Abstract
The obligate intracellular Gram-negative bacterium Chlamydia psittaci often causes avian chlamydiosis and influenza-like symptoms in humans. However, the commercial subunit C. psittaci vaccine could only provide a partial protection against avian chlamydiosis due to poor cellular immune response. In our previous study, a recombinant herpesvirus of turkeys (HVT)-delivered vaccine against C. psittaci and Marek’s disease based on human cytomegalovirus (CMV) promoter (rHVT-CMV-pmpD) was developed and provided an effective protection against C. psittaci disease with less lesions and reduced chlamydial loads. In this study, we developed another recombinant HVT vaccine expressing the N-terminal fragment of PmpD (PmpD-N) based on human elongation factor-1 alpha (EF-1α) promoter (rHVT-EF-pmpD) by modifying the HVT genome within a bacterial artificial chromosome. The related characterization of rHVT-EF-pmpD was evaluated in vitro in comparison with that of rHVT-CMV-pmpD. The expression of PmpD-N was determined by western blot. Under immunofluorescence microscopy, PmpD-N protein of both two recombinant viruses was located in the cytoplasm and on the cell surface. Growth kinetics of rHVT-EF-pmpD was comparable to that of rHVT-CMV-pmpD, and the growth rate of rHVT-EF-pmpD was apparently higher than that of rHVT-CMV-pmpD on 48, 72, and 120 h postinfection. Macrophages activated by rHVT-EF-pmpD could produce more nitric oxide and IL-6 than that activated by rHVT-CMV-pmpD. In this study, a recombinant HVT vaccine expressing PmpD-N based on EF-1α promoter was constructed successfully, and a further research in vivo was needed to analyze the vaccine efficacy.
Keywords: Chlamydia psittaci, Marek’s disease, herpesvirus of turkeys, PmpD-N, elongation factor-1 alpha promoter
Introduction
Chlamydia psittaci is an emerging zoonotic pathogen of global significance, which can cause avian chlamydiosis (1), and infection in human with pneumonia, encephalitis, endocarditis, and even death (2). Study at home, abroad and in our laboratory had indicated that the seropositivity rate of C. psittaci was extremely high in poultry flocks (3–6). Currently, no efficacious commercial C. psittaci vaccine is available because the cellular and humoral immunity are both necessary to protect animals from this obligate intracellular bacterial infection (7).
The major outer membrane protein (MOMP) and the autotransported polymorphic membrane protein D (PmpD) of C. psittaci are proved to be good vaccine candidates (8–10). PmpD has more merit than MOMP because PmpD is conserved and can elicit early immune-mediated neutralization of an ongoing chlamydial infection (11, 12). The specific neutralizing antibody triggered by N-terminal fragment of PmpD (PmpD-N) may provide humoral immune protection against early infection (12).
A herpesvirus of turkeys (HVT) vector-based vaccine can deliver antigens to the surface of cells, and further effectively stimulate cellular immunity and humoral immunity (13). This characteristic is very useful for the development of an effective vaccine for the prevention and control of Chlamydia infection (14). Furthermore, a great many of HVT-based recombinant vaccines expressing protective antigens of avian pathogens, such as Newcastle disease virus, infectious bursal disease virus, and highly pathogenic avian influenza, were constructed, and excellent and long-term protective effect in chickens against both pathogens were proved (15–18).
In our previous study, a recombinant HVT expressing PmpD-N based on cytomegalovirus (CMV) promoter (rHVT-CMV-pmpD) was constructed to successfully elicit an exceptional cellular and humoral immunity, and finally proved to confer a partial protection in chickens against C. psittaci challenge infection (10). In this study, we developed another recombinant HVT vaccine expressing PmpD-N based on elongation factor-1 alpha (EF-1α) promoter (rHVT-EF-pmpD), and its morphological and immunological characterization was analyzed in comparison with rHVT-CMV-pmpD in vitro.
Materials and Methods
Chicken Cells and C. psittaci Strain
Primary chicken embryo fibroblast (CEF) cells were prepared from 10-day-old specific-pathogen-free embryos (Vital Merial Experimental Animal Co., Ltd., Beijing, China) (15). The HD11 chicken macrophage cell line was maintained in RPMI-1640 medium with 10% FBS (v/v). The DNA of C. psittaci strain CB7 was extracted and stored in our lad as reported previously (10).
