Abstract
Postoperative pancreatic fistula (POPF) remains the major postoperative cause of morbidity and mortality following pancreatic surgery. Since 2005, the International Study Group of Pancreatic Fistula (ISGPF) definition and classification has been adopted worldwide allowing the comparison among different surgical approaches and mitigation strategies. Over the last 11 years, several limitations have emerged from clinical practice and in 2016 the International Study Group for Pancreatic Surgery (ISGPS) updated the POPF definition and grading system. Objectives of this review article were to summarize modifications in the updated ISGPS definition and to illustrate their clinical impact.
Keywords: Distal pancreatectomy, pancreatic fistula, pancreatic surgery, pancreaticoduodenectomy, postoperative complications, postoperative pancreatic fistula (POPF)
Introduction
Postoperative pancreatic fistula (POPF) remains the major contributor to morbidity and mortality following pancreatic surgery occurring for 10–34% of all pancreatic resections in high volume centers (1). The leakage of pancreatic juice is caused by the impaired sealing of the pancreatic parenchyma on the site of the surgical resection (2) and can be associated with different clinical conditions. It might be asymptomatic or, when not adequately drained, it may contribute to the development of further complications as abdominal abscess, bleeding, wound infection, pneumonia, sepsis and postoperative mortality (1).
Over years, various surgical techniques and mitigations strategies have been investigated in order to reduce the incidence and severity of POPF. In 2005, 26 different definitions of pancreatic fistula were reported in literature, making it difficult to compare outcomes and surgical experiences among different centers (3). The International Study Group of Pancreatic Fistula (ISGPF) therefore provided a unique and universally accepted definition (2) that it has been adopted worldwide and applied to over 320,000 patients (4). Despite its overall acceptance and success, following studies point out some major limitations (4-7) and, in 2016, the International Study Group for Pancreatic Surgery (ISGPS) revised the POPF definition and introduced new criteria to better characterize the different severity grades.
The 2005 ISGPF definition and grading
Pancreatic fistula has been defined as “an abnormal communication between the pancreatic ductal epithelium and another epithelial surface containing pancreas-derived, enzyme-rich fluid” (2). POPF is clinically detected using the quantitative measurement of amylase content in the drainage fluid. According to the 2005 definition, POPF was diagnosed when the amylase content was greater than 3 times the upper normal serum value starting from the postoperative day (POD) 3.
ISGPF classified POPF into three different grades with increasing clinical severity. Grade A was characterized by drainage fluid rich in amylase but with no clinical sequelae. The grade B fistula required a change in the postoperative management and specific treatments to promote the healing of the fistula as parental and enteral nutrition and antibiotics. Finally, the grade C fistula required major deviations from the normal clinical pathway as invasive procedures including surgical reoperation. In this condition, sepsis and organ dysfunction might be present as death may occur.
Issues and controversies of 2005 definition and grading
The first issue regarding the 2005 definition concerned the inclusion of grade A in the POPF definition as a postoperative complication since these patients had an uneventful postoperative clinical course (1,5). As a consequence, most of the studies included grade B and grade C in a new category named “clinically relevant fistula” and grade A was mostly reported separated or integrated with the no fistula group (6,8-11). The second issue concerned the unclear distinction between grade B and grade C inclusion criteria (6). In particular, the classification of a POPF requiring an “invasive procedure” was ambiguous due to a partial inconsistency between the text and the summary table in the original paper (2). The placement of an invasive drain with a percutaneous approach has been defined as grade C in the text, whereas it was considered as a “possible” grade B (“yes/no”) procedure in the summary table. As a result, patients undergoing percutaneous drainage were often differently classified according to the various interpretations (6,12-14). Finally, a further concern regards the shift of grade A into a grade B POPF when patients were discharged with the drain in situ. This event occurred commonly in patient undergone distal pancreatectomy that usually does not experience further complications.
