Abstract
Cardiovascular disease (CVD) constitutes one of the leading causes of mortality worldwide, therefore representing a major public health concern. Despite recent advances in the treatment of patients with acute myocardial infarction (AMI), such as bypass surgery or percutaneous coronary intervention, pathological cardiac remodeling often predisposes survivors to fatal heart failure. In this context, the proven efficacy of stem cell-regenerative therapies constitutes a promising therapeutic perspective with is nevertheless slow down by safety and ethical concerns. Recent studies have underscored the capacity of stem cell-derived extracellular vesicles (EV) to recapitulate the regenerative properties of their parental cells therefore offering a therapeutic alternative to cell therapy in cardiovascular regenerative medicine. In this article, we review the functional relevance of using stem cell-derived EV as therapeutically agents and detail the identified molecular pathways that they used to exert their effects. We also discuss the advantages of using such an acellular regenerative therapy, in regard with parental stem cells, and address the limitations, which would need to be resolved, before their clinical translation.
Keywords: Extracellular vesicles (EV), cardiovascular disease (CVD), stem cell
Introduction
Cardiovascular disease (CVD) refers to pathologies that affect heart and blood vessels. CVD is the leading cause of morbidity and mortality worldwide, contributing to approximately one third of worldwide global deaths (1).
Events that contribute to CVD are multi-faceted, including both genetic and environmental factors among which obesity and type II diabetes represent major risk factors, moreover favored by the aging of the population. Recent advances in the treatment of patients with acute myocardial infarction (AMI), such bypass surgery or percutaneous coronary intervention, have greatly improved their survival. Nonetheless, the scar tissue and the associated irreversible loss of cardiomyocytes and subsequent tissue remodeling altogether affect the contractibility and the relaxation of ventricles. As a consequence, these functional alterations often predispose AMI survivors to ventricular arrhythmias and/or heart failure. Whereas strategies addressing direct cardiac tissue repair and regeneration are still challenging, the majority of treatment options aim to limit scar formation and adverse remodeling, while improving myocardial function. As a consequence, heart transplantation remains currently the unique option for fatal heart failure.
The demonstration that the injection of bone marrow (BM)-derived hematopoietic stem cells in the contracting wall bordering the infarcted zone in mice can repair myocardial damage and improve heart function has greatly stimulated expectations of stem cell-based cardiovascular therapies (2). This report has aroused enthusiasm for BM cell transplantation as a potential approach for repairing damaged hearts. Preclinical (3-5) and clinical studies (6,7) have nonetheless confirmed the potential of mesenchymal stem cells (MSC) to be used as treatment in AMI patients with efficacy and safety. Besides the use of total BM-derived stem cells, selected BM-subpopulations including endothelial progenitors, or stem cells from others sources such as adipose tissue, myocardium and skeletal muscle have also been evaluated for cell-based regenerative cardiovascular therapies (8).
Despite the demonstrated capacity of MSC to differentiate in multiple cardiac cell types, a number of studies nonetheless questioned the rational of MSC-associated beneficial observed effects due to the very poor engraftment and differentiation of injected cells in the infarct zone (9,10). In addition, beneficial effects of transplanted stem cells continue to be observed over several weeks, whereas 90% of cells die in the days following their injection into the myocardium (11). Such discrepancies have led to propose that MSC would rather exert their effects through a secretion, and not a direct differentiation mechanism (12). Such a hypothesis was further validated by the fact that MSC-conditioned culture medium alone recapitulated the efficacy of MSC in cardio protection (13,14).
Extracellular vesicles (EV), physiologically secreted by almost all cell types and retrieved in all body fluids (blood, lymph, milk, urine, etc.), appeared as good candidates able to recapitulate MSC beneficial effects. EV encompasses membrane vesicles, secreted in the extracellular space, which are surrounded by a lipid bilayer and enclosed cytosolic and genetic materials. Considered originally as cellular debris, their role as critical regulators of intercellular communication is now recognized and accumulating evidences also suggest their participation in pathophysiological situations. Of note, circulating levels of EV derived from blood vessel cells are increased in CVD, including AMI, leading to regard them as prognostic or diagnostic biomarkers [for review (15)]. Besides, their ability to carry and transfer biological information at the level of the organism highlight their potential as biological vectors (16).
Multiple evidences have underscored the capacity of stem cell-derived EV to recapitulate the regenerative properties of their parental cells therefore offering a therapeutic alternative to cell therapy in cardiovascular regenerative medicine.
In this article, we review the functional relevance of using stem cell-derived EV as therapeutically agents and detailed the identified molecular pathways that they used to exert their effects. We also discuss the advantages of using such an acellular regenerative therapy, in regard with parental stem cells, and address the limitations, which would need to be resolved, before their clinical translation.
Therapeutic potential of stem cell-derived EV in CVDs
Stem cell-based therapies have recently focused considerable attention due to the high plasticity of these cells to give rise to multiple lineages together with their immunomodulatory properties and low immunogenicity. Recent evidences have moreover highlighted the capacity of stem cell-derived EV to recapitulate cardiac beneficial properties of stem cells thereby highlighting stem cell-EV as promising therapeutic tools in regenerative cardiovascular medicine.
Stem cell-derived EV: functional extensions of parental stem cells
Three types of EV can be generally distinguished (17). Apoptotic bodies are generated during the apoptotic process, when the cell’s cytoskeleton breaks up. This causes the membrane to bulge outward, forming large membrane-bound EV (generally >1 µm), which may have engulfed portion of cytoplasm. Besides, microvesicles are shed from the plasma membrane as a consequence of cytoskeleton reorganization combined with the negatively charged phosphatidylserine exposure in the outer membrane (18,19): microvesicles size generally distributes between 50/100 nm to 1 µm. Exosomes are endosome-derived vesicles formed in multi-vesicular bodies and released after the fusion of this compartment with the plasma membrane (17) and constitute the smallest type of EV described, ranging between 30 and 100 nm. However, the terminology “exosomes” has been widely used for small EV, regardless of their endo-lysosomal cellular origin, therefore designing a mix of EV from different intracellular origins and with distinct functional properties. Moreover, specific subsets of proteins, longtime considered to be exosomal markers, rather appear to be EV-enriched proteins since they may also be retrieved in other EV-subtypes (20,21). Considering the biological complexity of EV, which is further influenced by the cellular context, there is so far no standardized analysis procedure to allow a clear separation of these different EV subtypes despite recent positions papers which have now established requirements (22) and methodological guidelines (23) to validate the undeniable presence of EV. Taking these considerations into account, we therefore chose to refer to the general terminology EV in the rest of the review, whatever the original term employed to design these vesicles in the original publications.