Generation of Recombinant HVT Expressing pmpD-N Gene Based on EF-1α Promoter
The recombinant HVT was generated as described previously (10). The recombinant HVT bacterial artificial chromosome (BAC) was based on EF-1α promoter, which was from the vector of pEF6/V5-His (Invitrogen, Carlsbad, CA, USA). A pair of primers used to amplify the expression cassette with the EF-1α promoter at the 5′ end and the BGH polyadenylation (poly A) site at the 3′ end was designed using Oligo 7 (Molecular Biology Insights, USA). The forward primer was 5′-GGTTAATTAACCTTCTAGGTCTTGAAAGGAGTGGGA-3′, and the reverse primer was 5′-GGTTAATTAAGCCTCAGAAGCCATAGAGCCCACC-3′. Both primers contained a PacI restriction site at their 5′ termini. The recombinant virus was designated as rHVT-EF-pmpD.
Plaque Assays and One-Step Growth Kinetics
The plaque size, morphology, and plaque-forming unit (PFU) of rHVT-EF-pmpD were compared with those of the same passage of rHVT-CMV-pmpD by the immunohistochemical assay as recorded previously (15). Briefly, recombinant viruses were 10-fold diluted and inoculated into the CEF cells, which were seeded in six-well plates. Four days later, ice-clod acetone was added into each well to fix the cells. Cells were washed by phosphate-buffered saline (PBS) and then blocked by blocking buffer (PBS containing 0.1% BSA). Cells were incubated with the wild type HVT-specific polyclonal antibodies raised in chickens (diluted 1:100), and subsequently reacted with horseradish peroxidase-labeled goat-anti-chicken IgG (1:4,000) (Sigma-Aldrich, Shanghai, China). Cells containing the conjugated antibody were identified by incubation at 37°C for 1 h in developing solution containing 1% (w/v) 3-amino-9-ethylcarbazole (Sigma) and 0.02% (v/v) H2O2 in 0.1 M sodium acetate (pH 4.8). Plaques were counted using an inverted microscope. Moreover, the growth rates of the recombinant viruses were studied on CEF cells by calculating the virus plaques at 12, 24, 48, 72, 96, and 120 h postinfection (16).
Identification of the Recombinant Viruses Using Immunoblot and Immunofluorescence
Immunoblot analysis was carried out as described previously (10). Briefly, 3,000 PFU of rHVT-CMV-pmpD, rHVT-EF-pmpD, and parental HVT were used to infect CEF cells seeded in T25 flasks. The cells were washed twice with PBS when the cytopathic effect occurred. Lysates of cells were subjected to 12% SDS-PAGE and electroblotted to polyvinylidene fluoride membranes, followed by 0.25% trypsin for 2 min. Membranes were incubated with the C. psittaci strain 6BC-specific polyclonal antibodies (diluted 1:100) previously prepared by our own lab, and then reacted with horseradish peroxidase-labeled goat-anti-chicken IgG (1:4,000) (Sigma-Aldrich, Shanghai, China). The PmpD-N glycoprotein bands were visualized using the enhanced DAB reagents (Tiangen, Beijing, China) according to the manufacturer’s instructions.
The expression and distribution of PmpD-N were determined in rHVT-CMV-pmpD-infected cells and rHVT-EF-pmpD-infected cells by immunofluorescence. First, CEF cells were infected with 3,000 PFU rHVT-CMV-pmpD and rHVT-EF-pmpD, respectively. The cells were probed with mouse anti-PmpD-N polyclonal serum of C. psittaci (previously prepared in our lab) and anti-HVT polyclonal serum (IVDC, Beijing, China) at a dilution of 1:100, and then overlaid with a mixture of goat anti-mouse IgG labeled with Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) and goat anti-chicken IgY labeled with Alexa Fluor 568 (1:600 dilution) (Invitrogen, Carlsbad, CA, USA). Moreover, cell nuclei were stained with 1:10,000 dilution of 4,6-diamidino-2-phenylindole (DAPI) for 1 min or longer. Finally, the rinsed cover slips were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and examined using a confocal microscope (Nikon, Tokyo, Japan).
Measurement of Nitric Oxide (NO) and IL-6
HD11 cells were infected by 400 PFU rHVT-CMV-pmpD and rHVT-EF-pmpD, respectively. Cell culture supernatants were collected on day 1, 2, and 4 postinfection. OD values of supernatants were measured at 540 nm. NO production by activated HD11 cells was assessed as nitrite content in conditioned media using Griess reagent as illustrated previously (19). Sodium nitrite was used as the standard.
The infected cells treated above were also collected on day 1, 2, and 4 after inoculation. The cells were washed twice with PBS. Total RNA of cells on different day’s postinfection was extracted. The quantity of IL-6 was measured by relative quantitative real time RT-PCR, and the internal control gene was GAPDH as described previously (20).
Statistical Analysis
Data were analyzed using the one-way ANOVA. A P-value of less than 0.05 was considered statistically significant.
