Abstract
Repair and regeneration of inflammation-induced bone loss remains a clinical challenge. LL37, an antimicrobial peptide, plays critical roles in cell migration, cytokine production, apoptosis, and angiogenesis. Migration of stem cells to the affected site and promotion of vascularization are essential for tissue engineering therapy, including bone regeneration. However, it is largely unknown whether LL37 affects mesenchymal stem cell (MSC) behavior and bone morphogenetic protein 2 (BMP2)-mediated bone repair during the bone pathologic remodeling process. By performing in vitro and in vivo studies with MSCs and a lipopolysaccharide (LPS)-induced mouse calvarial osteolytic bone defect model, we found that LL37 significantly promotes cell differentiation, migration, and proliferation in both unmodified MSCs and BMP2 gene-modified MSCs. Additionally, LL37 inhibited LPS-induced osteoclast formation and bacterial activity in vitro. Furthermore, the combination of LL37 and BMP2 markedly promoted MSC-mediated angiogenesis and bone repair and regeneration in LPS-induced osteolytic defects in mouse calvaria. These findings demonstrate for the first time that LL37 can be a potential candidate drug for promoting osteogenesis and for inhibiting bacterial growth and osteoclastogenesis, and that the combination of BMP2 and LL37 is ideal for MSC-mediated bone regeneration, especially for inflammation-induced bone loss.
Keywords: mesenchymal stem cells, LL37, antimicrobial peptide, bone regeneration, bone repair, inflammation, bone loss, osteolysis
Yang and colleagues found that the combination of antimicrobial peptide LL37 with bone morphogenetic protein 2 dramatically promotes mesenchymal stem cell mediated bone regeneration, providing a new therapeutic strategy for promoting bone regeneration, especially for inflammation-induced bone loss, such as osteomyelitis, periodontitis caused bone loss, and rheumatoid arthritis.
Introduction
Inflammation-induced bone diseases, such as periodontitis and rheumatoid arthritis, are characterized by loss of bone matrix constituents, eventually leading to resorption and bone loss.1, 2, 3 Several studies have reported that activation of the proteolytic enzymes such as matrix metalloproteinase (MMP) can initiate and propagate collagen destruction during inflammatory disease.4, 5, 6, 7 An imbalance between activation of MMP and downregulation of their endogenous inhibitors leads to pathologic breakdown of the extracellular matrix.8, 9 Inflammation is an integral part of the innate immune response required for maintaining a functional and physical barrier against microorganisms.10 However, overactive inflammatory responses are often harmful to the organism. It is difficult to regenerate bone following bone loss owing to inflammation. Thus, multiple regulatory mechanisms modulate the effectors of innate immunity to balance between host defense and inflammation.11 Neutrophils are one of the most prominent effector cells of the innate immune system,12 which are recruited to the site of infection and exert various antimicrobial effector mechanisms.13
Antimicrobial peptides (AMPs) are effector molecules of the innate immune system with various activities. LL37, a naturally occurring 37-aa sequence synthesized from the C terminus of human cationic antimicrobial protein 18 (hCAP-18), is one such antimicrobial peptide. It possesses bactericidal activity and mediates immunomodulation under physiological conditions.14 LL37 is a constituent of the body fluids and of several cell types, such as epithelial cells and immune cells.15, 16 When a wound occurs, LL37 is significantly elevated and helps to regulate cell proliferation, vascularization, and immunomodulation via the mitogen-activated protein kinase (MAPK) pathway at the injury site.17 It can also interact with cell membranes to affect cell surface receptors and enter cells.17 A previous study has demonstrated that LL37 can exert both pro- and anti-inflammatory effects, either directly by stimulating cells or indirectly by modulating the cellular response to cytokine(s) or signal(s).17 Additionally, LL37 can increase chemotaxis of neutrophils and immune cells as well as cytokine production at the wound healing site.17 Moreover, LL37 has a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria, including Staphylococus aureus and Pseudomonas aeruginosa.18 A recent study by Krasnodembskaya et al.19 showed that human MSCs possess direct antimicrobial activity, which is mediated in part by secretion of LL37. On the basis of these multiple biological functions, LL37 has been associated with the maintenance of host homeostasis, i.e., the regulation of wound healing, immune response, and neovascularization in injured tissues. However, the role of LL37 in bone tissue engineering is still elusive.
Recently, stem cells have been employed in the field of tissue engineering, especially because of their ability to differentiate into various lineages and promote vascularization and bone formation. Bone-marrow-derived mesenchymal stem cells (MSCs) are one of the populations of stem cells used most frequently in studies of bone regeneration.20, 21 These cells can be easily isolated than procuring autogenous grafts.20 Moreover, MSCs can be autologous to avoid the risk of disease transmission, graft rejection, and expensive costs associated with allogenic graft.21 MSCs can differentiate into an osteogenic lineage in the presence of several growth factors, including platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), transforming growth factor β (TGF-β), and bone morphogenetic proteins (BMPs). Of these growth factors, BMPs (especially BMP2) have the greatest potential to induce MSCs to differentiate into an osteogenic lineage.22 In our previous studies, we have reported the role of MSCs with BMP2 in osteogenic differentiation and in the repair and regeneration of critical sized bone defects in vivo.23, 24 Clinical studies generally employ BMP2 recombinant protein for bone regeneration. However, the advantage of using BMP2 gene modification rather than exogenous BMP2 protein includes lower costs and the ability to produce a stable level of BMP2 protein in BMP2- engineered MSCs.25
In this study, we tested the effect of LL37 on MSC behavior and MSC-mediated bone formation both in vitro and in vivo. We found that LL37, especially combined with BMP2, promoted MSC proliferation, migration, and osteogenic differentiation and inhibited osteoclast formation and bacterial infection in vitro. We further applied LL37 to a mouse calvarial lipopolysaccharide (LPS)-induced osteolytic model and analyzed the new bone formation in the area of the induced defects. We found that LL37 combined with BMP2-modified MSCs dramatically enhanced new blood vessel formation and ectopic bone formation in the LPS-induced bone defect site. These findings demonstrated for the first time that LL37 promotes osteogenesis and angiogenesis. The combination of LL37 and BMP2 can be a new therapeutic strategy to promote MSC-mediated bone regeneration, especially for inflammation-induced bone loss.
