| 11-11 | NCT01626495 | C.H.J. | 7/11/12 | 2/20/14 | third | CAR-T | T cell |
| 11-12 | NCT01029366 | C.H.J. | 12/15/11 | 9/9/13 | third | CAR-T | T cell |
| 11-13 | NCT01551043 | C.H.J. | 12/15/11 | 12/15/11 | third | CAR-T | T cell |
| 12-4 | NCT01626495 | C.H.J. | 7/2/14 | 11/9/16 | third | CAR-T | T cell |
| 12-16 | NCT01683279 | M.J. | 12/8/14 | 3/16/15 | third | CAR-T | CD4, CD8 |
| 13-4 | NCT01747486 | C.H.J. | 3/21/13 | 4/18/16 | third | CAR-T | T cell |
| 13-12 | NCT01567891, NCT01352286, NCT01350401, NCT01343043 | G.B.-S. | 9/5/15 | 3/9/16 | third | TCR | T cell |
| 13-15 | NCT01318317, NCT01815749 | S.F. | 2/13/14 | 2/13/14 | third | CAR-T | memory T |
| 14-9 | NCT02030847 | C.H.J. | 4/2/14 | 8/4/16 | third | CAR-T | T cell |
| 14-10 | NCT02030834 | C.H.J. | 5/5/14 | 5/2/16 | third | CAR-T | T cell |
| 14-12 | NCT01865617 | C.J.T. | 5/28/14 | 8/24/15 | third | CAR-T | CD4, CD8 |
| 14-18 | NCT02315612 | T.F. | 1/26/15 | 6/13/16 | third | CAR-T | T cell |
| 14-27 | NCT01815749 | S.F. | 2/12/15 | 2/12/15 | third | CAR-T | memory T |
| 15-9 | NCT02146924a | S.F. | 3/30/14 | 10/26/15 | third | CAR-T | memory T |
| 15-10 | NCT02153580 | S.F. | 3/30/15 | 10/26/15 | third | CAR-T | memory T |
| 15-11 | NCT02051257 | S.F. | 3/30/15 | 10/26/15 | third | CAR-T | memory T |
| 15-26 | NCT02028455 | M.J. | 12/8/14 | 3/18/16 | third | CAR-T | CD4, CD8 |
| 15-36 | NCT02208362 | S.F. | 10/26/15 | 10/26/15 | third | CAR-T | memory T |
| 16-1 | NCT02311621 | M.J. | 4/4/16 | 6/17/16 | third | CAR-T | CD4, CD8 |
TCR, T cell receptor; CAR-T, chimeric antigen receptor T cell; T, T cell.
1 patient treated on a compassionate-use basis linked to this study.
Table 2 provides information about RCL testing. A total of 499 assays were performed during the study period. The T cell manufacturing processes for initial studies included a parallel non-transduced control, which provided an opportunity to evaluate assay performance for false-positives. After 39 assays of non-transduced control samples were analyzed and found to be RCL negative, the NGVB requested investigators limit subsequent sample submission to transduced cell products only. A total of 460 transduced cell products are included in this analysis. All were RCL negative. The number of T cells within a product varied per protocol. The U.S. FDA requires 1% of the cell product (up to 1 × 108 cells) be screened for RCL, and over half of products tested contained or approached the 1 × 108 maximum. Clinical trials with lower numbers were those treating pediatric subjects or transducing T cell subsets. The total number of transduced cells tested from all studies was 2.4 × 1010.
Table 2.
