Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the gastrointestinal tract, and about 60% of them are found in the stomach. With the widespread application of endoscopy and endoscopic ultrasonography (EUS), more and more gastric GISTs are being found in an early stage (with a relative small diameter and no metastasis), giving the chance of complete resection. Endoscopic resection such as endoscopic band ligation (EBL), endoscopic submucosal dissection (ESD), endoscopic submucosal excavation (ESE), endoscopic full-thickness resection (EFTR) and submucosal tunneling endoscopic resection (STER), is a minimally invasive method compared with the conventional surgical approaches (open or laparoscopic), and has been demonstrated to be safe and effective for treating gastric GISTs. This review summarizes the recent advances on endoscopic resection of gastric GISTs, aiming to provide a rational management strategy for gastric GISTs.
Keywords: Endoscopic surgical procedures, gastric neoplasm, gastrointestinal stromal tumor (GIST)
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the gastrointestinal tract, and the estimated clinical incidence is 1 in 100,000 populations per year (1). GISTs can occur anywhere in the gastrointestinal tract, and in rare cases, in intra-abdominal sites (such as omentum, mesentery, and retroperitoneum), among which the stomach is the most common site (about 60%) (1). With the widespread use of endoscopy and endoscopic ultrasonography (EUS), more and more gastric GISTs are being found in an early stage, providing the chance of complete resection. Laparoscopic surgery (LAP) has been regarded as the standard methods for treatment of gastric GISTs <5 cm (2-4). Endoscopic resection takes advantages over LAP in reducing intraoperative blood loss, operating time and hospital stay without any compromise in success rate or increase in complications, and has been widely accepted as an alternative method for gastric GISTs originating from the MP layer (5-7). Available endoscopic methods include endoscopic band ligation (EBL), endoscopic submucosal dissection (ESD), endoscopic submucosal excavation (ESE), endoscopic full-thickness resection (EFTR), submucosal tunneling endoscopic resection (STER), and laparoscopic and endoscopic cooperative surgery (LECS) (8). This review summarizes recent advances on endoscopic resection of gastric GISTs, aiming to provide a rational management strategy for gastric GISTs.
Indications of endoscopic resection of gastric GISTs
Gastric submucosal tumors (SMTs) are found in 0.36% of middle-aged adults by health examination, and most of them are asymptomatic or have nonspecific symptoms (9). Once a gastric SMT is found, EUS is usually recommended to further determine the characteristics of the SMT, such as the originating layer, echo, lymph node, which is helpful to differentiating GISTs from other mesenchymal tumors. Specific findings of GIST on EUS include: low echo, inhomogeneous, anechoic or high echo (when tumors are malignant), and it is usually located in the third or fourth layer, rarely the second layer (10). If an SMT is highly suggestive of a GIST and is considered resectable, preoperative biopsy is not necessary (11). Periodical surveillance is recommended for small (<2 cm) asymptomatic gastric GISTs. However, it involves issues related to the patient’s compliance and stress, cost-effectiveness, and the risk associated with repeated endoscopic procedures and delayed diagnosis of malignancy (12,13). Moreover, it is believed that small gastric GISTs also have malignant potential and that the size of small gastric GISTs could increase significantly during follow-up (13,14). Therefore, some researchers suggested that once a gastric GIST was suspected, it should be resected by surgical or endoscopic approaches (13,15), although the NCCN guideline did not recommend immediate resection for GISTs <2 cm (2). Figure 1 shows the patient selection diagram of endoscopic resection for gastric GISTs in our hospital.
Figure 1.
The patient selection diagram of endoscopic resection for gastric GISTs in our hospital. Risk factors: ulceration or erosion at the site of tumor location; EUS shows irregular border, internal heterogeneity include anechoic area (i.e., necrosis) and echogenic loci (i.e., bleeding), heterogeneous enhancement, regional lymph node swelling; CT show metastasis or invasion out of the gastrointestinal tract; a Zubrod-ECOG performance status ≥2; have severe cardiopulmonary disease or blood coagulation disorders. GIST, gastrointestinal stromal tumor; EUS, endoscopic ultrasonography; CT, computed tomography; SMT, submucosal tumor.
Endoscopic methods for gastric GIST
EBL
EBL was first reported for treating esophageal varices (16), and was then applied to the treatment of gastrointestinal superficial lesions (17). Sun et al. (18) firstly reported the feasibility and safety of EBL in the treatment of gastric GISTs, and complete resection was achieved in 96.6% (28/29) of the cases, with a low complication rate (3.4%, 1/29) and recurrence rate (3.4%, 1/29). The standard procedure of EBL is as follows: aspirating the tumor into a transparent cap, releasing the band, cutting the overlying mucosal and submucosal layer and then dissecting the tumor. EUS is usually used to confirm whether the mass is completely confined within the band, and hemoclips are placed around the band to reduce the tension and potential perforation. Several clinical studies have demonstrated the safety and efficacy of EBL for gastric GISTs, with favorable complete rate, low complication and recurrence rate (19,20) (Table 1). The most common complications reported are perforation and bleeding (18-20,36). In addition, Meng et al. (5) demonstrated that EBL could reduce operation time, estimated blood loss, complications, hospital stay and cost, compared with ESD and LAP. The major disadvantage of EBL is the restriction of maximal resectable size (≤12 mm) due to the size of the transparent cap. And EBL is feasible only for GISTs originating from superficial muscularis propria layer. EBL is now less used and mostly be replaced by other endoscopic methods.