New 2016 ISGPS definition and grading
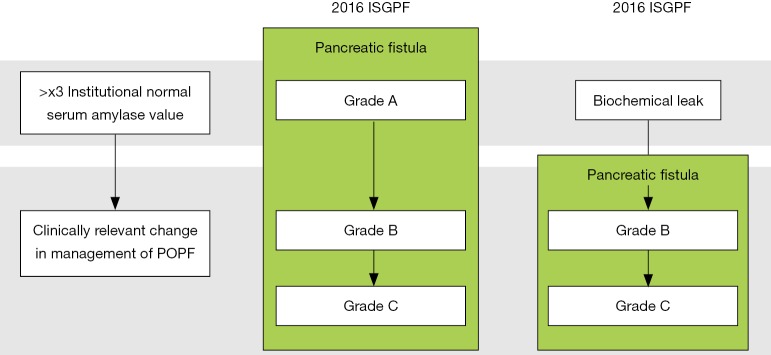
In 2016 the ISGPF reconvened as the ISGPS to update and revise the POPF definition and grading system (4). Although the pancreatic fistula definition was unchanged, the criteria for its diagnosis underwent a radical change. The increase in amylase content of the abdominal fluid alone is no longer sufficient to define POPF, but it has to be associated with an impaired clinical condition causally linked with the pancreatic leak (Figure 1). Grade A POPF is therefore no longer existing but it has been replaced with a new category characterized by an asymptomatic pancreatic leak called “biochemical leak” (BL). The drain in situ for an extended period following the discharge was explicitly approved for this condition.
Figure 1.
Change in POPF diagnosis. POPF, postoperative pancreatic fistula; ISGPF, the International Study Group of Pancreatic Fistula.
Finally, ISGPS provided a stricter border delineation of the grade B and grade C POPF (Table 1). Patients that experienced specific treatments to promote the fistula healing, including invasive procedures such as percutaneous or endoscopic drainage of abdominal collections and angiographic procedure, were included in the grade B. Whereas, grade C was limited to those patients that developed organ failure, required a reoperation or died because of the POPF. The ISGPS provided a specific definition of organ failure as “the need for reintubation, hemodialysis, and/or use of inotropic agents for >24 hours because of respiratory, renal, or cardiac insufficiency, respectively” (4) while sepsis was excluded from the criteria. Finally, it was specified that conditions or treatments mentioned above had to be POPF related in order to define the fistula grade.
Table 1. Change in Grade B and C discriminating criteria.
| Event | 2005 ISGPF | 2016 ISGPS |
|---|---|---|
| Percutaneous or endoscopic drainage | Grade C/Grade B (unclear) | Grade B |
| Angiographic procedures for POPF related bleeding | Grade C | Grade B |
| Organ Failure | Not included | Grade C |
| Sepsis | Grade C | Not included |
| All events and treatment on POPF classification must be POPF-related | No | Yes |
POPF, postoperative pancreatic fistula; ISGPF, the International Study Group of Pancreatic Fistula; ISGPS, the International Study Group for Pancreatic Surgery.
Clinical implications
Changes in the POPF definition and grading had relevant implications on its rate and classification (1). Applying the new definition in our series revealed a general reduction of the POPF incidence from about 34% to 27% with specific differences among surgical procedures. According to the previous definition, distal pancreatectomy was associated with a higher POPF rate compared to pancreaticoduodenectomy; whereas, with the updated classification, distal pancreatectomy is characterized by a lower than pancreaticoduodenectomy POPF rate, but by a higher BL incidence (1).