Number of RCL Assays Performed by Study and the Number of Cells Analyzed
| Study No. | Principal Investigator | No. of Samples Assayed | No. of Negative Control Samples | No. of Transduced Samples | Total No. of Control Cells Tested | Total No. of Transduced Cells Tested | Average No. of Transduced Cells per Assay | Median No. of Cells per Assay |
|---|---|---|---|---|---|---|---|---|
| 11-2 | C.H.J. | 5 | 2 | 3 | 2.00E+08 | 3.00E+08 | 1.00E+08 | 1.00E+08 |
| 11-3 | C.H.J. | 43 | 20 | 23 | 1.88E+09 | 2.05E+09 | 8.90E+07 | 1.00E+08 |
| 11-4 | C.H.J. | 4 | 2 | 2 | 2.00E+08 | 2.00E+08 | 1.00E+08 | 1.00E+08 |
| 11-11 | C.H.J. | 33 | 5 | 28 | 4.07E+08 | 2.24E+09 | 7.98E+07 | 1.00E+08 |
| 11-12 | C.H.J. | 27 | 9 | 18 | 4.11E+08 | 1.27E+09 | 7.04E+07 | 9.80E+07 |
| 11-13 | C.H.J. | 2 | 1 | 1 | 5.00E+07 | 5.00E+07 | 5.00E+07 | 5.00E+07 |
| 12-4 | C.H.J. | 41 | 0 | 41 | 0 | 3.20E+09 | 7.80E+07 | 1.00E+08 |
| 12-16 | M.J. | 3 | 0 | 3 | 0 | 8.75E+07 | 2.92E+07 | 3.07E+07 |
| 13-4 | C.H.J. | 35 | 0 | 35 | 0 | 2.64E+09 | 7.53E+07 | 8.73E+07 |
| 13-12 | G.B.-S. | 36 | 0 | 36 | 0 | 3.60E+09 | 1.00E+08 | 1.00E+08 |
| 13-15 | S.F. | 6 | 0 | 6 | 0 | 9.60E+07 | 1.60E+07 | 1.65E+07 |
| 14-9 | C.H.J. | 30 | 0 | 30 | 0 | 2.39E+09 | 8.11E+07 | 1.00E+08 |
| 14-10 | C.H.J. | 39 | 0 | 39 | 0 | 3.25E+09 | 8.33E+07 | 1.00E+08 |
| 14-12 | C.J.T. | 90 | 0 | 90 | 0 | 1.73E+09 | 1.92E+07 | 2.00E+07 |
| 14-18 | T.F. | 14 | 0 | 14 | 0 | 1.20E+08 | 8.57E+06 | 1.00E+07 |
| 14-27 | S.F. | 7 | 0 | 7 | 0 | 5.09E+07 | 7.26E+06 | 5.80E+06 |
| 15-9 | S.F. | 6 | 0 | 6 | 0 | 3.00E+07 | 5.00E+06 | 4.38E+06 |
| 15-10 | S.F. | 6 | 0 | 6 | 0 | 3.63E+07 | 6.04E+06 | 3.13E+06 |
| 15-11 | S.F. | 11 | 0 | 11 | 0 | 1.14E+08 | 1.03E+07 | 1.00E+07 |
| 15-26 | M.J. | 45 | 0 | 45 | 0 | 3.65E+08 | 8.30E+06 | 7.50E+06 |
| 15-36 | S.F. | 2 | 0 | 2 | 0 | 5.75E+06 | 2.88E+06 | 2.88E+06 |
| 16-1 | M.J. | 14 | 0 | 14 | 0 | 1.49E+08 | 1.06E+06 | 1.00E+07 |
| Total | 499 | 39 | 460 | 3.15E+09 | 2.40E+10 | |||
| M | 4.64E+07 | 5.26E+07 |
Subject Follow-Up
Not all products screened for RCL were infused into the intended research subject, due to non-RCL-related issues. As shown in Table 3, of the 460 transduced products tested for RCL, 409 were infused (89%). A number of trials utilized multiple products per subject, with the 409 products infused into 375 subjects. To help provide additional validation of the RCL testing method, subjects who underwent RCL testing at time points ≥ 30 days post-infusion were tabulated and listed in Table 2. A total of 296 of the 375 (79%) subjects had at least one RCL test performed after infusion; all analyses were negative for RCL. In all but one subject, RCL detection of peripheral blood was performed using a PCR for VSV-G envelope DNA.
Table 3.
Follow-Up Testing for RCLs in Subjects Infused with Gene-Modified T Cell Products
| Study No. | Principal Investigator | No. of Products Infused | No. of Subjects Infused | No. of Subjects with RCL Follow-Upa | Method of RCL Detection | Level of Sensitivity per DNA |
|---|---|---|---|---|---|---|
| 11-2 | C.H.J. | 2 | 2 | 1 | VSV-G DNA PCR | 25 copies per 1 μg |
| 11-3 | C.H.J. | 19 | 17 | 16 | VSV-G DNA PCR | 25 copies per 1 μg |
| 11-4 | C.H.J. | 2 | 2 | 2 | VSV-G DNA PCR | 25 copies per 1 μg |
| 11-11 | C.H.J. | 24 | 24 | 21 | VSV-G DNA PCR | 25 copies per 1 μg |
| 11-12 | C.H.J. | 14 | 13 | 13 | VSV-G DNA PCR | 25 copies per 1 μg |
| 11-13 | C.H.J. | 1 | 1 | 0 | VSV-G DNA PCR | 25 copies per 1 μg |
| 12-4 | C.H.J. | 36 | 36 | 34 | VSV-G DNA PCR | 25 copies per 1 μg |
| 12-16 | M.J. | 3 | 3 | 2 | VSV-G DNA PCR | 10 copies per 50 ng |
| 13-4 | C.H.J. | 32 | 32 | 23 | VSV-G DNA PCR | 25 copies per 1 μg |
| 13-12 | G.B.-S. | 31 | 31 | 24 | VSV-G DNA PCR | 5 copies per 100 ng |
| 13-15 | S.F. | 5 | 5 | 5 | VSV-G DNA PCR | 2.5 copies per 50 ng |
| 14-9 | C.H.J. | 25 | 25 | 14 | VSV-G DNA PCR | 25 copies per 1 μg |
| 14-10 | C.H.J. | 34 | 34 | 30 | VSV-G DNA PCR | 25 copies per 1 μg |
| 14-12 | C.J.T. | 76 | 76 | 49 | VSV-G DNA PCR | 10 copies per 1 μg |
| 14-18 | T.F. | 14 | 14 | 11 | VSV-G DNA PCR | 10 copies per 200 ng |
| 14-27 | S.F. | 7 | 7 | 6 | VSV-G DNA PCR | 2.5 copies per 50 ng |