Table 1. Studies about endoscopic resection for gastric GISTs.
| Ref. | N | Method | Mean tumor diameter [range] (mm) | Mean operation time (min) | Complete resection rate (%) |
Complication (%) | Recurrence (%) |
|---|---|---|---|---|---|---|---|
| Sun et al. (18) | 29 | EBL | 9.2 [7–12] | – | 96.6 | 1 bleeding | 3.4 |
| Nan et al. (19) | 24 | EBL | 8 [7–12] | – | 100 | 0 | 0 |
| Huang et al. (21) | 38 | EBL | <12 | – | 100 | 3 perforation | – |
| Nan et al. (20) | 177 | EBL | 8 [5–12] | – | 100 | 2 perforation | – |
| An et al. (22) | 168 | ESD | 15 [5–60] | 46.5 [33–181] | 100 | 2 bleeding, 71 gastric wall defect | 0 |
| He et al. (23) | 25/31† | ESD | 27 [20–50] | 70.16 [40–105] | 100 | 3 bleeding, 6 perforation | 0 |
| Zhang et al. (24) | 69 | ESE | 18.7 [7–30] | 41.07±10.79 | 100 | 6 bleeding, 23 perforation, 5 surgery-related complication | 0 |
| Huang et al. (21) | 18 | ESE | >15 | – | 100 | 0 | – |
| Wang et al. (25) | 86 | ESE | – | – | 100 | 5 bleeding, 9 perforation | 5.8 |
| Shi et al. (26) | 43/60‡ | ESE | 1.4 [5–50] | 38 | 100 | – | – |
| Wang et al. (27) | 30 | ESE | 22 [10–35] | 50±5 [20–120] | 100 | 6 perforation | 0 |
| Shi et al. (28) | 68 | EFTR | 26 | 41 | 100 | 1 Mallory-Weiss syndrome, 1 delayed bleeding | 0 |
| Mori et al. (29) | 16 | EFTR | 28.3 | 271 | 100 | 0 | 0 |
| Huang et al. (21) | 13 | EFTR | >20 | – | 100 | 0 | – |
| Lu et al. (30) | 36/47§ | STER | 14 [5–50] | 79.3 [45–150] | 100 | 3 peumoperitoneum | 0 |
| Li et al. (31) | 11/32§ | STER | 23 [10–50] | 51.8 [25–125] | 100 | Intraoperative: 1 bleeding, 6 peumoperitoneum; postoperative: 3 pneumothorax, 3 pleural effusion, 1 subphrenic infection | 0 |
| Mao et al. (32) | 10/56§ | STER | 18 [10–32] | 41.5 [20–65] | 100 | 9 gas-related complications with or without pleural effusion | 0 |
| Kikuchi et al. (33) | 10 | LECS | 24.1±7.6 | 253±45 | 100 | 1 intra-abdominal abscess | 0 |
| Qiu et al. (34) | 69 | LECS | 28±16 | 86.1 | – | 1 leakage, 1 bleeding | 0 |
| Hiki et al. (35) | 10 | LECS | 46±3 | 169±17 | 100 | 0 | – |
†, 25 of the 31 GISTs were located in the stomach; ‡, 43 of the 60 GISTs were located in the stomach; §, n/m, these 3 studies are about STER for gastric submucosal tumors, n of the m submucosal tumors are gastric GISTs. GISTs, gastrointestinal stromal tumors; EBL, endoscopic band ligation; ESD, endoscopic submucosal dissection; ESE, endoscopic submucosal excavation; EFTR, endoscopic full-thickness resection; STER, submucosal tunneling endoscopic resection; LECS, laparoscopic and endoscopic cooperative surgery.
ESD
ESD was firstly used to treat early stage gastric cancer (37), and was then applied to the treatment of gastric SMTs, including gastric GISTs (38,39). The standard procedure of ESD is as follows: making marking dots around the lesion, submucosal injection, precutting the mucosal and submucosal layer and then dissecting the tumor (Figure 2). Compared with EBL, ESD enables a larger resectable size and provides a higher en bloc resection rate. Although many clinical studies concerning the treatment of ESD for gastric SMTs (GISTs included) have been reported [see in detail in review (40)], only two studies have been published regarding ESD as a treatment for pure gastric GISTs (Table 1), and both of their results were exciting. Moreover, Meng et al. (41) demonstrated that the efficacy of ESD and LAP for treating small gastric GISTs was comparable, but ESD could reduce the operation time, estimated blood loss and hospital stay. Perforation and bleeding are the major complications associated with gastric ESD, whose incidence have been reported to range from 0% to 8.2% and 0% to 15.6%, and most of them can be successfully managed by appropriate endoscopic interventions [see in detail in review (42,43)]. Other rare but serious complications include aspiration pneumonia, stenosis, venous thromboembolism, and air embolism (44-47). Endoscopists should be aware of these complications and their associated risk factors (44-47), so as to prevent their occurrence and reduce the harm. And to achieve an en bloc resection, ESD is only recommended for SMTs originating from the superficial MP layer.