Results
LL37 Promotes MSC Proliferation and Osteogenic Differentiation
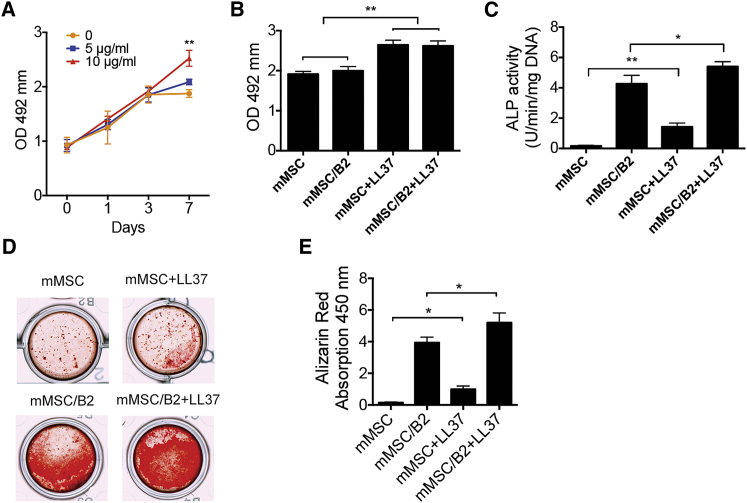
We first tested the proliferation activity of LL37 on mouse MSCs (mMSC) at different dosages. The results demonstrated that 10 μg/mL LL37 significantly increased the proliferation rate of mMSC at day 7 (Figure 1A). Subsequently, the proliferation rates of mMSC, BMP2-modified MSCs (mMSC/B2), mMSC+LL37, and mMSC/B2+LL37 groups were compared. mMSC/B2 showed the same proliferation rate as the mMSC group. LL37 (10 μg/mL) promoted cell proliferation in both mMSC and mMSC/B2 at day 7 (Figure 1B).
Figure 1.
LL37 Promotes Proliferation, Osteogenic Differentiation, and Mineralization of MSCs
(A) Cell proliferation activity of MSCs with LL37 (5 or 10 μg/mL) at a cell seeding density of 5 × 103 per well in a 96-well plate (n = 6). **p < 0.01. (B) Cell proliferation activity of mMSC, mMSC/B2, mMSC+LL37, and mMSC/B2+LL37 at day 7 (n = 6). **p < 0.01. (C) ALP activity of MSCs with LL37 (5 or 10 μg/mL) treatment (n = 3). The cells were induced with osteogenic media for 7 days. *p < 0.05; **p < 0.01. (D) Alizarin red staining of mMSC, mMSC/B2, mMSC+LL37, and mMSC/B2+LL37. Cells were cultured in osteogenic medium for 14 days. (E) Quantitative analysis of cell mineralization shown in (D) (n = 3). *p < 0.05.
We next examined the effect of LL37 on osteogenic differentiation by alkaline phosphatase (ALP) activity assay and alizarin red staining. ALP activity was measured at day 7 following osteogenic induction with 5 or 10 μg/mL LL37. LL37 was found to significantly increase the ALP activity (Figure 1C). A combination of LL37 and BMP2 dramatically enhanced the ALP activity (Figure 1C). The level of calcium mineral deposition was measured by alizarin red staining with 14-day osteogenic induction. In line with the ALP activity results, BMP2 and LL37 both increased calcium mineral deposition, whereas a combination of both led to further significant enhancement in osteoblast differentiation and mineralization (Figure 1D).
LL37 Promotes MSC Migration
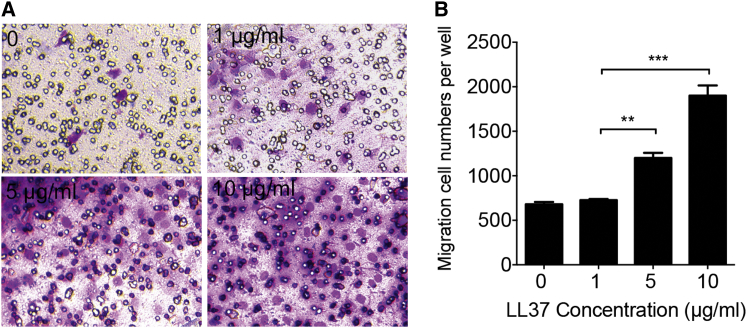
To determine whether LL37 is involved in the migration of MSCs, we performed in vitro migration assays using transwell chambers. MSCs were seeded in the chambers and treated with LL37 at indicated dosages. After MSCs were incubated with LL37 for 12 hr in the transwell system, the numbers of cells that moved through the membrane were determined following Giemsa staining (Figure 2A). The results showed that the number of MSCs that had moved through the membrane in the LL37 (10 μg/mL) group was 2.57-fold more than in the control group (0) (Figure 2B). These results indicated that LL37 promotes MSC migration in a dose-dependent manner.
Figure 2.
LL37 Promotes MSC Migration
(A) Giemsa staining of cells (purple) that migrated through a transwell membrane pore (8 μm, black circle) in response to a different concentration of LL37. (B) The calculated migration cell number is shown in (A). **p < 0.01; ***p < 0.001.