Stem cell-derived EV may be isolated in basal conditions, as demonstrated from exponentially growing embryonic stem cells (ESC), suggesting that the mechanism of EV release is inherent (24). However, secretion of MSC-derived EV is enhanced by external stimuli such as hypoxia (25) or inflammatory conditioning of parental cells (26) indicating that the surrounding environment of MSC would impact on EV release. Apart of such an effect of environmental stimuli on EV quantities, mass spectrometry analyses reveal also different protein content which translated into different functional properties therefore illustrating the complexity of the MSC paracrine regulation (25,26).
Several studies have revealed the ability of MSC-derived EV to mimic MSC features, including self-renewal, differentiation and maturation suggesting that they would represent functional extension of stem cells [for review (27)]. EV from MSC indeed carry numerous transcription factors among which are found MSC markers such as CD105, prominin-1/CD133 and KIT (24) but also many molecules characteristic of stem cell signatures (27). These EV might even support self-renewal and expansion of adult stem cells as demonstrated by the capacity of ESC-derived EV to reprogram hematopoietic progenitors through the delivery of specific proteins and mRNA (24). Hence, interactions of stem cell derived-EV with target cells appear to directly impact their phenotypic and functional characteristics. This could be achieved through a variety of mechanisms including transfer of molecules (membrane surface receptors, active lipids, membranous proteins…), ligand/receptors interaction, EV fusion with targeted plasma membrane cell or epigenetic reprogramming through horizontal genetic or transcription factors horizontal transfer (28). Overall, SC-derived EV appear to be critical components of intercellular communication via the transfer of functionally relevant biomolecules and, as such, have been harnessed for their therapeutic potential, in a similar fashion to their parent cells, in cardiovascular therapies (Table 1).
Table 1. Therapeutic benefits of stem cell-derived EV administration in cardiovascular disease models.
| EV source | Isolation method | Assay model | Therapy | Findings | Mechanisms | References |
|---|---|---|---|---|---|---|
| Native stem cells | ||||||
| Human BM-MSC | 100,000 ×g | Rat acute MI | 80 µg (in 20 µL) of MSC-EV derived from 2×106 BM-MSC by IM 30 min after LAD ligation | Reduce infarct size | Promote angiogenesis | Bian et al., 2014 (29) |
| Preserve systolic and diastolic performance | ||||||
| Improve blood flow recovery | ||||||
| Human UC-MSC | 100-kDa MW cut off ultrafiltration followed by 100,000 ×g on sucrose gradient | Rat acute MI | 400 µg (in 200 µL) of MSC-EV by IV right after LAD ligation | Preserve systolic function | Protect myocardial cells from apoptosis | Zhao et al., 2015 (30) |
| Reduce cardiac fibrosis | Promote angiogenesis | |||||
| Human ESC-MSC | Sequential 10 to 100 kDa filtration and sucrose gradient | Pig I/R | 3 or 0.4 µg MSC-EV (in 200 µL) by IV 5 min before reperfusion | Reduce infarct size | Undefined | Lai et al., 2010 (31) |
| Human ESC-MSC | 10-kDa MW cutoff ultrafiltration and 220 nm filtration | Pig I/R | 2 mg MSC-EV (in 1 mL) by IV 5 min before reperfusion, and additional IC bolus of 8 mg MSC-EV (in 4 mL) immediately following reperfusion | Reduce infarct size | Decrease oxidative stress and apoptosis | Timmers et al., 2007 (13) |
| Preserve systolic and diastolic performance | ||||||
| Human ESC-MSC | 10-kDa MW cutoff ultrafiltration and 220 nm filtration | Pig acute MI | 1 mg MSC-EV (in 2 mL) 4 h after LAD ligation by IV and during 7 days (twice daily) | Reduce infarct size | Increase capillaries density | Timmers et al., 2011 (32) |
| Preserve systolic and diastolic performance | ||||||
| Human ESC-MSC | 10-kDa MW cutoff ultrafiltration and 220 nm filtration | Pig I/R | 0.4 μg/mL MSC-EV by IV 5 min before reperfusion | Reduce infarct size | Decrease oxidative stress | Arslan et al., 2013 (33) |
| Preserve systolic function | Activation of pro-survival signaling | |||||
| Reduce local and systemic inflammation | Restore bioenergetics | |||||
| Human CPC | 200 nm filtration and ExoQuickTM | Rat acute MI | 30 or 300 µg CPC-EV by IM (in 150 µL) 60 min after LAD ligation | Improve cardiac function | Reduced cardiomyocyte apoptosis | Barile et al., 2014 (34) |
| Enhance angiogenesis | ||||||
| Human CPC | ExoQuickTM | Mouse acute and chronic MI | 2.8×109 CPC-EV at two peri-infarct sites (40 µL per site) after LAD ligation or three weeks later (for chronic model) | Improve cardiac function | Inhibition of cardiomyocyte apoptosis | Ibrahim et al., 2014 (35) |
| Enhance angiogenesis | ||||||
| Possibly related to miR-146a | ||||||
| Human CPC | 220 nm filtration and PEG precipitation | Mouse I/R | 25 μL exosomes from 5×105 CPC by IM immediately after LAD ligation | Inhibit cardiomyocyte apoptosis | Possibly related to miR-451 | Chen et al., 2013 (36) |
| Human CPC | Ultrafiltration and PEG precipitation | Pig I/R and MI | 15 mg of EV-CPC [≈33×1011 particles] by IC or IM 30 min after I/R, or 7.5 mg protein by IM [≈16.5×1011 particles] 4 weeks post-MI | Reduce infarct size | Undefined | Gallet et al., 2017 (37) |
| Decrease LV collagen content | ||||||
| Cardiomyocyte hypertrophy | ||||||
| Increase vessel density | ||||||
| Human ESC-CPC | 100,000 ×g | Mouse acute MI | 3x10 µL of EV derived from 5×105 ESC-CPC by IM 3 weeks after LAD ligation | Improve cardiac function | Undefined | Kervadec et al., 2016 (38) |
| Human EPC | 100,000 ×g | Mouse hindlimb ischemia | 100 µg of EPC-EV by IV right after left hindlimb of mouse | Enhance limb perfusion and reduce injury | Neovascularization | Ranghino et al., 2012 (39) |
| Possibly related to miR-126 | ||||||
| Human UC-MSC | 200 nm filtration and 100,000 ×g | Rat hindlimb ischemia | 10, 50 or 100 µg of UC-EV (in 200 μL) by IMusc 2 h after ligation | Improve blood flow recovery | Favor angiogenesis | Zhang et al., 2012 (40) |
| Genetically modified stem cells | ||||||
| Rat BM-MSC overexpressing GATA4 | 10-kDa MW cut off ultrafiltration and 220 nm filtration and ExoQuickTM | Rat acute MI | 50 µL of EV by IM derived from 4×106 MSCGATA-4 right after LAD ligation | Reduce infarct size | Transfer of antiapoptotic miRNA | Yu et al., 2015 (41) |
| Human UC-MSC overexpressing Akt | 100-kDa MW cut off ultrafiltration followed by 100,000 ×g on sucrose gradient | Rat acute MI | 400 µg of EV derived from UC-MSCAkt by IV right after LAD ligation | Improve cardiac function | Promote angiogenesis | Mangi et al., 2003 (42) |
| Activation of PDGF-D | ||||||
BM, bone marrow; ESC, embryonic stem cell; MSC, mesenchymal stem cell; CPC, cardiac progenitor cell; UC, Umbilical cord; CM, conditioned media; MI, myocardial infarction; I/R, ischemia/reperfusion; LV, left ventricule; LAD, left anterior descending artery; IV, Intravenous Injection; IC, Intracoronary Injection; IM, intramyocardial injection; IMusc, intramuscular injection; PDGF-D, platelet-derived growth factor D.