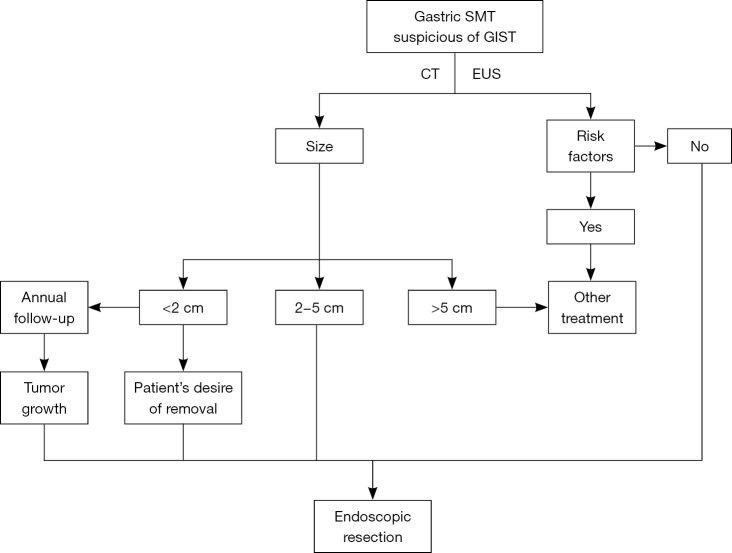
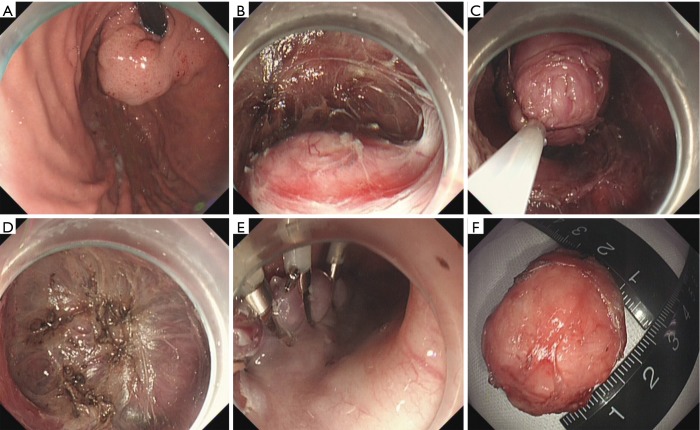
Figure 2.
Case illustration of endoscopic submucosal dissection. (A) We could see a submucosal tumor in the gastric fundus; (B) after making dots and submucosal injection, we precut the mucosal and submucosal layer using a dual knife, and the submucosal tumor is shown; (C,D) dissect the tumor with a dual knife; (E) close the wound with several clips; (F) the resected tumor.
ESE
Although ESD is effective for treating gastric GISTs, the en bloc resection rate sometimes is not that satisfactory, especially for those originating from deep MP layer. ESE, allowing deep excavation, is a better choice. ESE was first reported by Jeong et al. (48) for treating gastric SMTs (GISTs included) originating form the MP layer, with a high complete resection rate and acceptable complication rate. The standard procedure of ESE is as follows: making marking dots around the lesion, submucosal injection, precutting the mucosal and submucosal layer and excavating the tumor (Figure 3). The major difference between ESD and ESE procedure is the depth of endoscopic resection. As deep excavation was necessary during ESE, an insulated-tip knife is usually recommended during excavation to avoid or reduce unintentional injury, while in the ESD procedure, the dissection could achieved by other endoscopic knives such as dual knife, hook knife, etc. Several studies have demonstrated the safety and efficacy of ESE for gastric GISTs, with favorable complete rate and low recurrence rate (24,26,27) (Table 1). The most common complication reported is perforation, whose incidence was up to 33.3%. However, most of them could be successfully managed by endoscopy, only few needed surgical intervention. Other reported complications include bleeding, surgery-related complications, bacteremia (21,24,26,27,48,49). CO2 is recommended during the procedure, as it can reduce the pain score and increase the visual analog scale score, compared with air insufflation (26).
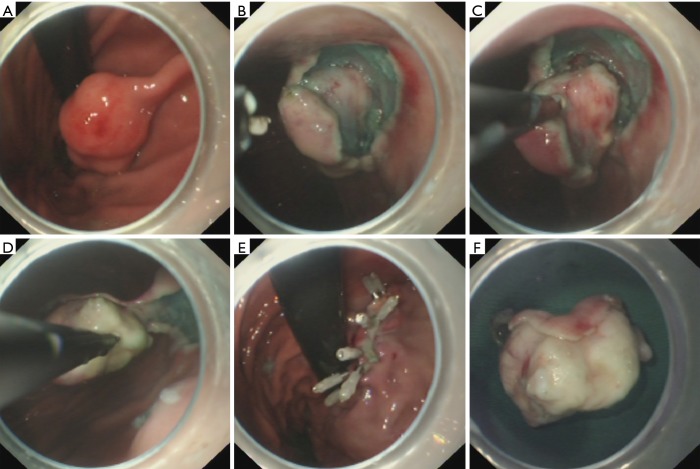
Figure 3.
Case illustration of endoscopic submucosal excavation. (A) We could see a submucosal tumor in the gastric corpus; (B) after making dots and submucosal injection, we precut the mucosal and submucosal layer covering the submucosal tumor to expose the tumor; (C) excavate the tumor from the muscularis propria layer; (D) the wound surface after tumor removal; (E) close the wound with several clips; (F) the resected tumor.
EFTR
EFTR was firstly reported by Suzuki and Ikeda for treating two rectal carcinoids and one duodenal carcinoid using the snaring technique (50), and then Ikeda et al. reported EFTR using the ESD technique on a porcine stomach (51). Wang et al. (52) firstly introduced EFTR into clinical practice for treating gastric GISTs. The standard procedure of EFTR is as follows: submucosal injection, precutting the mucosal and submucosal layer around the lesion, circumferential incision as deep as the MP layer around the lesion, incision into the serosal layer around the lesion, full-thickness resection of the tumor including the serosal layer and closing the gastric-wall defect (Figure 4). Although many clinical studies concerning EFTR for gastric SMTs have been published, only three studies are available about EFTR for pure gastric GISTs (21,28,29), and the clinical outcomes were promising (Table 1). In EFTR, perforation is not considered as a complication. Reported complications include bleeding, localized peritonitis, abdominal distention, etc., and the overall complication rates were very low [in detail in review (53,54)]. Furthermore, Wang et al. (55) found that the safety and efficacy of EFTR and LAP for small gastric GIST is comparable, however, EFTR could reduce the procedure time, intraoperative bleeding volume and hospital stay. Besides, 12 of the 33 cases needed intraoperative endoscopy to precise identify the GISTs in the LAP group.