| 15-9 | S.F. | 6 | 4 | 3b | VSV-G DNA PCR | 2.5 copies per 50 ng |
| 15-10 | S.F. | 6 | 6 | 5 | VSV-G DNA PCR | 2.5 copies per 50 ng |
| 15-11 | S.F. | 11 | 11 | 11 | VSV-G DNA PCR | 2.5 copies per 50 ng |
| 15-26 | M.J. | 45 | 23 | 19 | VSV-G DNA PCR | 10 copies per 50 ng |
| 15-36 | S.F. | 2 | 2 | 2 | VSV-G DNA PCR | 2.5 copies per 50 ng |
| 16-1 | M.J. | 14 | 7 | 5 | VSV-G DNA PCR | 10 copies per 50 ng |
| Total | 409 | 375 | 296 |
VSV-G, vesicular stomatitis virus G protein.
≥30 Days post-infusion.
2 subjects were tested by PCR, and 1 subject was tested by serology.
Assay Performance
The RCL assay used in this paper is composed of two culture phases: an initial amplification phase and a second indicator phase. Culture media are tested at the end of the amplification phase for HIV capsid protein (p24 ELISA). Media from the indicator phase are tested for HIV capsid protein, as well as a molecular-based assay (psi-gag PCR or PERT). Due to the large number of cells per test articles, over half of the assays contained only 2 test articles per assay. Successful performance of the amplification phase is critical, since failure to complete this portion of the assay requires resubmission of test articles and significantly delays test completion. Reviewing the 499 RCL test articles analyzed, there were 13 out of specifications or deviations noted in the amplification phase. Repeat testing was justified by prospective assay acceptance criteria or following a corrective and preventative action plan and applied to 11 samples. Defective culture flasks caused loss of cells in 2 test articles; both test articles contained 1 × 108 cells, which were split among 25 flasks. Investigators indicated that the remaining 9.6 × 107 was sufficient to meet the 1% FDA testing requirement, and the assay was completed without requiring additional test articles. For 2 test articles in one assay, the amplification-phase positive controls were negative, requiring resubmission of test articles. Two additional amplification-phase cultures were lost due to technician error. In 3 test articles from two different investigators, the test articles inhibited the growth of the C8166-45 cells. Resubmitted test articles also showed inhibition, and the assay was completed by increasing the number of C8166-45 cells at each passage. All resubmitted test articles discussed earlier completed the assay successfully and were found to be negative for RCL at the amplification and indicator phases. During review of all assays for this analysis, 2 assays were found to have a miscalculation in the number of C8166-45 cells added to the culture; the ratio of test article cells to C8166-45 cells was 1:4.35, which is below the minimum of 1:5 specified for this assay.
There were 8 indicator-phase assays that failed to meet acceptability criteria. Three of the assays failed due to technician error. One indicator-phase culture grew slowly, and a repeat assay using reserve material also showed slow growth of C8166-45 cells; sufficient cells were available for analysis. Four indicator assays were repeated because the positive controls were negative. Reserve amplification phase media were available for all the indicator-phase assays noted earlier; resubmission test articles were, therefore, not needed. All indicator-phase cultures using reserve amplification phase media were found to be negative for RCL.
Amplification- and indicator-phase culture media are subjected to p24 ELISA analysis to identify HIV capsid protein, a predicted component of any RCL. Each test article, as well as control, is run in duplicate wells. There were no instances when both duplicate wells were positive, which is an acceptance criterion for a positive assay. There were 15 instances when one well was below and one slightly above the limits of detection; 9 occurred in a test article, and 6 occurred in a negative-control sample. In all cases, repeat analysis with reserve material found the sample to be below the limits of detection (12.5 pg/mL). In addition, the test articles in question were negative by psi-gag PCR or PERT. All test articles tested by ELISA met the criteria for RCL-negative samples.