Therapeutic effects of stem cell-EV in CVD
Heart diseases
Since beneficial effects of transplanted MSC were often out of proportion to the surviving engrafted donor cells, the “paracrine hypothesis” has emerged according to which soluble factors derived from engrafted donor stem cells would be responsible for beneficial outcomes of the heart function. This hypothesis was further confirmed on animal models since treatment with conditioned medium from stem cells reduces infarct size in rat (43) and in porcine models (13,32) of myocardial ischemia-reperfusion (I/R) injury. Moreover, these cytoprotective effects were enhanced when conditioned medium arose from hypoxic MSC (43). Preservation of cardiac function was attributed to a vesicular fraction of the culture medium isolated by ultracentrifugation, enriched in tetraspanins, proteins markers of the exosomal fraction (13,31). Effectively, MSC-purified EV significantly reduced infarct size in animal models of I/R injuries (29-31,33). Cardiac repair following EV injection was illustrated by preserved systolic and diastolic cardiac performance, increased myocardial perfusion and attenuated left ventricular remodeling (13,29,32,33).
Because the use of EV directly produced from cells present in the heart itself would represent exciting perspectives, recent research has also focused on cardiac progenitor cells (CPC)-derived EV. CPC in semi-suspension culture form spherical clusters (cardiospheres) that are known to deliver paracrine signals to neighboring cells. Electron microscopic imaging further confirmed the ability of CPC in mouse myocardium and human cardiospheres to secrete EV (44). Of interest, EV isolated from cardiospheres also displayed pro-angiogenic and cardioprotective properties as demonstrated on animal models of cardiomyopathy (35) or myocardial I/R injury (34,36,37). Moreover, comparison between post-infarction administration of EV released by human ESC-derived cardiovascular progenitors and administered donor cells demonstrate that EV recapitulate the beneficial effects of the parental cells in the treatment of chronic heart failure (38).
Vascular diseases
Besides heart diseases, peripheral arterial disease, caused by atherosclerotic occlusion of the leg arteries, is an important manifestation of systemic atherosclerosis. The lack of proper blood perfusion to the extremities can potentially progress to severe alteration as limb ischemia.
Paracrine mediators released from endothelial progenitor cells (EPC) have been implicated in neoangiogenesis following ischemia. For instance, intramuscular injection of conditioned medium from EPC has been shown to be as effective as EPC transplantation for promoting tissue revascularization and functional recovery (45). Furthermore, intravenous administration of EV fraction isolated from EPC in a murine model of hindlimb ischemia significantly improved neovascularization and favors regeneration (39). Similarly, intramuscular injections of EV collected from hypoxic human umbilical cord MSC-derived EV in a rat hindlimb ischemia model improve significantly the blood flow recovery, in a similar proportion than parental cells (40). Both of these studies pinpoint EV as major mediators of the effectiveness of MSC therapy by promoting angiogenesis.
Of interest, stem cell-derived EV act at different levels to preserve endothelium integrity and to prevent from the development of vascular diseases. Thus, stem cell derived-EV are able to modulate the key processes of angiogenesis including proliferation, migration, tube formation of endothelial cells and promote angiogenesis related gene expression and protein expression (46-49). Horizontal transfer of different miRNA (39,48,50,51), as well as EV-encapsulated pro-angiogenic factors (46) have both been involved in the regulation of these EV-related angiogenic properties.
Molecular mechanisms underlying the therapeutic potential of stem cell-EV in CVDs
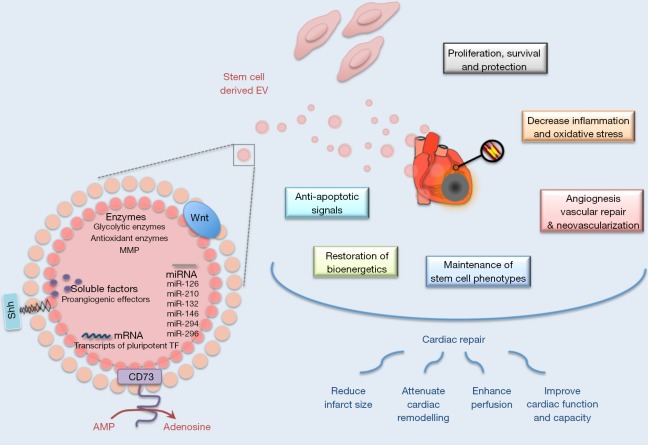
Whereas EV constitutes complex carriers of proteins, lipids and nucleic acids, some of their cargos have been specifically involved to elicit biological responses in recipient cells. Although EV content vary according to cell sources and environmental stimuli, some mechanisms attributed to specific molecules have been shown to be conserved whatever the EV donor cells or the pathophysiological situation. Of interest, this therapeutic potential can be further enhanced by genetic modification of MSC in order to specifically load EV with such molecules and therefore to engineer bioactive cargos. As presented in Figure 1, the therapeutic potential of stem cell-derived EV in regenerative cardiovascular therapies would be related to a variety of molecular mechanisms.