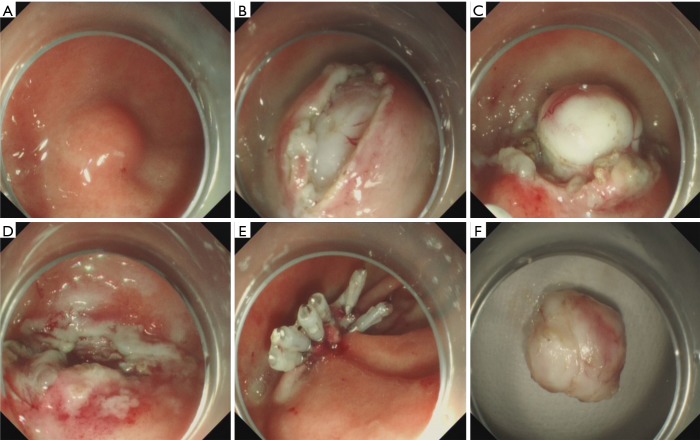
Figure 4.
Case illustration of endoscopic full-thickness resection. (A) We could see a submucosal tumor in the gastric corpus; (B) after submucosal injection, we precut and remove the mucosal and submucosal layer to expose the tumor; (C,D) endoscopic full-thickness resection of the tumor, we could see the abdominal cavity through the “active perforation”; (E) close the wound with several clips; (F) the resected tumor.
STER
STER was initially used as a therapeutic technique for treating esophageal and cardia SMTs (56-59). The standard procedure is as follows: submucosal injection, creating tunnel entry, submucosal tunnel creation, finding and dissecting the SMT, and then closing the tunnel entry (Figure 5). Compared with other endoscopic methods, STER possesses multiple advantages including the maintenance of mucosal integrity, the facilitation of an increased healing rate and a decreased risk of pleural/abdominal infection (60-62). Several studies have demonstrated the safety and efficacy of STER for treating gastric SMTs, half of whom were gastric GISTs (30-32). Zhang et al. (63) found that compared with endoscopic nontunneling methods (ESD and EFR), STER has no distinct advantages in treating relatively small gastric SMTs, but Tan et al. (64) found that the safety and efficacy between STER and EFTR were comparable, but patients who received EFTR needed more clips to close the gastric wall defect. Common complications of STER include gas-related complications, bleeding, pleural effusion, mucosal injury, etc. Although the overall incidence of complications is relatively high, only a small part of them need therapeutic intervention (59,65), suggesting STER is a safe and effective method.
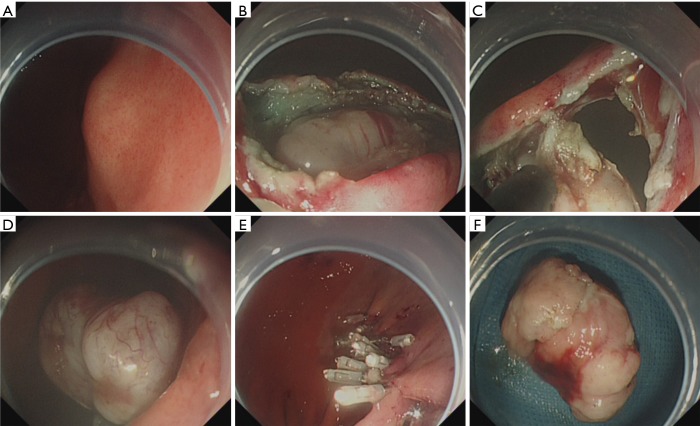
Figure 5.
Case illustration of submucosal tunneling endoscopic resection. (A) We could see a submucosal tumor in the gastric fundus; (B) after submucosal injection, a longitudinal mucosal incision was made 3 cm above the tumor, and a submucosal tunnel was created between the submucosal and muscularis propria layer, and then the submucosal tumor was visible; (C) carefully dissected the tumor from the muscularis propria layer and remove the tumor; (D) the wound surface after tumor removal; (E) close the tunnel entry with several clips; (F) the resected tumor.
LECS
All the above endoscopic methods have limitations in terms of rumor size and location, thus the concept of LECS was devised, consisting of endoscopic surgery in the form of endoscopic mucosal incision and LAP (35). In this advanced technique, incision lines are confirmed endoscopically and accurately determined by application of an endoscopic mucosal/submucosal incision technique, while the seromuscular layer is incised laparoscopically and the incision line is closed using a laparoscopic stapling device, resulting in minimal dissection of the normal gastric wall with minimal gastric transformation. Currently, LECS has been recommended by NCCN as a treatment for gastric GIST less than 50 mm in diameter regardless of the tumor location (2). Since it’s first reported by Hiki et al. (35), two other studies have explored the efficacy of LECS for gastric GISTs and have shown exciting results (33,34). In addition, Balde et al. (66) found that although ESD had a shorter operation time, the rate of intraoperative complications was lower in the LECS group. Ojima et al. (67) found that compared with LECS, endoscopic intragastric surgery (EIGS) had a higher perioperative complications rate and a longer time to resumption of first oral intake.
Postoperative management
All the patients are kept nil per os (NPO) for at least 72 h, a liquid diet for 5 days, and returned gradually to a normal diet within 2 weeks. And intravenous proton pump inhibitor (PPI) and antibiotics were recommended for at least 3 days. For patients with GISTs located in the gastric fundus, they are asked to keep a semireclining position for 3 days. A contrast roentgenography is performed on postoperative day 3 to check for any occurrence of leakage. Ultrasound was applied to check the presence of any abdominal or pelvic dropsy.