LL37 Inhibits LPS-Induced Osteoclast Formation and Bacterial Activity
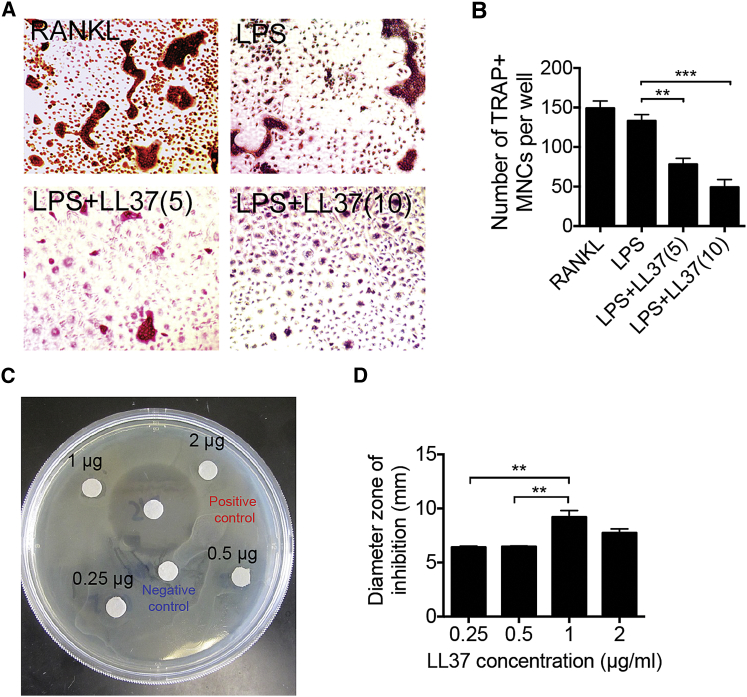
In addition to the direct effect of LL37 on osteogenic differentiation, we also characterized the effect of LL37 on osteoclast formation. Osteoclast precursor cells from bone marrow were cultured in vitro and treated with macrophage colony stimulating factor (M-CSF) and LPS. Tartrate-resistant acid phosphatase (TRAP) staining results showed that LPS induced the formation of osteoclasts in RANKL-pretreated cells (Figure 3A). LL37 dramatically inhibited LPS-induced osteoclast formation in a dose-dependent manner. The osteoclast number in the group with 10 μg/mL LL37 treatment was only 0.33-fold of that in the LL37 non-treated group (LPS) (Figure 3B). These results suggested that LL37 could inhibit inflammation-induced osteoclast formation.
Figure 3.
LL37 Shows Anti-bacterial Activity and Inhibits LPS-Induced Osteoclast Formation
(A) TRAP staining of the osteoclast formation by M-CSF/RANKL (RANKL) or M-CSF/LPS (LPS), as indicated. LL37 was added to the culture at 5 μg/mL (5) or 10 μg/mL (10). (B) Quantification of TRAP+ MNCs shown in (A) (n = 3). **p < 0.01. (C) Photographs of disk diffusion assay showing the inhibition zones of E. coli treated with LL37. Positive control is penicillin-streptomycin solution, and negative control is PBS. (D) Measurement of the diameter of inhibition zone in (C). n = 3. **p < 0.01.
LL37 is a known antibacterial peptide. We also tested its antibacterial activity by disk diffusion assay. We found that LL37 at 1 μg/mL had the greatest antibacterial effect (Figure 3C). The activity was recorded as diameter of the zone of inhibition, which is significantly increased in a dose-dependent manner until at the dose of 1μg/mL, then dropped down at 2 μg/mL (Figure 3D).
LL37 Combined with BMP2-Modified MSCs Dramatically Promotes Bone Regeneration in Inflammatory Calvarial Osteolytic Defects
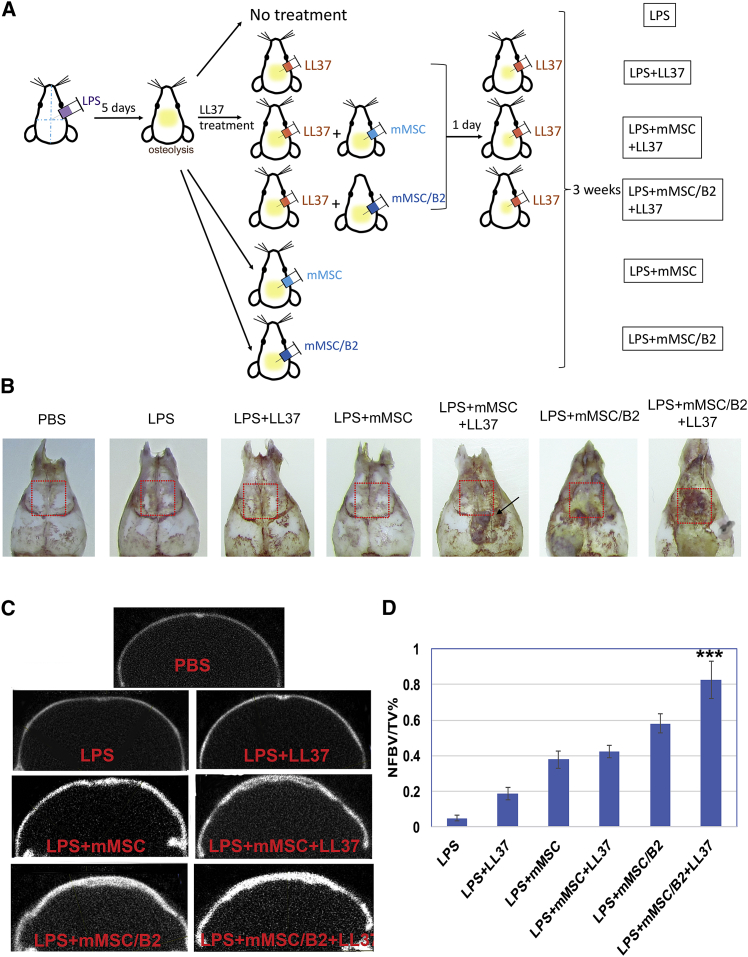
To evaluate the potential effect of LL37 on bone repair and regeneration in vivo, we used an LPS-induced mouse osteolytic calvarial defect model. LPS was injected into the calvaria to induce inflammation and bone loss. 5 days after injection, the mice were harvested and calvaria bones were isolated to analyze bone lysis. We found that LPS injection caused bone lysis in calvaria bones in all of the studied mice (see Figure S1). Furthermore, PBS-treated mice without an osteolytic defect were used as controls. Mice with LPS treatment were divided into 6 groups, as demonstrated in Figure 4A. Calvarial bones were harvested 3 weeks after the last treatment. Our results showed that more new bone was formed in the LPS+mMSC+LL37, LPS+mMSC/B2, and LPS+mMSC/B2+LL37 groups than in the PBS-treated normal bone group (no defect), LPS, LPS+LL37, and LPS+mMSC groups (Figure 4B).
Figure 4.
LL37 with BMP2-Modified MSCs Protects against LPS-Induced Osteolysis
(A) Schematic of LPS-induced osteolysis and treatment for each group. (B) Representative images showing calvarial bone harvested from each group. Red dot box is the ROI for Micro-CT quantitative analysis. (C) Representative coronal MicroCT image of each group. (D) Quantitative analysis of the ratio of newly formed bone volume (BV) to total volume (TV). Newly formed bone volume = total bone volume from each group at 3 weeks − bone volume from the LPS-injected group at day 5. ***p < 0.001 for the mMSC/B2+LL37 group versus each of the other groups.