Figure 1.
Schematic representation of molecular mechanisms underlying the therapeutic potential of stem cell-derived EV in regenerative cardiovascular therapies. EV, extracellular vesicles; Shh, sonic hedgehog; TF, tissue factor; CD, cluster of differentiation; AMP, adenosine monophosphate; MMP, matrix metalloproteinases.
MSC-EV contain angiogenic paracrine effectors
Whereas secreted factors support the proangiogenic potential of MSC, numerous angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), milk fat globule-EGF factor 8 (MFG-E8), angiopoietin like 1 (ANGPTL1) and thrombopoietin can also be retained in MSC-EV (52,53). Most notably, nuclear factor-kappaB (NFkB) signaling pathway proteins were specifically enriched in MSC-EV and identified as key mediators of angiogenesis induction in endothelial cells (53). Moreover, matrix metalloproteinases were identified in EV preparations isolated from different stem cell types (54,55) and shown to maintain their activity in target cells (55). Knowing the importance of these enzymes in matrix remodeling, metalloproteinases-associated EV could thereby modified invasion and migration properties of recipient cells.
Activation of anti-inflammatory and pro-survival pathways by stem cell-EV in the recipient cells
Suppression of inflammation by MSC-EV participates to improve cardiac function (33). For instance, a reduction of 45% of infarct size following MSC-EV administration in a murine model of I/R was associated with decreased local and systemic inflammation within 24 hours. In addition, MSC-EV were also found to increase adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (reduced form) (NADH) levels thereby restoring bioenergetics of the cells (33). Various ATP-generating enzymes (including glycolytic enzymes) associated to MSC-EV have been postulated to provide energy needed to prevent cell death and to support tissue repair [for review, (56)]. Furthermore, oxidative stress was reduced via peroxiredoxins and glutathione S-transferases contained in MSC-derived EV (33). Finally, MSC-EV also mediate cell survival and proliferation at the damaged sites through activation of extracellular signal-regulated kinase (ERK) and Akt signaling pathways (33,47). In agreement, EV derived from Akt-modified umbilical cord-derived MSC are more effective in AMI therapy through promoting angiogenesis than unmodified umbilical cord-MSC-EV (42). The importance of Akt pathway was further confirmed by the fact that PI3K/Akt inhibitor treatment abolished angiogenic effects of EV derived from MSC overexpressing C-X-C chemokine receptor 4 (CXCR4) in a model of AMI (57). Such activations may be consecutive to the enzymatic production of adenosine, a potent activator of both ERK and Akt (58), by EV-associated glycosylphosphatidylinositol (GPI)-anchored ecto-5’-nucleotidase CD73 (33). These pathways may be moreover activated through different molecular mechanisms as demonstrated by the elegant work of Deregibus et al. (50) who described that EPC-EV-mediated angiogenic effects were associated with the shuttling of mRNA in endothelial cells associated with the PI3K/Akt signaling pathway.
EV-associated miRNA mediated therapeutic effects
One of the most documented functional characteristics of EV is their ability to transfer nucleic acids between cells. Transriptomic approaches revealed that MSC-EV shuttle selected pattern of miRNA when compared to their parental cells (59,60). A selective packaging of nucleic acids into EV likely occurs and might be controlled by the recognition of sequence motifs present in miRNAs by sumoylated protein heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) (61). This compartmentalization may be regulated by environmental stimuli such as hypoxia, which can modulate immunomodulatory and regenerative properties of MSC-derived EV (43) but also enhanced expression of proangiogenic miRNAs in EPC-derived EV (62). Moreover, it appears that many of miRNA-associated MSC-derived EV are part of signaling network involved in multiorgan development, cell survival and differentiation, thus leading to consider EV-mediated genetic material exchange as active players in cell-fate determination decision (63).
From a therapeutic point of view, EV-mediated miRNA transfer is particularly attractive inasmuch some miRNA have been specifically associated with the pro-angiogenic, cardioprotective or even anti-apoptotic effects associated with improvement of cardiac or vascular function. Among those, miR-210, miR-132 and miR-146a-3p were the ones most highly enriched in CPC-derived EV (34). By regulating ephrin A3 and protein tyrosine phosphatase 1b (PTP1b), miR-210 was shown to inhibit apoptosis in cardiomyocytes (34). miR-132 down-regulated its target, RasGAP-p120, enhancing tube formation in endothelial cells (34). The contribution of the miR-146a-3p to the greater EV effects, by comparison to CPC, was specifically addressed through the use of miR-146a hairpin inhibitor or miR-146a mimic. Although such experiments demonstrated the ability of miR-146 to reproduce the cardiomyogenic and antiapoptotic effects in a chronic AMI model, it was not sufficient to confer the overall comprehensive therapeutic benefit (35). Such results suggest that others miRNA (or molecular agents) exert synonymous or perhaps synergistic effects with miR-146a.
Besides, miR-294, found specifically enriched in ESC-derived EV, constitutes also an interesting target. Indeed, the cardioprotective effect of ESC-derived EV after AMI is specifically tied to miR-294 delivery to CPC promoting increased survival, cell cycle progression, and proliferation (64).
Finally, RNA cargo from EPC-derived EV is also likely to play a role in biological or therapeutic responses such as angiogenesis (50) or protection against angiotensin-II-induced cardiac hypertrophy and apoptosis (65). Particularly, EV-associated miR-126 and miR-296 both participate in the improvement of neovascularization observed in a model of hindlimb ischemia in SCID mice (39). Of interest, miR-126 has been previously identified as a critical regulator of angiogenesis and vascular integrity (66,67) and shown to be also enriched in EV derived from endothelial cells (68,69). Treatments with these different types of miR-126-enriched EV all promote vascular regeneration. Indeed, systemic treatment of hypercholesterolemic apolipoprotein E null-mice with miR-126-enriched EV limit atherosclerosis and increased Sca1+ cell incorporation into aortic plaques (68). Besides, miR-126-associated EV promote in vivo reendothelialization (69). Of particular interest, miRNA profiling in patients with coronary artery diseases has revealed significantly reduced levels of miR-126 in comparison with healthy controls (70) or even total loss circulating miR-126 in patients with diabetes mellitus (71). Recent publication moreover revealed that obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived EV by impairing miR-126 content (72). Therefore, development of EV-based miR-126 therapeutic strategy might be promising in the treatment of CVD.