The resected specimens are fixed, embedded with paraffin and then sectioned. Hematoxylin and eosin and immunohistochemical staining (CD117, CD34, Dog-1, Ki67, SMA, etc.) are carried out to determine whether the SMT is a GIST or not. If the SMT is highly suspected of a GIST but all the markers above are negative, KIT and/or PDGFRA mutation should be detected (68). A risk category should obtained based on the tumor size, mitotic index and primary tumor site using the modified NIH classification system (69), classifying them as very low risk, low risk, intermediate risk and high risk, which is helpful to predict recurrence. For those patients classified as intermediate or high risk, additional surgery and/or adjuvant treatment (imatinib, etc.) are recommended.
Postoperative follow-up is necessary for GISTs patients who received endoscopic resection, and the surveillance interval varies according to the risk classification. For patients with high or intermediate risk, abdominal and pelvic CT or EUS every 3–4 months is recommended in the first 3 years after endoscopic treatment, and then every 6 months until 5 years after treatment and then annually thereafter. For those with very low or low risk, CT and/or EUS are recommended every 6 months in the first 5 years (68,70). Surveillance endoscopy is recommended to be performed at 3 months, and 12 months after treatment to observe healing of the wound and to check for any residual tumor.
Conclusions and perspectives
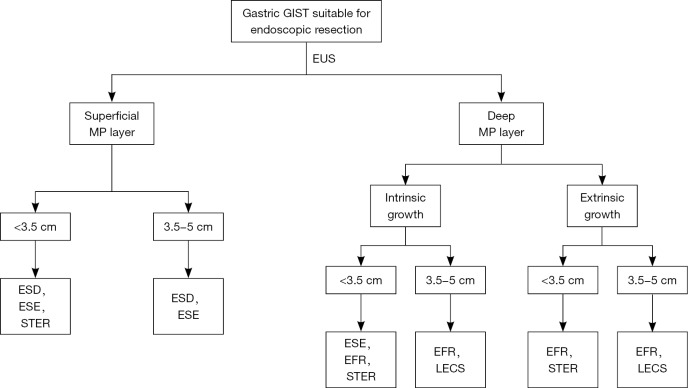
Unpredictable malignant potential and rare lymph node metastasis provide the theoretical basis of minimally invasive treatment of gastric GISTs. Currently, many studies concerning endoscopic resection for gastric GISTs have been published, and the primary results were exciting (Table 1). However, the follow-up of these studies were relative short (usually <2 years), thus warranting a long-term follow-up. What’s more, few studies that focused on the comparison among different endoscopic methods or between endoscopic and surgical methods have been published (Tables 2,3). Thus more evidence is required to recommend endoscopic resection as the first-line treatment of gastric GISTs. In our hospital, we use an algorithm as proposed in Figure 6.
Table 2. Comparison of different endoscopic methods for gastric GISTs.
| Ref. | Method | N | Mean tumor diameter (mm) | Mean operation time (min) |
En bloc resection rate (%) |
Complication (%) | Follow-up time (months) | Recurrence (%) |
|---|---|---|---|---|---|---|---|---|
| Meng et al. (5) | EBL vs. ESD | 72 vs. 27 | 10.68 vs. 11.78 | 17.11 vs. 65.26 | – | 1.39 vs. 18.52 | 6 vs. 7 | 15 vs. 9.1 |
| Tan et al. (64) | STER vs. EFTR | 20 vs. 32 | 17.8 vs. 15.4 | 74.9 vs. 69.1 | 95 vs. 96.9 | 5 vs. 15.6 | 10.9 vs. 23.8 | 0 vs. 0 |
| Zhang et al. (63) | Nontunneling vs. STER | 78 vs. 19 | 15 vs. 20 | 50 vs. 75 | 95.9 vs. 94.1 | 26.9 vs. 36.8 | – | 0 vs. 0 |
| Balde et al. (66) | LECS vs. ESD | 30 vs. 30 | 15 vs. 15 | 96.5 vs. 41.5 | 100 vs. 100 | 3.3 vs. 26.7 | – | 0 vs. 14.3 |
| Ojima et al. (67) | LECS vs. EIGS | 21 vs. 26 | 25 vs. 23 | 139 vs. 108 | 100 vs. 100 | 4.8 vs. 40 | 21 vs. 61 | 4.8 vs. 4 |
GISTs, gastrointestinal stromal tumors; EBL, endoscopic band ligation; ESD, endoscopic submucosal dissection; EFTR, endoscopic full-thickness resection; STER, submucosal tunneling endoscopic resection; LECS, laparoscopic and endoscopic cooperative surgery; EIGS, endoscopic intragastric surgery.
Table 3. Comparison between endoscopic and surgical methods for gastric GISTs.
| Ref. | Method | N | Mean tumor diameter (mm) | Mean operation time (min) | Complete resection rate (%) | Complication (%) | Follow-up time (months) | Recurrence (%) |
|---|---|---|---|---|---|---|---|---|
| Meng et al. (5) | EBL vs. LAP | 72 vs. 48 | 10.68 vs. 12.02 | 17.11 vs. 90.81 | – | 1.39 vs. 4.17 | 6 vs. 6 | 15.00 vs. 11.76 |
| Meng et al. (41) | ESD vs. LAP | 75 vs. 51 | 14.4 vs. 14.6 | 63.59 vs. 79.12 | – | 2.67 vs. 1.96 | 40.1 vs. 40.9 | 2.67 vs. 1.96 |
| Wang et al. (55) | EFTR vs. LAP | 35 vs. 33 | 13 vs. 16 | 91 vs. 155 | 100 vs. 100 | 11.4 vs. 13.3 | – | 0 vs. 0 |
| Wu et al. (71) | EFTR vs. LAP | 50 vs. 42 | – | 85 vs. 88 | 100.0 vs. 92.9 | 0 vs. 4.8 | – | 0 vs. 0 |
| Huang et al. (72) | EFTR vs. LAP | 32 vs. 30 | – | 78.5 vs. 80.9 | 100.0 vs. 93.3 | 0 vs. 3.3 | – | 0 vs. 0 |
| Wang et al. (52) | EFTR vs. LAP | 66 vs. 43 | 15 vs. 11 | 53.6 vs. 139 | 98.4 vs. 100.0 | 24.2 vs. 14.0 | – | 0 vs. 0 |
| Dong et al. (73) | EFTR vs. MLIGS | 10 vs. 8 | 16.5 vs. 27.5 | 120 vs. 85 | 100 vs. 100 | 10 vs. 0 | – | 0 vs. 0 |
GISTs, gastrointestinal stromal tumors; EBL, endoscopic band ligation; ESD, endoscopic submucosal dissection; EFTR, endoscopic full-thickness resection; LAP, laparoscopic surgery; MLIGS, modified laparoscopic intragastric surgery.
