Abstract
A 37‐year‐old Japanese man experienced fever and headache 8 days after returning to Japan following a 6‐month stay in Nigeria. He visited two clinics but was sent home from each with a diagnosis of common cold. He was eventually brought to the emergency department with an altered mental status. Severe P. falciparum malaria was confirmed; his initial parasitemia index was 5.4%. He recovered fully with antimalarial treatment. This case suggests that primary care physicians should obtain recent travel history and consider malaria for any febrile patient who has returned from a malaria‐endemic area.
Keywords: imported malaria, incubation period, Plasmodium falciparum
1. INTRODUCTION
Malaria should be considered and excluded for travelers returning with fever from malaria‐endemic areas. Even though the number of Japanese travelers abroad has been dramatically increasing, the annual number of imported malaria cases has been decreasing.1 As a result, the opportunity to encounter patients with malaria is very limited, particularly in primary care settings. However, the misdiagnosis of malaria can lead to a fatal outcome. Here, we report on a 37‐year‐old Japanese man returning from Nigeria who was later diagnosed with Plasmodium falciparum malaria after being misdiagnosed with common cold in two clinics.
2. CASE REPORT
The patient was a 37‐year‐old Japanese man without a significant medical history. Two weeks prior to admission, he had returned to Japan from Nigeria after a 6‐month business visit. He had taken no chemoprophylaxis for malaria. He had not used any repellent and was sometimes bitten by mosquitoes. Six days prior to admission, he experienced general malaise, fever, and headache. Five days prior to admission, he visited a clinic nearby and was diagnosed with common cold. Loxoprofen and cefaclor were prescribed. Three days prior to admission, he visited another clinic and was again diagnosed with common cold. His symptoms had not improved, and his mental state appeared altered. He was transferred to the Japanese Red Cross Narita Hospital by ambulance. On admission, his mental status was E4V4M6. His vital signs were as follows: blood pressure of 138/87 mm Hg, heart rate of 126 beats/min, body temperature of 38.5°C, respiratory rate of 28 breaths/min, and oxygen saturation on room air of 98%. His physical examination results revealed slight jaundice on the conjunctiva. Laboratory test results revealed anemia with a hemoglobin level of 12.5 g/dL, thrombocytopenia with a platelet count of 17 000 /μL, high bilirubinemia with a total bilirubin level of 5.1 mg/dL, and elevated inflammatory markers, with elevated C‐reactive protein levels of 23.14 mg/dL. The examination of his peripheral blood smear showed P. falciparum‐infected erythrocytes with a parasitemia index of 5.4% (Figure 1). A rapid immunographic test (Binax NOW Malaria Test; Binax, Portland, OR) result for P. falciparum was positive. P. falciparum infection was later confirmed by performing polymerase chain reaction. We administered intravenous quinine. After an initial increase in his parasitemia index to 8.6%, it decreased to <0.1% on hospital day 3. Intravenous quinine was replaced with artemether/lumefantrine and was continued for 3 days. He recovered fully and was discharged on hospital day 10.
Figure 1.