Stem cell-EV and developmental signaling pathways
Stem cell-derived EV might be considered as functional extensions of their parental cells. As such, molecular analyses revealed stem cell-EV were highly enriched in transcripts of pluripotent transcription factors such as Oct-4, Nanog and Rex-1 (24). EV also traffic effectors of the stem-like phenotype such as Wnt (73,74), beta-catenin (75) or sonic hedgehog (Shh) (76,77) that might contribute to mimic a spectrum of stem like phenotypes and involved in embryogenic processes.
Besides such a cell-fate determination role, these factors are also critical in EV-related regenerative processes like angiogenesis. For example, the activation of Wnt/eta-catenin is critical in the induction of angiogenesis by umbilical cord MSC-EV. Of interest, the knockdown of Wnt4 in EV derived from these MSC abrogated eta-catenin nuclear translocation in endothelial cells and also inhibited there in vivo proangiogenic effects (49).
The morphogen Shh is also known to be an important modulator of angiogenic processes (78). Our laboratory has demonstrated that Shh-enriched EV (EVShh+) from T lymphocytes stimulated angiogenic processes by inducing functional and mature blood vessel formation through modulation of, reciprocally, pro- and anti-angiogenic factors (79) and anti-apoptotic signals (80,81). In addition, these EVShh+ were able to enhance in vivo post-ischemic neovascularization (80) and exert cardioprotection against I/R injuries (82). Of note, injection CD34+ hematopoietic stem cells overexpressing Shh in the infarct border zone in mice after AMI preserves cardiac function, likely by transferring Shh-associated EV to recipient cells (83). Thus, Shh-loaded EV represent future promising therapeutic tools in CVD (84).
Another approach to enhance the cardioprotective potential of MSC-derived EV is by overloading on EV a transcription factor known to be a key regulator of numerous cardiac genes and cell surviving pathways, such as GATA-4. Yu et al. (41) used such a strategy by overexpressing GATA-4 in MSC. These authors demonstrated that administration of MSC-GATA-4-derived EV in a rat model of AMI restored cardiac function and reduced infarct size. Of note, these MSC-derived EV overexpressing GATA-4 mediated protection by transferring certain anti-apoptotic miRNAs contained within EV upon transplantation in the damaged tissue. Moreover, CPC-derived EV, able to ensure cardioprotection after AMI, was enriched with miR-451, by comparison to their parental cells, which is a GATA4-responsive miRNA (36). Therefore, the cardioprotective effect of CPC-derived EV may partially rely on miRNA transcripts enrichment controlled by the expression of the early developmental heart factor GATA-4.
Finally, specific types of heat shock proteins (HSPs), which exert chaperones functions in protein folding, can also be transferred by EV. Of interest, Hsp70 and Hsp20-associated EV have been shown to exert cardioprotective effects in animal models of cardiac dysfunction (85,86). Although HSP proteins have been retrieved in cardiomyocyte-derived EV, no studies reported so far their specific presence in stem cell-derived EV. Nonetheless, transplantation of cardiac stem cells (Sca1+), beforehand treated with a heat shock, exert beneficial outcomes in mouse ischemic hearts by EV transfer of heat shock factor 1 (HSF-1). Indeed, HSF-1 directs ischemic cardiomyocytes toward a prosurvival phenotype through upregulation of Hsp70 pathway (87). EV modulation of specific HSP pathways, displaying cytoprotective effects, may therefore have significant therapeutic value in translational stem cell-based therapy.
Towards clinical translation: expectations and limitations
The emergence of stem cell-based therapy has opened the door to non-pharmacological treatments of heart failure, especially for patients where therapeutic options prove to be inadequate or ineffective. To date, numerous clinical trials confirm the efficacy of different cell types including embryonic, induced pluripotent, mesenchymal and cardiac-derived stem cells (88-90). Nonetheless, the use of ESC raises political and ethical questions. Alternatively, undifferentiated induced pluripotent stem cells, which can be derived from somatic cells and demonstrate an embryonic-like state with a strong regenerative capacity towards cardiomyocytes, makes run the risk of teratoma formation. Cardiac stem cells might therefore be a privileged source due to the specific expression of cardiac markers and their efficient differentiation into cardiomyocytes and vascular endothelial cells. Nonetheless, the difficulty to isolate such cells may be a limited factor since ex vivo cell expansion would be needed before transplantation. In this regard, MSC present the advantage to be easily isolated from BM or adipose tissue and have demonstrated potent regenerative capacities and low immunogenicity. However, adult stem cells may present a restrictive plasticity and their therapeutic potential may be moreover impaired by cardiovascular risk actors as diabetes, hypertension or aging (91-93). Therefore, additional studies would be needed to control and support the efficacy of these cells on a long-term basis.
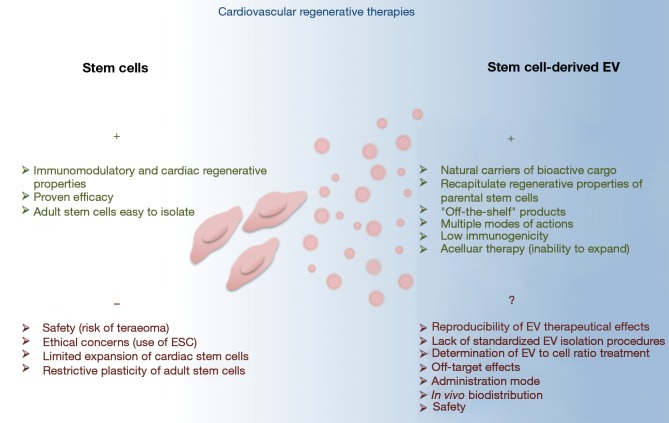
The fact that EV administration in numerous preclinical animal models recapitulates the benefits of stem cells transplantation has highlighted EV-based therapies as counterparts to address the safety concerns and practical limitations associated with the use of stem/progenitor cells (Figure 2). EV is naturally cell permanent, and their lipid bilayer coat protects their bioactive cargo from degradation as particles shuttle from cell to cell. Moreover, their membranous structures confer them a particular stability over time, making them real “off-the-shelf” products allowing careful maintenance of stability, integrity and biological activity during their manufacture, storage and subsequent administration. EV might exert their beneficial effects in the neighboring site of injection through multiple mechanisms including molecules transfer, ligands/receptors interaction, membrane fusion or even internalization into recipient cells (28). In addition, regenerative properties of stem cell-derived EV have been shown to lie on different molecular mechanisms, either involving molecules or nucleic acids, therefore reproducing the complex paracrine pattern of the parental cell donors. Finally, EV administration, with regard to stem cells transplantation, may provide specific advantages for patient safety including a lower propensity to trigger innate and adaptive immune responses, and inability to directly form tumors.