Between June 2011 and March of 2013, 84 test articles were analyzed by the psi-gag PCR assay. One assay did not meet criteria, due to a positive signal detected in both the test article and the negative control. The p24 ELISA result for this test article was negative in both the amplification and indicator phases. The assay was repeated, and both the negative control and the test article were negative; the test article was considered negative for RCL. After March of 2013, the PERT assay replaced psi-gag PCR. There were 3 PERT assays where the standard curve did not meet acceptability criteria and 1 assay where the positive control did not meet acceptance criteria. The assays were repeated using reserve material and met acceptance criteria. In analyzing 417 test articles by PERT, there were 4 test articles and 2 negative-control test articles that were just above baseline. The corresponding p24 analysis was negative, and repeat PERT testing using reserve samples was negative for reverse transcriptase. Upon retesting, all test articles met the criteria for RCL-negative samples.
Discussion
Inadvertent exposure of subjects to pathogens that contaminate gene therapy products is a top safety concern. Lentiviral vectors have been designed to minimize the chance of recombination, and, to date, RCL has not been detected in research materials generated with third-generation vector systems. In 2011, we also showed that 16 lentiviral vector products manufactured for clinical trial use had no evidence of RCL.29 Continued testing at the NGVB has failed to detect RCL using material submitted from a variety of manufacturing facilities. In this paper, we provide further evidence for the biosafety of lentiviral vector production systems. Using a sensitive RCL assay, there was no evidence of replicating virus in 460 T cell products. The products tested span 26 clinical trials at 6 different institutions. Follow-up analysis of treated research subjects also found no evidence of RCL. Given the lack of documented RCL in a large number of T cell products, the design features of third-generation lentiviral vectors, and the lack of documented RCL in clinical vector lots, RCL testing of T cell products does not appear to provide additional assurances of safety, and testing requirements should be re-evaluated. Our findings also have implications for research laboratories and suggest a need for re-evaluation of biosafety requirements for third-generation lentiviral vectors.
In developing a detection assay for RCL, we developed an assay that was exhaustive by intent. The assay uses both p24 ELISA and a molecular detection assay (psi-gag PCR or PERT) to provide redundancy in order to minimize false-positive or false-negative results due to technical error. Since we do not know the components of an RCL, two assays aimed at different components of the RCL may also increase the chance of RCL detection. For example, an RCL containing endogenous human retroviral or other unpredicted sequences may not be recognized in assays aimed at detecting HIV-1. The addition of the indicator phase also ensures that the assay detects a true RCL by requiring passage from amplification-phase cells to naive C8166-45 cells. The assay has been validated to detect 1 IU of RCL per 106 test article cells, and in this study, there was a total of 2.4 × 1010 transduced T cells tested.
The design of third-generation lentiviral vectors suggests that the risk of RCL is significantly lower than that of MLV-based vectors.40 The ability to segregate the vector components onto four plasmids, the use of self-inactivating (SIN) long terminal repeats (LTRs), and the retention of Rev dependence all contribute to the safety profile of lentiviral vectors.41, 42 HIV-1 also depends on a number of accessory genes and regulatory sequences that are deleted in lentiviral vector systems, further limiting the growth potential of any RCL. Furthermore, lentiviral vectors are generally produced by transient transfection, which limits the time for recombination events. Whether the experience in vector products generated by transient transfection will extend to lentiviral packaging cell lines awaits further studies. The most current lentiviral lines do suppress gene expression until shortly before vector harvest and also incorporate many of the safety features described earlier.43, 44 This predicts a significantly greater safety profile than the MLV-based packaging cell lines, but additional experience is required to establish whether these will consistently generate RCL-free vector products. Similarly, the propensity for RCL development in HIV-1-based vectors with significant differences in vector design, method of manufacture, or envelope will require bridging or full validation studies. Also, our findings do not extend to non-HIV-1-based lentiviruses or other retroviral vector systems.
In general, the RCL assay described here performed well. Of the 499 test articles analyzed, there were 11 test articles that required resubmission due to technical issues. Three of the 11 required resubmission due to growth inhibition of the C8166-45 cells. Growth inhibition appeared to be related to the cellular product; there were no apparent issues of microbial contamination, samples were run on different assays and were obtained from different investigators, and there was no evidence of p24 in the amplification-phase media. The assays were repeated, and increasing the number of cells at each split allowed the assays to be completed. Interestingly, the three products in question (representing 3 of 499 products test or 0.6%) were CD4 T cell subset samples.
There were occasions when a single replicate well in p24 or PERT was above background, while all other analyses for p24 and PERT were negative. This occurred in test articles and negative controls. Given the greater number of test articles run per assays, compared to the negative control, there is no evidence of a higher frequency occurring in test articles. Both p24 ELISA and PERT assays are performed in plates; the most likely cause of the sporadic positive wells is aerosolization of positive-control materials during set up and handling of the plates.