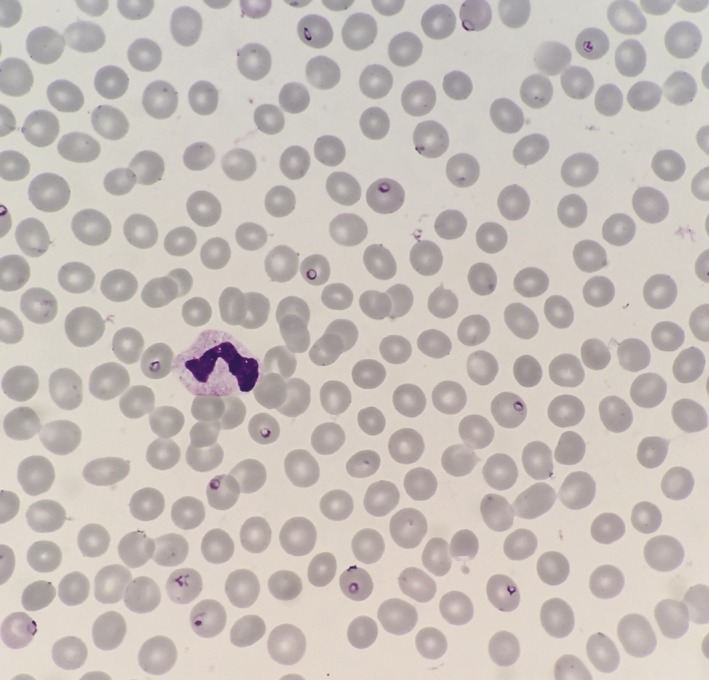
Microscopic image of thin films showing Plasmodium falciparum parasitemia index of 5.4%
3. DISCUSSION
Malaria should be ruled out in all travelers who are returning from malaria‐endemic areas.2, 3 This approach is crucial because malaria, particularly P. falciparum malaria, results in a fatal outcome unless diagnosed and treated immediately. The fatality rate of P. falciparum malaria cases was reported to be 0.73% in the UK compared with 0.05% for nonfalciparum malaria.4 The area with the highest risk of P. falciparum malaria is sub‐Saharan Africa, followed by parts of Oceania.5 The typical incubation period of P. falciparum malaria is 9–14 days.6 Although the use of chemoprophylaxis may prolong the incubation period, >90% of reported cases of P. falciparum malaria manifest within 1 month of return.7 Therefore, any febrile traveler who has returned from sub‐Saharan Africa within a month should be suspected of having P. falciparum malaria. In the present case, the patient started to have fever 7 days after he had returned to Japan from Nigeria. Based on the incubation period, malaria should have been suspected, instead of diagnosing common cold.
Obtaining a recent travel history and determining malaria‐endemic areas are key clinical strategies for suspecting malaria. Fever is the most common symptom of patients with malaria, and other symptoms include chills, malaise, headaches, myalgias, coughing, and gastrointestinal symptoms.8 Because these symptoms are not specific for malaria, patients with malaria tend to be misdiagnosed with common cold unless their travel history has been carefully obtained and malaria has been considered in the differential diagnosis. In the present case, the patient himself informed the doctors that he had recently returned from Nigeria; however, they did not come up with malaria as the differential diagnosis based on his travel history, which suggests that physicians should not only ask the recent travel history but also understand malaria‐endemic areas.
The present case also suggests us that even primary care physicians in Japan need to know when to suspect malaria and seek support from a specialist in infectious diseases, even though they rarely encounter patients with imported malaria. The diagnosis of malaria in nonendemic countries is difficult because patients frequently present to healthcare facilities without tropical medicine expertise or expert diagnostic capabilities. Kain et al. have reported that 59% of malaria cases are initially misdiagnosed in nonendemic North American settings.9 In Japan, the annual number of malaria cases has recently been reported as approximately 50 cases, and P. falciparum malaria accounts for more than half of them (Figure 2). As a consequence, primary care physicians in Japan encounter patients with malaria only rarely. However, the diagnostic delay leads to a fatal outcome. Whitty et al. reported that the odds of dying from malaria were much higher in those presenting in regions of the UK where fewer cases of malaria were seen. They supposed that in regions where travel to malaria‐ridden areas was less common, doctors seeing febrile returning travelers may be less aware of the risk of malaria.4
Figure 2.