Figure 2.
Comparison of advantages and limitations between the use of stem cells and the use of stem cell-derived EV in cardiac regenerative therapies. EV, extracellular vesicles; ESC, embryonic stem cells.
Besides all these considerations, which make stem cell-derived EV promising therapeutic tools, a number of issues would still need to be addressed before their clinical use. A major question is the reproducibility of EV clinical lots. Different EV isolation protocols are currently used to isolate EV therefore leading to mixed or even different EV subpopulations (Table 1). Whereas low-speed centrifugation will pellet large vesicles (namely apoptotic bodies and/or microvesicles), the classically used 100,000 ×g ultracentrifugation will concentrate smaller vesicles (typically exosomes) which might also include microvesicles depending whether the supernatant was or not submitted to pre-centrifugation or filtration. Alternative isolation protocols using commercially available columns/kits or size-exclusion chromatography will also select different EV subtypes than those isolated by differential centrifugations. Recent proteomic studies separating these different EV subclasses have shown that they display different protein and lipid signatures, and thereby different functional properties (21,94). In addition, EV production and content are both influenced by the cell source and stimuli used for their production. As an example, EV derived from TNFalpha-treated cardiomyocytes unpack pro-inflammatory signals which may mediate unwanted biological responses (95). The pathophysiological environment of the patient might also influence its stem cell-derived EV features. Indeed, obesity (72), but also cardiovascular medication (96,97), have been shown to modify EV content and thus influence their biological properties. EV to cell ratio stoichiometry can also be determinant for EV biological responses as illustrated by the anti-angiogenic effects of high concentration of endothelial cell-derived EV through oxidative stress activation, whereas low concentrations of these EV preparations display pro-angiogenic properties (98). Finally, uncertainties also reside in the therapeutic mode used for EV administration. In vivo EV biodistribution is determined by cell source, route of administration and targeting (99). Dynamic imaging of in vivo biodistribution of EV following their intravenous injections in rodents revealed that they were predominantly accumulating, within a few hours, in liver, spleen, gastrointestinal tract and lungs (99,100). In addition, EV from different sources display different innate homing capabilities in vivo, which may be determined by the repertoire of surface receptors and extracellular matrix-binding proteins acquire. In this context, one can exclude that injected EV will also induce advert effects on undamaged tissues. Therefore, the biodistribution, as well as the long-term effects and safety of administered EV, would need to be explored and controlled.
Conclusions
Recent studies have compiled evidences that stem cells represent an abundant source of EV, able to act as important mediators in cell communication thereby influencing phenotype and function of recipient cells. More importantly, numerous experimental data and preclinical models have demonstrated the excellent potential of stem cell-derived EV to be used as therapeutic tools in CVD, and potentially to face the limitations inherent of stem cell regenerative therapies. Of interest, stem cell-derived EV mostly recapitulate parental cells efficacy in repair processes notably by combining anti-inflammatory and anti-apoptotic properties together with angiogenesis and regenerative properties. Therapeutic potential of EV is achieved through multiple molecular mechanisms including the transfer of factors or nucleic acids within the target cell, which will mediate EV-beneficial effects.
Translation of these preclinical evidences in humans will represent relevant advantages with regard to the safety and ethical questions, production of clinical lots and final costs, by comparison to stem cell-based approaches. EV moreover represents stable circulating carriers, which are able to overcome biological barriers and display intrinsic cell targeting properties. Nonetheless, EV represent a mix of biological complex vesicles, whose content vary according to cell source and pathophysiological environment, and whose production and isolation would need to be better controlled to ensure reproducibility of biological therapeutically effects. As natural delivery carriers for a wide set of recipient cells, EV-based strategies may also lead to produce potential off-target effects.
Waiting to resolve these limitations, an alternative strategy might reside on engineering biomimetic EV by packaging deliberately specific components into the vesicles and thereby orientating their tissue targeting and/or the awaiting biological responses (101,102). One has however to consider that beneficial effects of stem cell-derived EV result from a complex interplay of multiple factors. Thus, it may be counterproductive to deconstruct natural biological EV content.
Although EV have wined their stripes in the field of intercellular communication and attracting projectors on their numerous therapeutically properties, future investigations will definitely need to provide insights on how streamlining their isolation and production processes in order to constitute a real alternative to stem cells-based CVD regenerative therapies for a safe, efficient and standardized clinical use.