Figure 6.
Algorithm on endoscopic management of gastric GISTs in our hospital. GIST, gastrointestinal stromal tumor; EUS, endoscopic ultrasonography; MP, muscularis propria; ESD, endoscopic submucosal dissection; ESE, endoscopic submucosal excavation; STER, submucosal tunneling endoscopic resection; EFR, endoscopic full-thickness resection; LECS, laparoscopic and endoscopic cooperative surgery.
Furthermore, to expand the role of endoscopy on the treatment of gastric GISTs, several technical problems need to be resolved. Firstly, we need to find ways to reduce complications of endoscopic resection, especially perforation. Although several devices such as over-the- scope clip have been proposed [see in review (74,75)], most of them are not suitable for large GISTs, thus warranting the development of new devices. Secondly, there is a possibility of pseudocapsule injury during endoscopic resection of a gastric GIST, providing the risk of peritoneal seeding. Thus a more secure endoscopic method is needed, and it should be performed by a well-trained endoscopist. Recently, novel hybrid techniques, such as combination of laparoscopic and endoscopic approaches to neoplasia with non exposure technique (CLEANNET) (76) and non-exposed endoscopic wall-inversion surgery (NEWS) (77,78), could avoid exposing malignant SETs to the peritoneal cavity. In conclusion, technical modifications and improvements are required to define the role of endoscopy for treating gastric GISTs.
Acknowledgements
Funding: This work was supported by the Chinese National Key Disciplines and Development and Reform Commission of Hunan Province (XFGTZ2014713).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. 10.1016/S0140-6736(13)60106-3 [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-44. [DOI] [PMC free article] [PubMed]
- 3.Chen QL, Pan Y, Cai JQ, et al. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: an updated systematic review and meta-analysis. World J Surg Oncol 2014;12:206. 10.1186/1477-7819-12-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda M, Hiki N, Nunobe S, et al. Long-term and surgical outcomes of laparoscopic surgery for gastric gastrointestinal stromal tumors. Surg Endosc 2014;28:2317-22. 10.1007/s00464-014-3459-0 [DOI] [PubMed] [Google Scholar]
- 5.Meng Y, Cao C, Song S, et al. Endoscopic band ligation versus endoscopic submucosal dissection and laparoscopic resection for small gastric stromal tumors. Surg Endosc 2016;30:2873-8. 10.1007/s00464-015-4571-5 [DOI] [PubMed] [Google Scholar]
- 6.Joo MK, Park JJ, Kim H, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc 2016;83:318-26. 10.1016/j.gie.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 7.Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31. 10.1007/s00464-011-1644-y [DOI] [PubMed] [Google Scholar]
- 8.Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J Gastrointest Endosc 2015;7:192-205. 10.4253/wjge.v7.i3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5:20-3. 10.1007/BF00591381 [DOI] [PubMed] [Google Scholar]
- 10.Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc 2013;25:479-89. 10.1111/den.12149 [DOI] [PubMed] [Google Scholar]
- 11.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy 2017;49:695-714. 10.1055/s-0043-109021 [DOI] [PubMed] [Google Scholar]
- 12.Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:64-7. [DOI] [PubMed] [Google Scholar]
- 13.Koga T, Hirayama Y, Yoshiya S, et al. Necessity for resection of gastric gastrointestinal stromal tumors </= 20 mm. Anticancer Res 2015;35:2341-4. [PubMed] [Google Scholar]
- 14.Fang YJ, Cheng TY, Sun MS, et al. Suggested cutoff tumor size for management of small EUS-suspected gastric gastrointestinal stromal tumors. J Formos Med Assoc 2012;111:88-93. 10.1016/j.jfma.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Yegin EG, Duman DG. Small EUS-suspected gastrointestinal stromal tumors of the stomach: An overview for the current state of management. Endosc Ultrasound 2016;5:69-77. 10.4103/2303-9027.180469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Newihi HM, Achord JL. Emerging role of endoscopic variceal band ligation in the treatment of esophageal varices. Dig Dis 1996;14:201-8. 10.1159/000171551 [DOI] [PubMed] [Google Scholar]
- 17.Nwakakwa V, Fleischer D. Endoscopic mucosal resection of the esophagus: band ligation technique. Gastrointest Endosc Clin N Am 2001;11:479-88, vi. [PubMed] [Google Scholar]
- 18.Sun S, Ge N, Wang C, et al. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg Endosc 2007;21:574-8. 10.1007/s00464-006-9028-4 [DOI] [PubMed] [Google Scholar]
- 19.Nan G, Siyu S, Shiwei S, et al. Hemoclip-reinforced and EUS-assisted band ligation as an effective and safe technique to treat small GISTs in the gastric fundus. Am J Gastroenterol 2011;106:1560-1. 10.1038/ajg.2011.144 [DOI] [PubMed] [Google Scholar]
- 20.Nan G, Siyu S, Sheng W, et al. The role of hemoclips reinforcement in the ligation-assisted endoscopic enucleation for small GISTs in gastric fundus. Biomed Res Int 2014;2014:247602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang LY, Cui J, Liu YX, et al. Endoscopic therapy for gastric stromal tumors originating from the muscularis propria. World J Gastroenterol 2012;18:3465-71. 10.3748/wjg.v18.i26.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An W, Sun PB, Gao J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc 2017;31:4522-31. 10.1007/s00464-017-5511-3 [DOI] [PubMed] [Google Scholar]