Consistently, MicroCT results showed that the LPS+mMSC/B2+LL37 group exhibited the highest newly formed bone volume than the other groups (Figures 4C and 4D), demonstrating that the combination of BMP2-modified MSCs and LL37 can significantly promote bone regeneration after inflammation-induced bone loss. Other treatment groups also showed increased bone volume compared to the PBS and LPS groups (Figure 4C). Bone volumes in the LPS+mMSC+LL37 group were much higher than those in the LPS+mMSC and LPS+LL37 groups, indicating that the combination of LL37 and MSCs can promote bone regeneration.
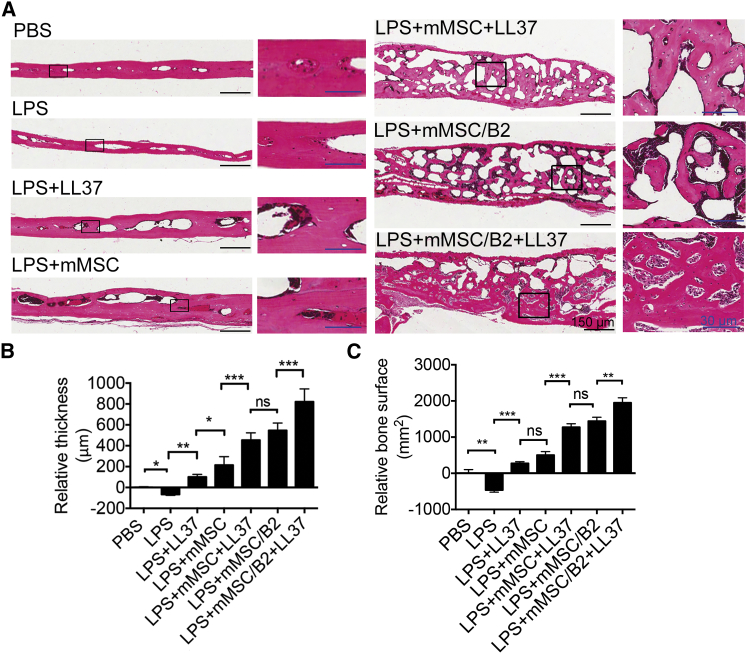
To further examine the newly formed bone, we performed histological assessment with H&E staining (Figure 5). Compared with normal calvarial bone (PBS group), the LPS group showed significantly decreased bone thickness, demonstrating that bone defects still existed at 3 weeks following LPS osteolytic induction. Introduction of mMSC (LPS+mMSC) to the defect area also helped the bone repair process. However, groups that were treated with LL37 twice showed significant bone regeneration, with increased bone thickness and bone surface area in the LPS-induced bone loss model (Figures 5A–5C). Although the LPS+mMSC group showed greater bone thickness, the bone surface area was comparable with that in the LPS+LL37 group. Mice treated with a combination of LL37 and mMSC/B2 showed much more bone formation than did the other groups. It is very clear from the histological section that the newly formed bones are cancellous bone with large pores, demonstrating that bone formation is in the active bone remodeling stage. The LPS+mMSC/B2 group showed a similar level of new bone formation to the LPS+mMSC+LL37 group, further confirming that LL37 has a comparable role to BMP2 in promoting bone formation in the inflammation-induced bone defect model. Therefore, treatment with a combination of mMSC/B2 and LL37 led to the greatest bone thickness and bone surface area. Although the newly formed bones were still spongy bone in the mice treated with LPS+mMSC/B2+LL37, the pore size and number were reduced compared with the groups treated with LPS+mMSC/B2 and LPS+mMSC+LL37, demonstrating that mMSC/B2 plus LL37 has robust osteogenic activity.
Figure 5.
Histological Evaluation of Newly Formed Bone in LPS-Induced Osteolysis
(A) H&E staining of calvarial bone for the analysis of new bone formation. The sagittal section through the midline of defects is shown. Lower magnification, scale bar (black), 150 μm; higher magnification, scale bar (blue), 30 μm. (B) Quantitative analysis of relative bone thickness shown in (A) (n = 3). *p < 0.05; **p < 0.01; ***p < 0.0001; ns, not statistically significant. (C) Quantitative analysis of relative bone surface shown in (A). **p < 0.01; ***p < 0.0001; ns, not statistically significant.
Because the in vitro results showed that LL37 can inhibit osteoclast formation, we evaluated the effect of LL37 on osteoclast formation in vivo by TRAP staining of calvarial bone sections 3 weeks post injury (Figure S2). The result showed that no statistical difference was observed among the PBS, LPS, and LPS+LL37 groups (Figure S3B). Furthermore, in the LPS+mMSC group, the TRAP-positive cell number was approximately double that in those three groups. The groups treated with LL37 plus MSCs or MSC/B2 (LPS+mMSC+LL37 and LPS+mMSC/B2+LL37) and LPS+mMSC/B2 had higher TRAP-positive cells than did LPS+mMSC. However, there is no significant difference in LL37 treated and non-treated MSCs and MSCs/B2 groups (Figures S2A and S2B), suggesting that LL37 does not directly promote osteoclast formation in vivo in the late stage of bone regeneration.