Acknowledgements
The authors were supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Angers University, Agence Nationale de la Recherche MilkChEST [n°ANR-12-BSV6-0013-04], GIS APIS-GENE, Société Francophone du Diabète (SFD). J Amosse is funded by a PhD from the French Ministry of Education and Research.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Townsend N, Wilson L, Bhatnagar P, et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232-45. 10.1093/eurheartj/ehw334 [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5. 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- 3.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93-8. 10.1161/hc0102.101442 [DOI] [PubMed] [Google Scholar]
- 4.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A 2005;102:11474-9. 10.1073/pnas.0504388102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 2002;73:1919-25; discussion 26. [DOI] [PubMed]
- 6.Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 2007;50:1761-7. 10.1016/j.jacc.2007.07.041 [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167:989-97. 10.1001/archinte.167.10.989 [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther 2016;7:82. 10.1186/s13287-016-0341-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 2006;27:1114-22. 10.1093/eurheartj/ehi818 [DOI] [PubMed] [Google Scholar]
- 10.den Haan MC, Grauss RW, Smits AM, et al. Cardiomyogenic differentiation-independent improvement of cardiac function by human cardiomyocyte progenitor cell injection in ischaemic mouse hearts. J Cell Mol Med 2012;16:1508-21. 10.1111/j.1582-4934.2011.01468.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol 2005;23:845-56. 10.1038/nbt1117 [DOI] [PubMed] [Google Scholar]
- 12.Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle 2007;6:2884-9. 10.4161/cc.6.23.5095 [DOI] [PubMed] [Google Scholar]
- 13.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2007;1:129-37. 10.1016/j.scr.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 14.Shabbir A, Zisa D, Suzuki G, et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol 2009;296:H1888-97. 10.1152/ajpheart.00186.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulanger CM, Loyer X, Rautou PE, et al. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017;14:259-72. 10.1038/nrcardio.2017.7 [DOI] [PubMed] [Google Scholar]
- 16.Martínez MC, Andriantsitohaina R. Extracellular vesicles in metabolic syndrome. Circ Res 2017;120:1674-86. 10.1161/CIRCRESAHA.117.309419 [DOI] [PubMed] [Google Scholar]
- 17.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 18.Morel O, Jesel L, Freyssinet JM, et al. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 2011;31:15-26. 10.1161/ATVBAHA.109.200956 [DOI] [PubMed] [Google Scholar]
- 19.Kunzelmann-Marche C, Freyssinet JM, Martinez MC. Regulation of phosphatidylserine transbilayer redistribution by store-operated Ca2+ entry: role of actin cytoskeleton. J Biol Chem 2001;276:5134-9. 10.1074/jbc.M007924200 [DOI] [PubMed] [Google Scholar]
- 20.Bobrie A, Colombo M, Krumeich S, et al. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 2012. 10.3402/jev.v1i0.18397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durcin M, Fleury A, Taillebois E, et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles 2017;6:1305677. 10.1080/20013078.2017.1305677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913. 10.3402/jev.v3.26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res 2017;120:1632-48. 10.1161/CIRCRESAHA.117.309417 [DOI] [PubMed] [Google Scholar]
- 24.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847-56. 10.1038/sj.leu.2404132 [DOI] [PubMed] [Google Scholar]
- 25.Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 2013;8:e68451. 10.1371/journal.pone.0068451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilpinen L, Impola U, Sankkila L, et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles 2013. 10.3402/jev.v2i0.21927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nawaz M, Fatima F, Vallabhaneni KC, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int 2016;2016:1073140. 10.1155/2016/1073140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tual-Chalot S, Leonetti D, Andriantsitohaina R, et al. Microvesicles: intercellular vectors of biological messages. Mol Interv 2011;11:88-94. 10.1124/mi.11.2.5 [DOI] [PubMed] [Google Scholar]
- 29.Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 2014;92:387-97. 10.1007/s00109-013-1110-5 [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Sun X, Cao W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int 2015;2015:761643. 10.1155/2015/761643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214-22. 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 32.Timmers L, Lim SK, Hoefer IE, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 2011;6:206-14. 10.1016/j.scr.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 33.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 2013;10:301-12. 10.1016/j.scr.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 34.Barile L, Lionetti V, Cervio E, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovascular research 2014;103:530-41. 10.1093/cvr/cvu167 [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014;2:606-19. 10.1016/j.stemcr.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Wang Y, Pan Y, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 2013;431:566-71. 10.1016/j.bbrc.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2017;38:201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kervadec A, Bellamy V, El Harane N, et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 2016;35:795-807. 10.1016/j.healun.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 39.Ranghino A, Cantaluppi V, Grange C, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol 2012;25:75-85. 10.1177/039463201202500110 [DOI] [PubMed] [Google Scholar]
- 40.Zhang HC, Liu XB, Huang S, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev 2012;21:3289-97. 10.1089/scd.2012.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 2015;182:349-60. 10.1016/j.ijcard.2014.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195-201. 10.1038/nm912 [DOI] [PubMed] [Google Scholar]
- 43.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367-8. 10.1038/nm0405-367 [DOI] [PubMed] [Google Scholar]
- 44.Barile L, Gherghiceanu M, Popescu LM, et al. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J Biomed Biotechnol 2012;2012:354605. 10.1155/2012/354605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One 2009;4:e5643. 10.1371/journal.pone.0005643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Chen C, Wei L, et al. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 2016;18:253-62. 10.1016/j.jcyt.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 47.Shabbir A, Cox A, Rodriguez-Menocal L, et al. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 2015;24:1635-47. 10.1089/scd.2014.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang X, Zhang L, Wang S, et al. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci 2016;129:2182-9. 10.1242/jcs.170373 [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Wu X, Zhang X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl Med 2015;4:513-22. 10.5966/sctm.2014-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007;110:2440-8. 10.1182/blood-2007-03-078709 [DOI] [PubMed] [Google Scholar]
- 51.Kang T, Jones TM, Naddell C, et al. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl Med 2016;5:440-50. 10.5966/sctm.2015-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopatina T, Bruno S, Tetta C, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal 2014;12:26. 10.1186/1478-811X-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem Cells 2016;34:601-13. 10.1002/stem.2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vrijsen KR, Sluijter JP, Schuchardt MW, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 2010;14:1064-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Bucan V, Baehre H, et al. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol 2015;47:244-52. 10.3892/ijo.2015.3001 [DOI] [PubMed] [Google Scholar]