- 23.He Z, Sun C, Zheng Z, et al. Endoscopic submucosal dissection of large gastrointestinal stromal tumors in the esophagus and stomach. J Gastroenterol Hepatol 2013;28:262-7. 10.1111/jgh.12056 [DOI] [PubMed] [Google Scholar]
- 24.Zhang JS, Ye LP, Wang CY, et al. Endoscopic submucosal enucleation of small gastric gastrointestinal stromal tumors with cross-shaped incision: report of sixty-nine cases. Hepatogastroenterology 2012;59:440-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Li Y, Luo H, et al. Efficacy analysis of endoscopic submucosal excavation for gastric gastrointestinal stromal tumors. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:352-5. [PubMed] [Google Scholar]
- 26.Shi WB, Wang ZH, Qu CY, et al. Comparison between air and carbon dioxide insufflation in the endoscopic submucosal excavation of gastrointestinal stromal tumors. World J Gastroenterol 2012;18:7296-301. 10.3748/wjg.v18.i48.7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Shen L. Efficacy of Endoscopic Submucosal Excavation for Gastrointestinal Stromal Tumors in the Cardia. Surg Laparosc Endosc Percutan Tech 2016;26:493-6. 10.1097/SLE.0000000000000330 [DOI] [PubMed] [Google Scholar]
- 28.Shi D, Li R, Chen W, et al. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc 2017;31:837-42. 10.1007/s00464-016-5041-4 [DOI] [PubMed] [Google Scholar]
- 29.Mori H, Kobara H, Fujihara S, et al. Establishment of the hybrid endoscopic full-thickness resection of gastric gastrointestinal stromal tumors. Mol Clin Oncol 2015;3:18-22. 10.3892/mco.2014.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Jiao T, Li Y, et al. Heading toward the right direction--solution package for endoscopic submucosal tunneling resection in the stomach. PLoS One 2015;10:e0119870. 10.1371/journal.pone.0119870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li QL, Chen WF, Zhang C, et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 2015;29:3640-6. 10.1007/s00464-015-4120-2 [DOI] [PubMed] [Google Scholar]
- 32.Mao XL, Ye LP, Zheng HH, et al. Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus 2017;30:1-7. 10.1093/dote/dow023 [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi S, Nishizaki M, Kuroda S, et al. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer 2017;20:553-7. 10.1007/s10120-016-0641-1 [DOI] [PubMed] [Google Scholar]
- 34.Qiu WQ, Zhuang J, Wang M, et al. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis 2013;14:469-73. 10.1111/1751-2980.12076 [DOI] [PubMed] [Google Scholar]
- 35.Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. 10.1007/s00464-007-9696-8 [DOI] [PubMed] [Google Scholar]
- 36.Xing XB, Wang JH, Chen MH, et al. Perforation posterior to endoscopic band ligation of a gastric submucosal tumor. Endoscopy 2012;44 Suppl 2 UCTN:E296-7. [DOI] [PubMed]
- 37.Yamamoto H, Yube T, Isoda N, et al. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc 1999;50:251-6. 10.1016/S0016-5107(99)70234-8 [DOI] [PubMed] [Google Scholar]
- 38.Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006;63:243-9. 10.1016/j.gie.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 39.Lee IL, Lin PY, Tung SY, et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy 2006;38:1024-8. 10.1055/s-2006-944814 [DOI] [PubMed] [Google Scholar]
- 40.Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc 2016;8:418-24. 10.4253/wjge.v8.i11.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng Y, Li W, Han L, et al. Long-term outcomes of endoscopic submucosal dissection versus laparoscopic resection for gastric stromal tumors less than 2 cm. J Gastroenterol Hepatol 2017;32:1693-7. 10.1111/jgh.13768 [DOI] [PubMed] [Google Scholar]
- 42.Oda I, Suzuki H, Nonaka S, et al. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013;25 Suppl 1:71-8. 10.1111/j.1443-1661.2012.01376.x [DOI] [PubMed] [Google Scholar]
- 43.Saito I, Tsuji Y, Sakaguchi Y, et al. Complications related to gastric endoscopic submucosal dissection and their managements. Clin Endosc 2014;47:398-403. 10.5946/ce.2014.47.5.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyahara K, Iwakiri R, Shimoda R, et al. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion 2012;86:273-80. 10.1159/000341422 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015;21:12635-43. 10.3748/wjg.v21.i44.12635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park CH, Lee SK. Preventing and controlling bleeding in gastric endoscopic submucosal dissection. Clin Endosc 2013;46:456-62. 10.5946/ce.2013.46.5.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Libânio D, Costa MN, Pimentel-Nunes P, et al. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc 2016;84:572-86. 10.1016/j.gie.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 48.Jeong ID, Jung SW, Bang SJ, et al. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc 2011;25:468-74. 10.1007/s00464-010-1195-7 [DOI] [PubMed] [Google Scholar]
- 49.Li G, Zeng S, Chen Y, et al. Bacteremia after Endoscopic Submucosal Excavation for Treating the Gastric Muscular Layer Tumors. Gastroenterol Res Pract 2015;2015:306938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki H, Ikeda K. Endoscopic mucosal resection and full thickness resection with complete defect closure for early gastrointestinal malignancies. Endoscopy 2001;33:437-9. 10.1055/s-2001-14269 [DOI] [PubMed] [Google Scholar]