In addition to testing the T cell products, we collected data from the 26 clinical trials on post-infusion RCL testing. The U.S. FDA requires subjects infused with lentiviral transduced products be monitored for RCL, but currently there is no guidance requiring a specific assay. Interestingly, each investigator independently chose qPCR for the VSV-G envelope as the preferred method for RCL monitoring. To date, no evidence of VSV-G envelope DNA has been reported in the subjects enrolled in the 26 clinical trials surveyed.
In summary, RCL has not been detected in third-generation lentiviral vector products manufactured for clinical use.29 We now add to that experience by analyzing T cell products used in cancer immunotherapy. In 460 products tested, using a vigorous biologic assay for RCL, there was no evidence of RCL. Participants evaluated post-infusion of T cell products were also without evidence of RCL exposure. These findings suggest that current vector design and vector product screening provide a high level of assurance regarding the absence of replicating virus. Therefore, screening T cell products for RCL does not add additional assurance of safety and should no longer be required when the lentiviral vector product has been successfully screened for RCL.
Materials and Methods
Collection of Study Data
The NGVB is a National Heart, Lung and Blood Institute-sponsored resource that assists gene therapy investigators in meeting FDA-required testing (www.NGVBCC.org); all T cell product testing was performed centrally at the NGVB. For this study, RCL assays performed between June 2011 and August 2016 were reviewed, and all investigators with at least one study of more than 10 subjects were invited to participate. Only samples intended for in vivo administration were included in the analysis. Investigators agreeing to participate were sent a list of their studies along with the test article’s name and the dates of assay initiation and completion. Participants were asked to supply the following information: (1) lentiviral vector type (second or third generation); (2) target cell type; (3) transgene type (TCR, CAR, or other); (4) whether the product was administered to the subject; (5) whether the clinical vector product was shown to be RCL free prior to use in the clinical trial; and (6) whether the subject was screened for RCL at ≥30 days after product administration. For subjects screened for RCL after product administration, the testing was not done centrally, and the method of screening was at the investigators’ discretion. All were screened by VSV-G, except for one patient tested by serology in a clinical pathology laboratory for HIV-1 p24 antigen.
Cell Line and Positive Control Preparation
The C8166-45 (derived from human umbilical cord lymphocytes) cell line was obtained from the AIDS Research and Reference Reagent Program (Rockville, MD), and HEK293T cells were obtained from the American Type Culture Collection (Manassas, VA). The former was maintained in RPMI 1640 media, while the latter were maintained in DMEM. Both media were obtained from Invitrogen (Carlsbad, CA) and supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen), and 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen; RPMI10 and D10, respectively). D10 also contains sodium pyruvate (100 mM) and L-glutamine (200 mM). C8166-45 cells are maintained in cell banks, and only those cells at ≤15 passages at the start of an assay are utilized. The attenuated HIV-1-positive control is generated by transfecting HEK293T cells with the pR8.71 plasmid (provided from Cell Genesys; 112 μg plasmid per 5 × 106 cells) followed by a media change at 24 hr. Cell-free supernatant (0.45-μm filter) is collected 48 hr after transfection. Material is stored at < −70°C and then thawed for potency assessment by preparing thirty-nine 50-mL flat-bottom culture tubes with 1 × 106 C8166-45 cells per tube and incubating cells overnight. On day 0, 6 tubes are centrifuged, and cells are resuspended in 1 mL of virus with Polybrene at 8 μg/mL. The six dilutions tested range from 10−3 to 10−8 of the frozen viral stock. Three negative-control tubes are also prepared. After a 4-hr incubation, cells are pelleted, resuspended in RPMI, and transferred to 6-well plates. Cells are maintained in log-phase growth for at 12 days, after which time, cells are pelleted, and the media are filtered (0.45 μm) and assessed for p24 by ELISA. Cultures are read as positive or negative based on the lower limits of detection of the ELISA assay (12.5 pg/mL).
RCL Assays
Amplification Phase
The RCL assay used here has previously been reported for screening lentiviral vector products29 and was used with the modifications described in the following text. Control triplicate negative- and positive-control cultures are prepared on day −1 by adding 12 mL RPMI10 and 5 × 106 C8166-45 cells into six 50-mL culture tubes (flat-bottom, 10 cm2). Test article flasks are prepared on day −1 based on the number of cells to be tested. A minimum of 1 test cell to 5 C8166-45 cells are required for the assay. Most investigators submit 108 cells, which require twenty-five 300-cm2 flasks. On day 0, the negative-control culture media are replaced with 1 mL RPMI10 and Polybrene (8 μg/mL), and the three positive controls are inoculated with 1 mL RPMI10, 5 IU R8.71 virus, and 8 μg/mL Polybrene. On day 0, the test article cells are introduced into the flasks containing C8166-45 cells. After 4 hr, cultures are centrifuged and media replaced with RPMI10. Cells are passed for 3 weeks with a minimum of 5 splits. Cultures in larger flasks are split into decreasing size flasks with cells cultured in 75 cm2 flasks by the end of week 3. The media are not conducive for primary T cell growth, and C8166-45 cells predominate at the end of the amplification phase.