LL37 Combined with BMP2-Modified MSCs Promotes Blood Vessel Growth in Inflammatory Calvarial Osteolytic Defects
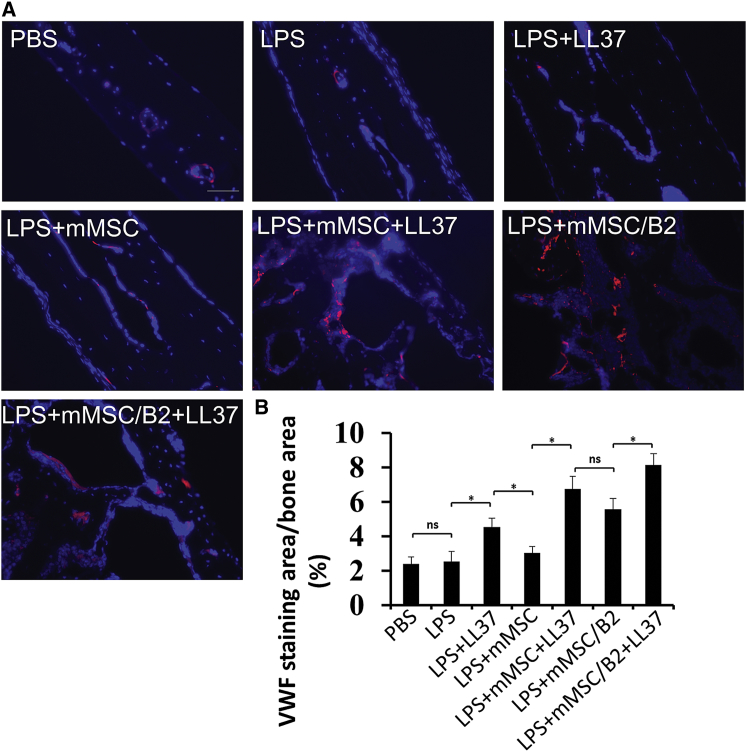
To investigate angiogenesis in calvarial bones after LPS injection and LL37 treatment, we performed immunofluorescence staining with antibodies against von Willebrand Factor (VWF) and smooth muscle α-actin (ACTA2) in calvarial bone sections (Figures 6, S3, and S4) and mouse liver sections (positive control, Figure S4). We studied blood vessel growth by quantifying the ratio of the positive staining area to the total bone area. As shown in Figures 6A and 6B, our data revealed that this ratio was similar in the PBS group and LPS group. Meanwhile, introduction of mMSC (LPS+mMSC) to the defect area did not significantly affect blood vessel growth compared to that in the LPS group. However, LL37 treatment significantly enhanced blood vessel formation, as attested by an increased VWF+ area. All LL37-treated groups showed a larger staining area when compared with LL37-untreated groups. As shown in Figure 6B, the VWF expression area was almost doubled in the LPS+LL37 group and tripled in the LPS+mMSC/B2+LL37 group compared to that in the LPS group. Taken together, these data indicate that LL37 promotes blood vessel growth in inflammatory calvarial osteolytic defects.
Figure 6.
Section of Calvarial Bone Stained with Antibody against VWF
(A) Representative images from each group. Scale bar, 100 μm. (B) Quantitative analysis of relative staining area per bone area (%) in (A) (n = 3). *p < 0.05; ns, not statistically significant.
Discussion
LL37, an antimicrobial peptide, exerts multiple biological effects on various types of cells.17 It has been associated with the regulation of wound healing, immune responses, and neovascularization in injured tissues.17, 18, 19 Further studies suggested that LL37 can facilitate the recruitment of MSCs to the bone defect region to promote bone repair.26, 27 However, whether the combination of LL37 and MSCs and/or BMP2 can enhance bone regeneration, especially in a model of inflammation-induced bone loss, was previously unknown. This study appears to be the first to evaluate the combination of LL37 and BMP2-modified MSCs in the treatment of inflammatory bone loss. The results of this study show that a combination of LL37 and BMP2-modified MSCs promotes bone regeneration after inflammation-induced bone loss and emphasize the potential of this construct to inhibit inflammation and lead to bone repair and regeneration.
In this study, we found that a combination of LL37 and BMP2-modified MSCs could significantly increase new bone formation in vivo in areas of LPS-induced bone loss; compared with other treatments, this combination led to greater bone thickness and surface area. Compared to the PBS control group, much more new bone formed in the LPS+mMSC/B2+LL37 group, which not only completely filled the bone loss defect, but also markedly increased the thickness of the calvaria bone, demonstrating a robust effect of LL37 and MSCs/B2 on osteogenesis and suggesting that LL37 is more effective when combined with unmodified or BMP2-modified MSCs to treat inflammation-induced bone defects. Notably, we also found that although bone thickness was slightly greater in the LPS+mMSC/B2 group, it was not significantly different from that in the LPS+mMSC+LL37 group, suggesting that LL37 has similar effects to BMP2 (a powerful activator of osteogenic differentiation) when combined with MSCs. Interestingly, even the group treated with LL37 alone exhibited increased new bone formation, which is comparable to the mMSC group, compared with the LPS group (defect only), suggesting that LL37 can treat inflammation-caused bone loss as effectively as MSCs.
LPS-neutralizing activities of LL37 have been well characterized in vitro and in vivo.28 LPS endotoxins are heteropolymeric components of the outer layer membrane of Gram-negative bacteria, with strong immunotoxic properties.29 LPS is released upon cell death, activating mononuclear phagocytes (monocytes and macrophages) to produce and release proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6).29 Studies have demonstrated that LL37 neutralizes LPS by binding to it with high affinity, inhibiting the interaction between LPS and LBP (LPS-binding protein) as well as reducing the expression of proinflammatory factors.29, 30 Our results showed that LL37 dramatically inhibited LPS-induced osteoclast formation in a dose-dependent manner. Additionally, disk diffusion assay results showed that LL37 also has antibacterial effects. These results suggest that LL37 could inhibit bacterial growth and neutralize LPS to inhibit inflammation in addition to osteoclast formation. However, our in vivo results showed that the groups with more newly formed bone had more TRAP+ osteoclasts. These results indicate that the increased osteoclast formation is likely because in the later stage, newly formed bones are underway of the active bone remodeling phase (Figures 5 and S3). These findings corroborate the published report from Nakamura et al.,31 which showed that basic fibroblast growth factor (FGF) injection into tibial fractures in a dog could promote bone regeneration as well as increase osteoclast numbers due to increase in the bone remodeling process.
MSCs are the pivotal progenitors for bone regeneration.32, 33, 34 Therefore, there is agreement among scientists that the efficient recruitment of MSCs can accelerate bone regeneration.34 Notably, in our study, we found that the mMSC/B2 group showed the same proliferation rate as the mMSC group. However, LL37 significantly increased the mMSC proliferation and migration. These findings are consistent with the previous studies showing that LL37 promotes the migration of dental pulp cells and the proliferation and migration of human adipose-derived stromal/stem cells though upregulating early growth response-1 (EGR1) and stromal cell-derived factor-1 (SDF1).35 Thus, these results provide additional evidence that the combination of LL37 and mMSC/B2 can dramatically enhance bone regeneration not only through inhibiting bacterial growth and LPS-induced bone loss, but also by promoting MSC migration and osteogenesis.