- 56.Lai RC, Yeo RW, Tan KH, et al. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med 2013;8:197-209. 10.2217/rme.13.4 [DOI] [PubMed] [Google Scholar]
- 57.Kang K, Ma R, Cai W, et al. Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells Int 2015;2015:659890. 10.1155/2015/659890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Arslan F, Ren Y, et al. Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned by temporal and quantitative changes in the cardiac proteome. J Proteome Res 2012;11:2331-46. 10.1021/pr201025m [DOI] [PubMed] [Google Scholar]
- 59.Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014;551:55-64. 10.1016/j.gene.2014.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010;5:e11803. 10.1371/journal.pone.0011803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nature communications 2013;4:2980. 10.1038/ncomms3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantaluppi V, Biancone L, Figliolini F, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell transplantation 2012;21:1305-20. 10.3727/096368911X627534 [DOI] [PubMed] [Google Scholar]
- 63.Quesenberry PJ, Dooner MS, Goldberg LR, et al. A new stem cell biology: the continuum and microvesicles. Trans Am Clin Climatol Assoc 2012;123:152-66; discussion 66. [PMC free article] [PubMed] [Google Scholar]
- 64.Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 2015;117:52-64. 10.1161/CIRCRESAHA.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu S, Zhang W, Chen J, et al. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS One 2014;9:e85396. 10.1371/journal.pone.0085396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 2008;15:272-84. 10.1016/j.devcel.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 2008;15:261-71. 10.1016/j.devcel.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2009;2:ra81. 10.1126/scisignal.2000610 [DOI] [PubMed] [Google Scholar]
- 69.Jansen F, Yang X, Hoelscher M, et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 2013;128:2026-38. 10.1161/CIRCULATIONAHA.113.001720 [DOI] [PubMed] [Google Scholar]
- 70.Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res 2010;107:677-84. 10.1161/CIRCRESAHA.109.215566 [DOI] [PubMed] [Google Scholar]
- 71.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res 2012;110:508-22. 10.1161/CIRCRESAHA.111.247445 [DOI] [PubMed] [Google Scholar]
- 72.Togliatto G, Dentelli P, Gili M, et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes (Lond) 2016;40:102-11. 10.1038/ijo.2015.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gross JC, Chaudhary V, Bartscherer K, et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol 2012;14:1036-45. 10.1038/ncb2574 [DOI] [PubMed] [Google Scholar]
- 74.Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015;33:2158-68. 10.1002/stem.1771 [DOI] [PubMed] [Google Scholar]
- 75.Chairoungdua A, Smith DL, Pochard P, et al. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 2010;190:1079-91. 10.1083/jcb.201002049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martínez MC, Larbret F, Zobairi F, et al. Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood 2006;108:3012-20. 10.1182/blood-2006-04-019109 [DOI] [PubMed] [Google Scholar]
- 77.Liégeois S, Benedetto A, Garnier JM, et al. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 2006;173:949-61. 10.1083/jcb.200511072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 2001;7:706-11. 10.1038/89083 [DOI] [PubMed] [Google Scholar]
- 79.Benameur T, Soleti R, Porro C, et al. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS One 2010;5:e12688. 10.1371/journal.pone.0012688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agouni A, Mostefai HA, Porro C, et al. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J 2007;21:2735-41. 10.1096/fj.07-8079com [DOI] [PubMed] [Google Scholar]
- 81.Soleti R, Lauret E, Andriantsitohaina R, et al. Internalization and induction of antioxidant messages by microvesicles contribute to the antiapoptotic effects on human endothelial cells. Free Radic Biol Med 2012;53:2159-70. 10.1016/j.freeradbiomed.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 82.Paulis L, Fauconnier J, Cazorla O, et al. Activation of Sonic hedgehog signaling in ventricular cardiomyocytes exerts cardioprotection against ischemia reperfusion injuries. Sci Rep 2015;5:7983. 10.1038/srep07983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mackie AR, Klyachko E, Thorne T, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 2012;111:312-21. 10.1161/CIRCRESAHA.112.266015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol 2014;5:370. 10.3389/fimmu.2014.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vicencio JM, Yellon DM, Sivaraman V, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 2015;65:1525-36. 10.1016/j.jacc.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Gu H, Huang W, et al. Hsp20-Mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 2016;65:3111-28. 10.2337/db15-1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng Y, Huang W, Meng W, et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 2014;32:462-72. 10.1002/stem.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menasché P, Vanneaux V, Hagege A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011-7. 10.1093/eurheartj/ehv189 [DOI] [PubMed] [Google Scholar]
- 89.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413-30. 10.1161/CIRCRESAHA.116.303614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samanta A, Dawn B. Meta-analysis of preclinical data reveals efficacy of cardiac stem cell therapy for heart repair. Circ Res 2016;118:1186-8. 10.1161/CIRCRESAHA.116.308620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Govaert JA, Swijnenburg RJ, Schrepfer S, et al. Poor functional recovery after transplantation of diabetic bone marrow stem cells in ischemic myocardium. J Heart Lung Transplant 2009;28:1158-65.e1. 10.1016/j.healun.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You D, Cochain C, Loinard C, et al. Hypertension impairs postnatal vasculogenesis: role of antihypertensive agents. Hypertension 2008;51:1537-44. 10.1161/HYPERTENSIONAHA.107.109066 [DOI] [PubMed] [Google Scholar]
- 93.Ayala-Lugo A, Tavares AM, Paz AH, et al. Age-dependent availability and functionality of bone marrow stem cells in an experimental model of acute and chronic myocardial infarction. Cell Transplant 2011;20:407-19. 10.3727/096368909X519283 [DOI] [PubMed] [Google Scholar]
- 94.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016;113:E968-77. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu X, Deng L, Wang D, et al. Mechanism of TNF-alpha autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1alpha, presented by exosomes. J Mol Cell Cardiol 2012;53:848-57. 10.1016/j.yjmcc.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 96.Gerrits AJ, Koekman CA, Yildirim C, et al. Insulin inhibits tissue factor expression in monocytes. J Thromb Haemost 2009;7:198-205. 10.1111/j.1538-7836.2008.03206.x [DOI] [PubMed] [Google Scholar]
- 97.Zu L, Ren C, Pan B, et al. Endothelial microparticles after antihypertensive and lipid-lowering therapy inhibit the adhesion of monocytes to endothelial cells. Int J Cardiol 2016;202:756-9. 10.1016/j.ijcard.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 98.Ou ZJ, Chang FJ, Luo D, et al. Endothelium-derived microparticles inhibit angiogenesis in the heart and enhance the inhibitory effects of hypercholesterolemia on angiogenesis. Am J Physiol Endocrinol Metab 2011;300:E661-8. 10.1152/ajpendo.00611.2010 [DOI] [PubMed] [Google Scholar]
- 99.Wiklander OP, Nordin JZ, O'Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 2015;4:26316. 10.3402/jev.v4.26316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014;8:483-94. 10.1021/nn404945r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology 2011;29:341-5. 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- 102.Vader P, Mol EA, Pasterkamp G, et al. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 2016;106:148-56. 10.1016/j.addr.2016.02.006 [DOI] [PubMed] [Google Scholar]