Indicator Phase
At the end of the 3-week culture, test-article and negative- and positive-control culture cells are resuspended in fresh RPMI10; media are collected after 24 hr and then filtered (0.45 μm). Naive C8166-45 cells (1 × 106 C8166-45 cells in 4 mL) are incubated overnight and then cultured with the filtered media for 4 hr in the presence of Polybrene (8 μg/mL). For a test article, the material from all flasks is pooled and used to inoculate 2 test-article indicator-phase flasks. After 6 days in cultures, cells are resuspended in fresh media, collected 24 hr later, filtered (0.45 μm), and analyzed for evidence of RCL. All samples in this paper were assessed for RCL using the p24 ELISA assay, a commercially available kit (Alliance HIV-1 p24 ELISA Kit, PerkinElmer, Waltham, MA) as previously described.29 All measurements were conducted in duplicate. For test articles, any value above the limits of detection (12.5 pg/mL) is considered positive. For the positive controls, values above the upper limits of detection (100 pg/mL) are expected.
The psi-gag PCR assay detects recombinants between the psi and gag viral genes and is described in Sastry et al.,11 with a modification to include a second PCR for human beta globin to validate that the test article DNA is of sufficient quantity and quality. The psi-gag PCR assay required PCR amplification followed by Southern blot analysis with 32P-labeled probes and added significant time to the assay turnaround. A validation study was performed demonstrating the equivalency of the PERT assay30 to the psi-gag PCR assay, and samples after March 2013 were evaluated by PERT. The limit of detection in the PERT assay is 100 reverse transcriptase (RT) molecules per 25 μL; positive controls at the end of the indicator phase are expected to be in excess of 104 RT molecules per 25 μL.
Acceptance criteria are defined as follows. At the end of the indicator phase, the assay is acceptable if (1) all 3 negative controls are negative for p24 antigen; (2) all 3 negative controls are negative for psi-gag sequences or PERT; and (3) at least one of the positive control flasks is positive for p24 antigen and psi-gag at the indicator phases. If the controls are acceptable and the 2 test article flasks are negative for p24 antigen and psi-gag PCR/PERT, then the test article is reported as negative for RCL. If the test article is positive for both p24 and psi-gag, the sample is interpreted as RCL positive. If both test article flasks are positive in one assay but negative in the other (e.g., positive for p24 and negative for psi-gag PCR/PERT), the indicator phase is repeated and extended for >14 days before samples are harvested and tested. If one of two test article flasks are positive in one assay and negative in the other assay, then both assays are repeated with reserve samples (e.g., one flask above and one below background in the ELISA assay and both flasks negative in psi-gag PCR/PERT). The only time the indicator phase is not repeated is when one of the 10 test article analyses is above background (each test article has 2 flasks from the indicator phase, and each flask is tested in duplicate for 24 and in triplicate by PERT, for a total of 10 analyses per test article). In this case, the discrepant sample is retested from reserve material from the initial indicator phase. If the repeat testing is negative, the sample is considered negative for RCL.
Author Contributions
Conceptualization, methodology, and investigation, K.C. and L.D.; Resources, C.J.T., M.J., S.F., G.B.-S., T.F., A.C., D.G.M., and C.H.J.; Writing – First Draft, K.C.; Writing – Review and Editing, all authors. Funding acquisition, K.C. (for the NGVB) and C.J.T., M.J., S.F., G.B.-S., T.F., D.G.M., and C.H.J. (for clinical trial samples).
Acknowledgments
The authors would like to thank Sue Koop, Jing Yao, Stephanie Baker, Lilith Reeves, and Scott Witting for assistance and the NGVB technicians involved with RCL testing. The National Gene Vector Biorepository is supported by a grant from the NHLBI (P40HL116242; principal investigator, K.C.). K.C. is a consultant for PACT Pharma; there was no financial conflict with the manuscript work. Authors would also like to acknowledge the following: C.T. is funded by NCT01865617. M.J. is a founder and equity holder in Juno Therapeutics, Inc., where he serves as a consultant. S.F. receives laboratory support from Mustang Biotech and acknowledges the contributions of Jamie Wagner and Christine Brown. G.B.-S. is an employee of Adaptimmune, compensated by salary and stock options, and acknowledges the contribution of Shuguang Bi. T.F. receives funding from the Intramural Research Program at the NIH and acknowledges the clinical team in the Pediatric Oncology Branch, the Hematologic Malignancies section of the Pediatric Oncology Branch, and the Cell Processing Section at the NIH Clinical Center. D.G.M. receives research funding from Juno Therapeutics. C.H.J. has a sponsored research grant from Novartis and receives royalties from the University of Pennsylvania for intellectual property (IP) licensed to Novartis, and is the scientific founder of Tmunity Therapeutics. A.C. has equity interests in Tmunity Therapeutics. C.H.J. and A.C. acknowledge the contributions of Katherine Marcucci, Julie Jadlowsky, Megan Suhoski, and Bruce Levine.