In conclusion, this study provides the first evidence that the novel combination of LL37 and mMSC/B2 stimulates new bone formation, and represents a potential treatment for bone loss, especially in inflammatory conditions. On the basis of the data presented here, it appears that this system can be useful not only in osteolytic defects, but also in the filling of defects, with limited accessibility, as in periodontal bone repair or marrow cavities as well as inflammatory bone loss. Its use in minimally invasive techniques, such as in situ fracture fixation and percutaneous vertebroplasty to fill the lesions and strengthen osteoporotic bone, is another area to explore further.
Materials and Methods
Preparation and Culture of MSCs
MSCs were prepared as previously described.23 Briefly, bone marrow was flushed from femurs and tibias of C57BL/6J mice aged 6–8 weeks. Separated bone marrow cells were cultured in α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L ʟ-glutamine, and 100 U/mL penicillin. Non-adherent cells were removed by changing fresh medium at day 3. Adherent cells were passaged at day 7 with TrypLE. At passage 4, cells were analyzed for MSC surface markers by flow cytometry.
BMP2 Gene Transfer
BMP2 adenovirus (Ad-BMP2) was produced and tittered as previously described.23, 24 Ad-BMP2 transfection was in serum-free medium for 4 hr and followed by incubation in 2% serum for an additional 24 hr.
Cell Proliferation
To investigate the effects of LL37 on the cellular proliferation of MSCs, MTS assay was performed using the MTS cell proliferation kit as per the manufacturer’s instructions (Promega). Cells were treated with different concentrations of LL37 and at different time points, as indicated in the figures. Optical density (OD) values were measured at 540 nm using a microplate reader. MSC group alone was used as a control group. Furthermore, the cell proliferation rate of MSCs treated with BMP2 and cultured with or without LL37 was assayed.
ALP Activity Assay and Alizarin Red Staining
MSCs were induced with osteogenic medium (α-MEM culture medium supplemented with 10 mM beta-glycerophosphate [Sigma, St. Louis, MO], 50 μg/mL ascorbic acid [Sigma] and 10−8 M dexamethasone [Sigma]) for 7 days (for ALP activity assay) or 14 days (for alizarin red staining).36, 37 Cells incubated with different doses of LL37 were harvested at the above time points, the lysis supernatant were added to the assay buffer (100 mM glycine, 1 mM MgCl2, pH 10.5, and p-nitrophenyl phosphate solution [50 mM in 0.1 M glycine buffer]) at 37°C for 5–15 min, and the reaction was stopped by NaOH solution. OD value was measured at 405 nm and normalized to DNA content. Alizarin red staining was performed with fresh 40 mM alizarin red S solution (pH 4.4) at room temperature. Quantification was made by destaining with 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH 7.0).
TRAP Staining
In order to study the effect of the LL37 on LPS-induced osteoclast differentiation, fresh bone marrow cells were cultured with α-MEM containing 10% FBS, M-CSF (100 ng/mL), and 10 ng/mL RANKL for 36 hr at a cell density of 1 × 105 cells/well in 24-well plates. Then, the medium was changed to α-MEM containing 10% FBS, M-CSF (100 ng/mL), LPS (100 ng/mL), and LL37 for 5 days, followed by TRAP staining using a TRAP staining kit (Sigma).
Cell Migration Activity
Cell migration was measured by a transwell assay following manufacturer instruction and as described.38 Briefly, 1 × 105 cells were seeded onto the inside membrane (8-μm pore size) of inserts in the presence of LL37 (0, 1, 5, and 10 μg/mL) in the basolateral plates. Cells were incubated for 12 hr at 37°C, and the cell culture inserts were then removed from the wells and the upper part of each membrane were wiped with a cotton-tipped swab to remove non-migrated cells. Then, two separated experiments and methods were used to analyze the migrated cells. In one method, the migrated cells in the lower chamber were fixed, permeabilized, and stained with Giemsa solution (Gibco). Then, the purple cells in the lower chamber per well will be counted. In the second method, the migrated cells in the lower chamber were digested, centrifuged, and re-suspended in 100 μL of PBS, and then the cell number from each well was counted by a cell counter.
Antibacterial Test
For antimicrobial susceptibility methods, the bacterial suspensions were prepared by suspending 3–5 well-isolated colonies from E. coli agar plates into 3 mL of cation-adjusted Mueller Hinton broth (CAMHB; BD Diagnostic Systems, adjusted to pH 5.9) and the turbidity was adjusted to be equivalent to a 0.5 McFarland standard. For disk diffusion and broth microdilution, 100 μL of the 0.5 McFarland suspension was further diluted into 10 mL of CAMHB, which was used as the final inoculum.39 For agar dilution, no further dilution was applied.
For the disk diffusion method, the bacterial suspension prepared above was inoculated onto the entire surface of a Mueller-Hinton agar (MHA) plate (pH 5.9) using a sterile cotton-tipped swab to form an even lawn. Six sterile paper disks (6 mm in diameter; BD 25 Diagnostic Systems) impregnated, respectively, with 20 μL of LL37 solution (0.25, 0.5, 1, and 2 μg/μL), and penicillin-streptomycin solution (positive control) and PBS (negative control) were placed on the surface of each MHA plate using a sterile pair of forceps. The plates were incubated aerobically at 37°C for 24 hr, and the diameter of inhibition zone was then measured with a ruler or caliper.
LPS-Induced Calvaria Osteolytic Model
The in vivo experimental protocols were reviewed and approved by the University at Buffalo Animal Care and Use Committee. LPS (Sigma, 02:B6, L8274, 25 mg/kg body weight) or PBS (sham group) were injected subcutaneously into the tissue pocket surrounding the calvaria and near the midline of the skull between the ears and eyes in 8-week C57BL mice.40 5 days after injection, 12 mice were harvested for analyzing bone lysis. The other 36 mice were injected with 10 μg of LL37 (per mouse) and then followed by injection of 1 × 106 unmodified MSCs or BMP2-modified MSCs. 1 day later, 10 μg of LL37 (per mouse) were injected again. After each treatment, the mice were treated with carprofen for 2 days to minimize pain or discomfort according to the protocol. 3 weeks after treatment, animals were euthanized using CO2 and the calvarial bones were harvested and fixed with 10% neutral buffered formalin (NBF). Those 36 mice were divided into 6 groups randomly: group 1, LPS (no treatment); group 2, LPS+LL37; group 3, LPS+mMSC; group 4, LPS+mMSC+LL37; group 5, LPS+ mMSC/B2; and group 6, LPS+ mMSC/B2 + LL37 (Figure 3A). A group with 4 animals receiving PBS injection only (no defect) was used as a control group.