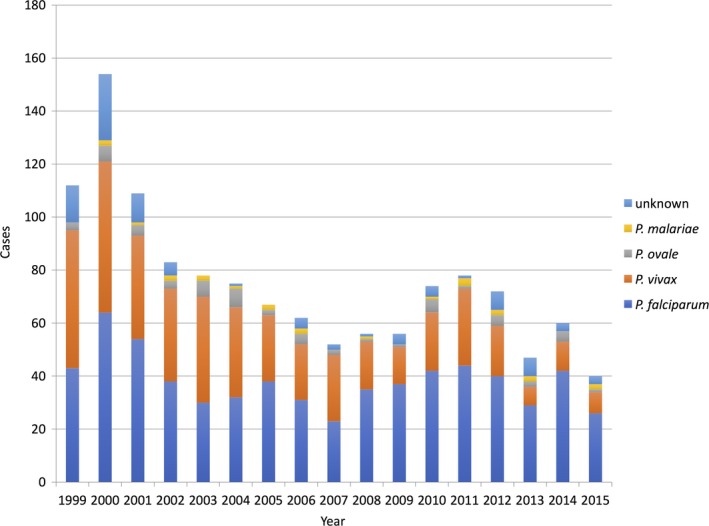
Number of notified malaria cases in Japan. All data on this figure are adapted from the National Epidemiological Surveillance of Infectious Diseases Annual Surveillance Data at the National Institute of Infectious Diseases.10 P. malariae: Plasmodium malariae; P. ovale: Plasmodium ovale; P. vivax: Plasmodium vivax; P. falciparum: Plasmodium falciparum
The best diagnostic strategy for malaria depends on the local endemic situation and the availability of the test in the facility. Microscopic examination remains the gold standard for diagnosis; however, it requires adequate training and experience.11 Antigen‐based rapid diagnostic test may be another useful option in settings with limited laboratory facilities;12 however, it remains unlicensed in Japan and is only available in some hospitals as a part of clinical study. Polymerase chain reaction has high sensitivity for detecting small number of parasites;13 however, its availability is usually limited in research laboratories. In Japanese primary care settings, any febrile patient with a recent travel history to a malaria‐endemic area should be referred to a hospital that has an experience of diagnosing and treating imported tropical diseases, unless the primary care facility is capable of accurately ruling out malaria.
In conclusion, we encountered the case of a patient with P. falciparum malaria who had been misdiagnosed as having common cold in two clinics. Although patients with malaria rarely visit clinics in Japan, primary care physicians should obtain recent travel history and consider malaria as a differential diagnosis for any febrile patient who has returned from malaria‐endemic areas.
CONFLICT OF INTEREST
The author has stated explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGEMENT
I would like to thank Dr. Shigeyuki Kano, Department of Tropical Medicine and Malaria, Research Institute, National Center for Global Health and Medicine for performing the diagnostic PCR test.
Hase R. Diagnostic delay for imported malaria: A case of Plasmodium falciparum malaria misdiagnosed as common cold. J Gen Fam Med. 2018;19:27–29. https://doi.org/10.1002/jgf2.149
REFERENCES
- 1. NIID . Reported cases of malaria infections based on NESID, 2006‐first half of 2014. Infect Agents Surveill Rep 2014;35:224–326. [Google Scholar]
- 2. Lo Re V III, Gluckman SJ. Fever in the returned traveler. Am Fam Physician. 2003;68:1343–50. [PubMed] [Google Scholar]
- 3. Thwaites GE, Day NP. Approach to fever in the returning traveler. N Engl J Med. 2017;376:548–60. [DOI] [PubMed] [Google Scholar]
- 4. Checkley AM, Smith A, Smith V, et al. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ. 2012;344:e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freedman DO. Clinical practice. Malaria prevention in short‐term travelers. N Engl J Med. 2008;359:603–12. [DOI] [PubMed] [Google Scholar]
- 6. Rietveld AEC, Newman RD. Malaria In: Control of communicable diseases manual, 20th edn Heymann David L. (ed). DC: APHA Press; 2015. [Google Scholar]
- 7. Eliades MJ, Shah S, Nguyen‐Dinh P, et al. Malaria surveillance–United States, 2003. MMWR Surveill Summ. 2005;54:25–40. [PubMed] [Google Scholar]
- 8. Suh KN, Kain KC, Keystone JS. Malaria. CMAJ. 2004;170:1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kain KC, Harrington MA, Tennyson S, Keystone JS. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27:142–9. [DOI] [PubMed] [Google Scholar]
- 10. National Institute of Infectious Diseases . National Epidemiological Surveillance of Infectious Disease Annual Surveillance Data (Notifiable Diseases, Category IV) 2016 [Internet] [cited 2017, August 9]. Available from https://www.niid.go.jp/niid/ja/survei/2085-idwr/ydata/6561-report-ja2015-20.html
- 11. Wilson ML. Laboratory diagnosis of malaria: conventional and rapid diagnostic methods. Arch Pathol Lab Med. 2013;137:805–11. [DOI] [PubMed] [Google Scholar]
- 12. Stauffer WM, Cartwright CP, Olson DA, et al. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clin Infect Dis. 2009;49:908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cordray MS, Richards‐Kortum RR. Emerging nucleic acid‐based tests for point‐of‐care detection of malaria. Am J Trop Med Hyg. 2012;87:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