References
- 1.Corrigan-Curay J., Kiem H.P., Baltimore D., O’Reilly M., Brentjens R.J., Cooper L., Forman S., Gottschalk S., Greenberg P., Junghans R. T-cell immunotherapy: looking forward. Mol. Ther. 2014;22:1564–1574. doi: 10.1038/mt.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June C.H., Riddell S.R., Schumacher T.N. Adoptive cellular therapy: a race to the finish line. Sci. Transl. Med. 2015;7:280ps7. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 3.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Ravin S.S., Wu X., Moir S., Anaya-O’Brien S., Kwatemaa N., Littel P., Theobald N., Choi U., Su L., Marquesen M. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016;8:335ra57. doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 9.Castiello M.C., Scaramuzza S., Pala F., Ferrua F., Uva P., Brigida I., Sereni L., van der Burg M., Ottaviano G., Albert M.H. B-cell reconstitution after lentiviral vector-mediated gene therapy in patients with Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. 2015;136:692–702.e2. doi: 10.1016/j.jaci.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuate S., Marino M.P., Reiser J. Analysis of partial recombinants in lentiviral vector preparations. Hum. Gene Ther. Methods. 2014;25:126–135. doi: 10.1089/hgtb.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sastry L., Xu Y., Johnson T., Desai K., Rissing D., Marsh J., Cornetta K. Certification assays for HIV-1-based vectors: frequent passage of gag sequences without evidence of replication-competent viruses. Mol. Ther. 2003;8:830–839. doi: 10.1016/j.ymthe.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Koldej R., Cmielewski P., Stocker A., Parsons D.W., Anson D.S. Optimisation of a multipartite human immunodeficiency virus based vector system; control of virus infectivity and large-scale production. J. Gene Med. 2005;7:1390–1399. doi: 10.1002/jgm.803. [DOI] [PubMed] [Google Scholar]
- 13.Muenchau D.D., Freeman S.M., Cornetta K., Zwiebel J.A., Anderson W.F. Analysis of retroviral packaging lines for generation of replication-competent virus. Virology. 1990;176:262–265. doi: 10.1016/0042-6822(90)90251-l. [DOI] [PubMed] [Google Scholar]
- 14.Bodine D.M., McDonagh K.T., Brandt S.J., Ney P.A., Agricola B., Byrne E., Nienhuis A.W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 1990;87:3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarpa M., Cournoyer D., Muzny D.M., Moore K.A., Belmont J.W., Caskey C.T. Characterization of recombinant helper retroviruses from Moloney-based vectors in ecotropic and amphotropic packaging cell lines. Virology. 1991;180:849–852. doi: 10.1016/0042-6822(91)90105-k. [DOI] [PubMed] [Google Scholar]
- 16.Otto E., Jones-Trower A., Vanin E.F., Stambaugh K., Mueller S.N., Anderson W.F., McGarrity G.J. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum. Gene Ther. 1994;5:567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- 17.Bosselman R.A., Hsu R.-Y., Bruszewski J., Hu S., Martin F., Nicolson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Moloney murine leukemia virus structural genes via the metallothionein promoter. Mol. Cell. Biol. 1987;7:1797–1806. doi: 10.1128/mcb.7.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J. Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 20.Danos O., Mulligan R.C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci. USA. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A.D., Rosman G.J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 22.Miller A.D., Garcia J.V., von Suhr N., Lynch C.M., Wilson C., Eiden M.V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong H., Starkey W., Vile R.G. A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences. J. Virol. 1998;72:2663–2670. doi: 10.1128/jvi.72.4.2663-2670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett E., Miller A.R.-M., Goldman J.M., Apperley J.F., Melo J.V. Characterization of recombination events leading to the production of an ecotropic replication-competent retrovirus in a GP+envAM12-derived producer cell line. Virology. 2000;266:170–179. doi: 10.1006/viro.1999.0052. [DOI] [PubMed] [Google Scholar]
- 25.Urnovitz H.B., Murphy W.H. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin. Microbiol. Rev. 1996;9:72–99. doi: 10.1128/cmr.9.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornetta K., Wilson C.A. Safety of retroviral vectors: regulatory and technical considerations. In: Dropulic B., Carter B., editors. Concepts in Genetic Medicine. John Wiley & Sons; 2008. pp. 277–288. [Google Scholar]