Analysis of In Vivo Bone Formation
Fixed calvarial bones were examined using a Scanco Viva-CT 35 instrument at a resolution of 10 μm. The CT settings were used as follows: pixel matrix, 1,024 × 1,024; slice thickness, 10 μm. Scans were performed at 55 keV for calvarial bone. After scanning, the micro-CT images were conducted by density thresholds from 330 to 1,000 mg/cm3 HA and a 3D histomorphometric analysis was analyzed on ∼100 slides that covered the region of interest (ROI), as shown in the red box in Figures 4B and S1 using 3D visualization and analysis software (MicroView, Version 2.1.2, GE Healthcare Biosciences, London, ON, Canada). Newly formed bone volume (NFBV) = bone volume (BV) of ROI at 3 weeks in each group − BV of ROI in the LPS-injected group at 5 days. The percentage of NFBV/TV, or bone volume/total volume, was used for comparison in this study.
For histological analysis, half of each calvarial bone from each group were decalcified and cut into 5-μm sections. The sections were then stained with H&E. Bone thickness and bone surface area was quantified using NIH ImageJ software. Briefly, the scale was corrected with a scale bar by “set scale.” Then, a straight line was drawn to cross the full thickness of calvaria bone. The length of this line was measured, which equals the bone thickness. The thickness of each group was subtracted by the thickness of the PBS group and expressed as relative thickness. For the bone surface area, the same procedure was performed except using “freehand selection” instead of “straight line.” The results were expressed as relative bone surface.
Statistical Analysis
Statistical analysis was performed using SPSS-17.0 software. Where indicated, experimental data were reported as mean ± SD of triplicate independent samples. Data were analyzed using one-way ANOVA, and Tukey’s honest SD (HSD) test was applied as a post hoc test if statistical significance was to be determined. A p value of ≤ 0.05 was considered statistically significant.
Author Contributions
S.Y., Z.L., and X.Y. conceived, designed, and directed the overall project; M.L., Y.Z., G.F., and C.N.I designed the selected experiments; Z.L., X.Y., M.L., G.F., S.Y., Y.Z., C.N.I., and S.Y. performed experiments and interpreted results; S.Y., Z.L., X.Y., Y.Z., M.L., and G.F. wrote the manuscript.
Conflicts of Interest
The authors declare that they have no competing financial interests.
Acknowledgments
We thank Drs. Martin Heyworth and Vishwa Deepak for their critical reading and editing of the manuscript. Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research and the National Institute of Aging, part of the NIH, under Award Numbers DE023105 and AG048388 to S.Y. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2017.09.011.
Supplemental Information
References
- 1.Hardy R., Cooper M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009;201:309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 2.Souza P.P., Lerner U.H. The role of cytokines in inflammatory bone loss. Immunol. Invest. 2013;42:555–622. doi: 10.3109/08820139.2013.822766. [DOI] [PubMed] [Google Scholar]
- 3.Redlich K., Smolen J.S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 4.Trombone A.P., Ferreira S.B., Jr., Raimundo F.M., de Moura K.C., Avila-Campos M.J., Silva J.S., Campanelli A.P., De Franco M., Garlet G.P. Experimental periodontitis in mice selected for maximal or minimal inflammatory reactions: increased inflammatory immune responsiveness drives increased alveolar bone loss without enhancing the control of periodontal infection. J. Periodontal Res. 2009;44:443–451. doi: 10.1111/j.1600-0765.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 5.Balli U., Cetinkaya B.O., Keles G.C., Keles Z.P., Guler S., Sogut M.U., Erisgin Z. Assessment of MMP-1, MMP-8 and TIMP-2 in experimental periodontitis treated with kaempferol. J. Periodontal Implant Sci. 2016;46:84–95. doi: 10.5051/jpis.2016.46.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibarra J.M., Jimenez F., Martinez H.G., Clark K., Ahuja S.S. MMP-activated fluorescence imaging detects early joint inflammation in collagen-antibody-induced arthritis in CC-chemokine receptor-2-null mice, in-vivo. Int. J. Inflamm. 2011;2011:691587. doi: 10.4061/2011/691587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivelä-Rajamäki M., Maisi P., Srinivas R., Tervahartiala T., Teronen O., Husa V., Salo T., Sorsa T. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J. Periodontal Res. 2003;38:583–590. doi: 10.1034/j.1600-0765.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers U.R., Kevorkian L., Surridge A.K., Waters J.G., Swingler T.E., Culley K., Illman S., Lohi J., Parker A.E., Clark I.M. Expression and function of matrix metalloproteinase (MMP)-28. Matrix Biol. 2009;28:263–272. doi: 10.1016/j.matbio.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton P.G., Bitar M., Gellynck K., Parkar M., Brown R.A., Young A.M., Knowles J.C., Nazhat S.N. Dense collagen matrix accelerates osteogenic differentiation and rescues the apoptotic response to MMP inhibition. Bone. 2008;43:377–385. doi: 10.1016/j.bone.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Xiao T.S. Innate immunity and inflammation. Cell. Mol. Immunol. 2017;14:1–3. doi: 10.1038/cmi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurabi A., Pak K., Ryan A.F., Wasserman S.I. Innate immunity: orchestrating inflammation and resolution of otitis media. Curr. Allergy Asthma Rep. 2016;16:6. doi: 10.1007/s11882-015-0585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Seny D., Cobraiville G., Leprince P., Fillet M., Collin C., Mathieu M., Hauzeur J.P., Gangji V., Malaise M.G. Biomarkers of inflammation and innate immunity in atrophic nonunion fracture. J. Transl. Med. 2016;14:258. doi: 10.1186/s12967-016-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2015). Innate immunity, inflammation & microbiology. J. Invest. Dermatol. 135 (Suppl 1), S87–S98. [DOI] [PubMed]