The redefinition of the criteria for each grade lead to 10% of patients downgraded from grade C to grade B POPF (1). Grade C POPF became “more severe” thanks to the more stringent inclusion criteria with increased hospital costs (147% more) than before. Grade C is now a rarer condition associated with a higher mortality rate (44%), a higher reoperation rate (69%), organ failure (89%), requiring intensive care admission (83%) and where sepsis is always present. Consequently, the rate of grade B increased mostly due to the inclusion of patients undergone “invasive procedure”, but not a reoperation, with a concomitant 28% increase in the median hospital costs. These changes didn’t modify significantly the overall rate of patients requiring intensive cares, enteral and parental nutrition, hospital length of stay and the readmission rate demonstrating that, invasive procedures, such as percutaneous or endoscopic drainage placement and as angiographic procedures, are associated with rapid improvements in clinical conditions and better outcomes than reoperation. According to this new definition, patients with a grade B POPF may also experience postoperative death when not POPF related. However, this event occurred in less than 1% of cases (1) indicating that mortality following pancreatic surgery is almost invariably POPF related. Regarding the inclusion of organ failure as grade C criteria, we found that it was almost always associated with the reoperation and the POPF-related death occurrence (1). For this reason, further studies are needed to clarify its inclusion as an independent criterion for the grade C definition.
We observed that, from the 2005 study, the new scheme and definition increase the ability to stratify patients undergone pancreatic surgery in three discrete groups of patients that significantly differ in terms of length of stay, management setting, clinical impact, hospital readmission rate, and hospital costs (1).
Conclusions
POPF is a common complication following pancreatic surgery that might prove fatal. Since 2005 ISGPF provided a common definition and classification that was worldwide-accepted and allowed a standardized report of surgical results. The 2016 updated version defined POPF as a relevant clinical event and developed a new classification system that improves the previous by stratifying different conditions for clinical and economic outcomes.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pulvirenti A, Marchegiani G, Pea A, et al. Clinical Implications of the 2016 International Study Group on Pancreatic Surgery Definition and Grading of Postoperative Pancreatic Fistula on 775 Consecutive Pancreatic Resections. Ann Surg 2017 J. [Epub ahead of print]. [DOI] [PubMed]
- 2.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. 10.1016/j.surg.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Bassi C, Butturini G, Molinari E, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg 2004;21:54-9. 10.1159/000075943 [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Pratt WB, Maithel SK, Vanounou T, et al. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg 2007;245:443-51. 10.1097/01.sla.0000251708.70219.d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackert T, Hinz U, Pausch T, et al. Postoperative pancreatic fistula: We need to redefine grades B and C. Surgery 2016;159:872-7. 10.1016/j.surg.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Hassenpflug M, Hinz U, Strobel O, et al. Teres Ligament Patch Reduces Relevant Morbidity After Distal Pancreatectomy (the DISCOVER Randomized Controlled Trial). Ann Surg 2016;264:723-30. 10.1097/SLA.0000000000001913 [DOI] [PubMed] [Google Scholar]
- 8.Vallance AE, Young AL, Macutkiewicz C, et al. Calculating the risk of a pancreatic fistula after a pancreaticoduodenectomy: a systematic review. HPB (Oxford) 2015;17:1040-8. 10.1111/hpb.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassi C, Buchler MW, Fingerhut A, et al. Predictive factors for postoperative pancreatic fistula. Ann Surg 2015;261:e99. 10.1097/SLA.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 10.Malleo G, Pulvirenti A, Marchegiani G, et al. Diagnosis and management of postoperative pancreatic fistula. Langenbecks Arch Surg 2014;399:801-10. 10.1007/s00423-014-1242-2 [DOI] [PubMed] [Google Scholar]
- 11.Roberts KJ, Sutcliffe RP, Marudanayagam R, et al. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy: A UK Multicenter Study. Ann Surg 2015;261:1191-7. 10.1097/SLA.0000000000000997 [DOI] [PubMed] [Google Scholar]
- 12.Kim WS, Choi DW, Choi SH, et al. Clinical validation of the ISGPF classification and the risk factors of pancreatic fistula formation following duct-to-mucosa pancreaticojejunostomy by one surgeon at a single center. J Gastrointest Surg 2011;15:2187-92. 10.1007/s11605-011-1726-0 [DOI] [PubMed] [Google Scholar]
- 13.Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann Surg 2016;263:440-9. 10.1097/SLA.0000000000001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid-Lombardo KM, Farnell MB, Crippa S, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg 2007;11:1451-8; discussion 1459. 10.1007/s11605-007-0270-4 [DOI] [PubMed] [Google Scholar]