- 27.U.S. Food and Drug Administration . U.S. Department of Health and Human Services, Food and Drug Administration; 2006. Guidance for Industry: Supplemental Guidance on Testing for Replication Competent Retrovirus in Retroviral Vector Based Gene Therapy Products and During Follow-up of Patients in Clinical Trials Using Retroviral Vectors.https://www.fda.gov/OHRMS/DOCKETS/98fr/994114gd.pdf [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration . U.S. Food and Drug Administration; 1998. Guidance for Human Somatic Cell Therapy and Gene Therapy. [Google Scholar]
- 29.Cornetta K., Yao J., Jasti A., Koop S., Douglas M., Hsu D., Couture L.A., Hawkins T., Duffy L. Replication-competent lentivirus analysis of clinical grade vector products. Mol. Ther. 2011;19:557–566. doi: 10.1038/mt.2010.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sastry L., Xu Y., Marsh J., Cornetta K. Product-enhanced reverse transcriptase (PERT) assay for detection of RCL associated with HIV-1 vectors. Hum. Gene Ther. 2005;16:1227–1236. doi: 10.1089/hum.2005.16.1227. [DOI] [PubMed] [Google Scholar]
- 31.Miskin J., Chipchase D., Rohll J., Beard G., Wardell T., Angell D., Roehl H., Jolly D., Kingsman S., Mitrophanous K. A replication competent lentivirus (RCL) assay for equine infectious anaemia virus (EIAV)-based lentiviral vectors. Gene Ther. 2006;13:196–205. doi: 10.1038/sj.gt.3302666. [DOI] [PubMed] [Google Scholar]
- 32.Chang L.J., Urlacher V., Iwakuma T., Cui Y., Zucali J. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 1999;6:715–728. doi: 10.1038/sj.gt.3300895. [DOI] [PubMed] [Google Scholar]
- 33.Segall H.I., Yoo E., Sutton R.E. Characterization and detection of artificial replication-competent lentivirus of altered host range. Mol. Ther. 2003;8:118–129. doi: 10.1016/s1525-0016(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasakumar N., Schuening F.G. A lentivirus packaging system based on alternative RNA transport mechanisms to express helper and gene transfer vector RNAs and its use to study the requirement of accessory proteins for particle formation and gene delivery. J. Virol. 1999;73:9589–9598. doi: 10.1128/jvi.73.11.9589-9598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escarpe P., Zayek N., Chin P., Borellini F., Zufferey R., Veres G., Kiermer V. Development of a sensitive assay for detection of replication-competent recombinant lentivirus in large-scale HIV-based vector preparations. Mol. Ther. 2003;8:332–341. doi: 10.1016/s1525-0016(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 36.Manilla P., Rebello T., Afable C., Lu X., Slepushkin V., Humeau L.M., Schonely K., Ni Y., Binder G.K., Levine B.L. Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector. Hum. Gene Ther. 2005;16:17–25. doi: 10.1089/hum.2005.16.17. [DOI] [PubMed] [Google Scholar]
- 37.Farley D.C., McCloskey L., Thorne B.A., Tareen S.U., Nicolai C.J., Campbell D.J., Bannister R., Stewart H.J., Pearson L.J., Moyer B.J. Development of a replication-competent lentivirus assay for dendritic cell-targeting lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2015;2:15017. doi: 10.1038/mtm.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corre G., Dessainte M., Marteau J.B., Dalle B., Fenard D., Galy A. “RCL-pooling assay”: a simplified method for the detection of replication-competent lentiviruses in vector batches using sequential pooling. Hum. Gene Ther. 2016;27:202–210. doi: 10.1089/hum.2015.166. [DOI] [PubMed] [Google Scholar]
- 39.McGarrity G.J., Hoyah G., Winemiller A., Andre K., Stein D., Blick G., Greenberg R.N., Kinder C., Zolopa A., Binder-Scholl G. Patient monitoring and follow-up in lentiviral clinical trials. J. Gene Med. 2013;15:78–82. doi: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]
- 40.Schambach A., Zychlinski D., Ehrnstroem B., Baum C. Biosafety features of lentiviral vectors. Hum. Gene Ther. 2013;24:132–142. doi: 10.1089/hum.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zufferey R., Dull T., Mandel R.J., Bukovsky A., Quiroz D., Naldini L., Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonner M., Ma Z., Zhou S., Ren A., Chandrasekaran A., Gray J.T., Sorrentino B.P., Throm R.E. Development of a second generation stable lentiviral packaging cell line in support of clinical gene transfer protocols. Mol. Ther. 2015;23(Suppl. 1):S35. [Google Scholar]
- 44.Throm R.E., Ouma A.A., Zhou S., Chandrasekaran A., Lockey T., Greene M., De Ravin S.S., Moayeri M., Malech H.L., Sorrentino B.P., Gray J.T. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–5110. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]