- 14.Lande R., Botti E., Jandus C., Dojcinovic D., Fanelli G., Conrad C., Chamilos G., Feldmeyer L., Marinari B., Chon S. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014;5:5621. doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 15.Elssner A., Duncan M., Gavrilin M., Wewers M.D. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson A., Sigel S., Ljunggren L. The antimicrobial peptide LL37 and its truncated derivatives potentiates proinflammatory cytokine induction by lipoteichoic acid in whole blood. Scand. J. Clin. Lab. Invest. 2010;70:512–518. doi: 10.3109/00365513.2010.521255. [DOI] [PubMed] [Google Scholar]
- 17.Ramos R., Silva J.P., Rodrigues A.C., Costa R., Guardão L., Schmitt F., Soares R., Vilanova M., Domingues L., Gama M. Wound healing activity of the human antimicrobial peptide LL37. Peptides. 2011;32:1469–1476. doi: 10.1016/j.peptides.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Thennarasu S., Tan A., Penumatchu R., Shelburne C.E., Heyl D.L., Ramamoorthy A. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys. J. 2010;98:248–257. doi: 10.1016/j.bpj.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasnodembskaya A., Song Y., Fang X., Gupta N., Serikov V., Lee J.W., Matthay M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe Y., Harada N., Sato K., Abe S., Yamanaka K., Matushita T. Stem cell therapy: is there a future for reconstruction of large bone defects? Injury. 2016;47(Suppl 1):S47–S51. doi: 10.1016/S0020-1383(16)30012-2. [DOI] [PubMed] [Google Scholar]
- 21.Kraus K.H., Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet. Surg. 2006;35:232–242. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 22.Ohata Y., Ozono K. [Bone and stem cells. The mechanism of osteogenic differentiation from mesenchymal stem cell] Clin. Calcium. 2014;24:501–508. [PubMed] [Google Scholar]
- 23.He X., Dziak R., Yuan X., Mao K., Genco R., Swihart M., Sarkar D., Li C., Wang C., Lu L. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One. 2013;8:e60473. doi: 10.1371/journal.pone.0060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X., Dziak R., Mao K., Genco R., Swihart M., Li C., Yang S. Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2 gene-modified mesenchymal stem cells for bone regeneration. Tissue Eng. Part A. 2013;19:508–518. doi: 10.1089/ten.tea.2012.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuda H., Wada T., Ito Y., Uchida H., Dehari H., Nakamura K., Sasaki K., Kobune M., Yamashita T., Hamada H. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector. Mol. Ther. 2003;7:354–365. doi: 10.1016/s1525-0016(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 26.Kittaka M., Shiba H., Kajiya M., Fujita T., Iwata T., Rathvisal K., Ouhara K., Takeda K., Fujita T., Komatsuzawa H. The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect. Peptides. 2013;46:136–142. doi: 10.1016/j.peptides.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Kittaka M., Shiba H., Kajiya M., Ouhara K., Takeda K., Kanbara K., Fujita T., Kawaguchi H., Komatsuzawa H., Kurihara H. Antimicrobial peptide LL37 promotes vascular endothelial growth factor-A expression in human periodontal ligament cells. J. Periodontal Res. 2013;48:228–234. doi: 10.1111/j.1600-0765.2012.01524.x. [DOI] [PubMed] [Google Scholar]
- 28.Ishida W., Harada Y., Fukuda K., Fukushima A. Inhibition by the antimicrobial peptide LL37 of lipopolysaccharide-induced innate immune responses in human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2016;57:30–39. [PubMed] [Google Scholar]
- 29.Schumann R.R. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: a short review. Res. Immunol. 1992;143:11–15. doi: 10.1016/0923-2494(92)80074-u. [DOI] [PubMed] [Google Scholar]
- 30.Larsen J.H. [The pathogenic significance of bacterial lipopolysaccharides: LPS syndrome. A review and hypothesis] Ugeskr. Laeger. 1976;138:538–542. [PubMed] [Google Scholar]
- 31.Nakamura T., Hara Y., Tagawa M., Tamura M., Yuge T., Fukuda H., Nigi H. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J. Bone Miner. Res. 1998;13:942–949. doi: 10.1359/jbmr.1998.13.6.942. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn L.T., Liu Y., Boyd N.L., Dennis J.E., Jiang X., Xin X., Charles L.F., Wang L., Aguila H.L., Rowe D.W. Developmental-like bone regeneration by human embryonic stem cell-derived mesenchymal cells. Tissue Eng. Part A. 2014;20:365–377. doi: 10.1089/ten.tea.2013.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Liao W., Zhao Q., Liu M., Xia W., Yang Y., Shao N. Angiogenesis and bone regeneration by allogeneic mesenchymal stem cell intravenous transplantation in rabbit model of avascular necrotic femoral head. J. Surg. Res. 2013;183:193–203. doi: 10.1016/j.jss.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Zomorodian E., Baghaban Eslaminejad M. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int. 2012;2012:980353. doi: 10.1155/2012/980353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajiya M., Shiba H., Komatsuzawa H., Ouhara K., Fujita T., Takeda K., Uchida Y., Mizuno N., Kawaguchi H., Kurihara H. The antimicrobial peptide LL37 induces the migration of human pulp cells: a possible adjunct for regenerative endodontics. J. Endod. 2010;36:1009–1013. doi: 10.1016/j.joen.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 36.He X., Liu Y., Yuan X., Lu L. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS ONE. 2014;9:e104061. doi: 10.1371/journal.pone.0104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan X., Cao J., He X., Serra R., Qu J., Cao X., Yang S. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat. Commun. 2016;7:11024. doi: 10.1038/ncomms11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y., Shim S.K., Kim H.A., Seon M., Yang E., Cho D., Bang S.I. CXC chemokine receptor 4 is essential for Lipo-PGE1-enhanced migration of human dermal fibroblasts. Exp. Dermatol. 2012;21:75–77. doi: 10.1111/j.1600-0625.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 39.Fiebelkorn K.R., Crawford S.A., McElmeel M.L., Jorgensen J.H. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J. Clin. Microbiol. 2003;41:4740–4744. doi: 10.1128/JCM.41.10.4740-4744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan X., Cao J., Liu T., Li Y.-P., Scannapieco F., He X., Oursler M.J., Zhang X., Vacher J., Li C. Regulators of G protein signaling 12 promotes osteoclastogenesis in bone remodeling and pathological bone loss. Cell Death Differ. 2015;22:2046–2057. doi: 10.1038/cdd.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.