- 51.Ikeda K, Fritscher-Ravens A, Mosse CA, et al. Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest Endosc 2005;62:122-9. 10.1016/S0016-5107(05)00517-1 [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Ren W, Fan CQ, et al. Full-thickness endoscopic resection of nonintracavitary gastric stromal tumors: a novel approach. Surg Endosc 2011;25:641-7. 10.1007/s00464-010-1189-5 [DOI] [PubMed] [Google Scholar]
- 53.Mori H, Kobara H, Nishiyama N, et al. Review of Pure Endoscopic Full-Thickness Resection of the Upper Gastrointestinal Tract. Gut Liver 2015;9:590-600. 10.5009/gnl14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt A, Meier B, Caca K. Endoscopic full-thickness resection: Current status. World J Gastroenterol 2015;21:9273-85. 10.3748/wjg.v21.i31.9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Feng X, Ye S, et al. A comparison of the efficacy and safety of endoscopic full-thickness resection and laparoscopic-assisted surgery for small gastrointestinal stromal tumors. Surg Endosc 2016;30:3357-61. 10.1007/s00464-015-4612-0 [DOI] [PubMed] [Google Scholar]
- 56.Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012;75:195-9. 10.1016/j.gie.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 57.Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012; 44:225-30. 10.1055/s-0031-1291659 [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Tan Y, Zhou Y, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 2015;27:776-80. 10.1097/MEG.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 59.Tan Y, Huo J, Liu D. Current status of submucosal tunneling endoscopic resection for gastrointestinal submucosal tumors originating from the muscularis propria layer (Review). Oncol Lett 2017;14:5085-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J, Lu X, Jiao T, et al. Endoscopic management of upper gastrointestinal submucosal tumors arising from muscularis propria. J Clin Gastroenterol 2014;48:667-73. 10.1097/MCG.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Ren W, Zhang Z, et al. Retrospective study of endoscopic submucosal tunnel dissection (ESTD) for surgical resection of esophageal leiomyoma. Surg Endosc 2013;27:4259-66. 10.1007/s00464-013-3035-z [DOI] [PubMed] [Google Scholar]
- 62.Tan Y, Lv L, Duan T, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc 2016;30:3121-7. 10.1007/s00464-015-4567-1 [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q, Wang F, Wei G, et al. Endoscopic resection of gastric submucosal tumors: A comparison of endoscopic nontunneling with tunneling resection and a systematic review. Saudi J Gastroenterol 2017;23:52-9. 10.4103/1319-3767.199116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan Y, Tang X, Guo T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 2017;31:3376-82. 10.1007/s00464-016-5350-7 [DOI] [PubMed] [Google Scholar]
- 65.Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy 2016;48:149-55. [DOI] [PubMed] [Google Scholar]
- 66.Balde AI, Chen T, Hu Y, et al. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc 2017;31:843-51. 10.1007/s00464-016-5042-3 [DOI] [PubMed] [Google Scholar]
- 67.Ojima T, Nakamura M, Nakamori M, et al. Laparoscopic and endoscopic cooperative surgery is a feasible treatment procedure for intraluminal gastric gastrointestinal stromal tumors compared to endoscopic intragastric surgery. Surg Endosc 2017. [Epub ahead of print]. 10.1007/s00464-017-5683-x [DOI] [PubMed] [Google Scholar]
- 68.Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat 2016;48:1155-66. 10.4143/crt.2016.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. 10.1016/j.humpath.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 70.Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:566-78. 10.1093/annonc/mdi127 [DOI] [PubMed] [Google Scholar]
- 71.Wu CR, Huang LY, Guo J, et al. Clinical Control Study of Endoscopic Full-thickness Resection and Laparoscopic Surgery in the Treatment of Gastric Tumors Arising from the Muscularis Propria. Chin Med J (Engl) 2015;128:1455-9. 10.4103/0366-6999.157651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang LY, Cui J, Wu CR, et al. Endoscopic full-thickness resection and laparoscopic surgery for treatment of gastric stromal tumors. World J Gastroenterol 2014;20:8253-9. 10.3748/wjg.v20.i25.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong HY, Wang YL, Jia XY, et al. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc 2014;28:1447-53. 10.1007/s00464-013-3375-8 [DOI] [PubMed] [Google Scholar]
- 74.Yılmaz B, Unlu O, Roach EC, et al. Endoscopic clips for the closure of acute iatrogenic perforations: Where do we stand? Dig Endosc 2015;27:641-8. 10.1111/den.12482 [DOI] [PubMed] [Google Scholar]
- 75.Akimoto T, Goto O, Nishizawa T, et al. Endoscopic closure after intraluminal surgery. Dig Endosc 2017;29:547-58. 10.1111/den.12839 [DOI] [PubMed] [Google Scholar]
- 76.Nabeshima K, Tomioku M, Nakamura K, et al. Combination of Laparoscopic and Endoscopic Approaches to Neoplasia with Non-exposure Technique (CLEAN-NET) for GIST with Ulceration. Tokai J Exp Clin Med 2015;40:115-9. [PubMed] [Google Scholar]
- 77.Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17:594-9. 10.1007/s10120-013-0291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim DW, Kim JS, Kim BW, et al. Non-Exposed Endoscopic Wall-Inversion Surgery for Gastrointestinal Stromal Tumor of the Stomach: First Case Report in Korea. Clin Endosc 2016;49:475-8. 10.5946/ce.2016.002 [DOI] [PMC free article] [PubMed] [Google Scholar]