Results
Generation of Recombinant HVT Expressing pmpD-N Gene Based on EF-1α Promoter
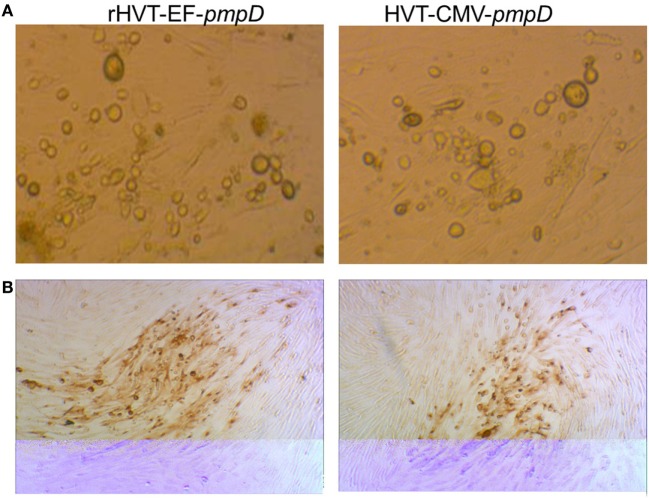
The pmpD-N gene with EF-1α promoter was successfully inserted into the HVT BAC region between UL45 and UL46. The recombinant HVT BAC DNA based on EF-1α promoter was transfected into CEF cells. Four days after transfection, typical plaques appeared in the CEF monolayers, these plaques being similar in size and morphology compared with those formed by rHVT-CMV-pmpD (Figure 1). The viral titration by staining plaques revealed no significant difference between rHVT-EF-pmpD and rHVT-CMV-pmpD.
Figure 1.
Cytopathic effect of rHVT-EF-pmpD and rHVT-CMV-pmpD on chicken embryo fibroblast (CEF) cells. (A) Morphology of the infected CEF cells induced by rHVT-EF-pmpD or rHVT-CMV-pmpD (magnifications 100×). (B) Immunohistochemical staining of CEF cells post inoculation with rHVT-EF-pmpD or rHVT-CMV-pmpD (magnifications 100×).
Expression of PmpD-N in rHVT-EF-pmpD Infected Cells
To confirm the expression of the PmpD-N protein, CEF cells infected with rHVT-EF-pmpD, rHVT-CMV-pmpD, and parental HVT were analyzed by immunoblot and immunofluorescence. A 43-kDa band corresponding to the PmpD-N polypeptide was identified with polyclonal antibodies generated against C. psittaci 6BC strain by immunoblot analysis (Figure 2A). Under immunofluorescence microscopy, PmpD-N protein of rHVT-EF-pmpD and rHVT-CMV-pmpD was both expressed in the cytoplasm and on the cell surface (Figures 2B,C).
Figure 2.
Confirmation of PmpD-N protein expression in herpesvirus of turkeys (HVT) vector by immunoblotting assay and indirect immunofluorescence. (A) The PmpD-N expression in rHVT-EF-pmpD was detected by immunoblot using Chlamydia psittaci strain 6BC-specific polyclonal antibodies. Lane M, pre-stained protein ladder; Lane 1, cell lysate post inoculation with rHVT-EF-pmpD; Lane 2, cell lysate post inoculation with rHVT-CMV-pmpD; Lane 3, cell lysate post inoculation with parental HVT. The black arrow indicates the approximate size of 43 kDa. (B) Indirect immunofluorescence analysis of PmpD-N expression in chicken embryo fibroblast (CEF) cells. CEF cells on glass coverslips were infected with rHVT-EF-pmpD, then incubated with mouse anti-PmpD-N polyclonal antibody of C. psittaci and chicken anti-HVT polyclonal serum, and then subsequently reacted with the goat anti-mouse IgG conjugated with Alexa Fluor 488 (green fluorescence, shown in the lower left panel) and goat anti-chicken IgY labeled with Alexa Fluor 568 (red fluorescence, shown in the lower right panel), respectively. Finally, cell nuclei were stained with diamidino-2-phenylindole (blue fluorescence, shown in the top right panel). The merged image is shown in the top left panel. The expression of the targeted protein is indicated by white arrows in the top left panel. (C) rHVT-CMV-pmpD control. CEF cells on glass coverslips were infected with rHVT-CMV-pmpD, and then the process of test and the panel meaning are the same as those shown in panel (B).
One-Step Growth Kinetics
Growth trends of both recombinant viruses were similar as revealed by calculating the virus plaques at various times postinfection (Figure 3). Growth rates displayed a significant increase between 24 and 96 h, and a sharp decrease from 96 to 120 h. Except for 96 h postinfection, the growth rate of the rHVT-EF-pmpD was consistently higher than that of rHVT-CMV-pmpD, with a consequential difference appearing on 48, 72, and 120 h postinfection.
Figure 3.
One-step growth kinetics of rHVT-EF-pmpD and rHVT-CMV-pmpD in vitro. The virus growth was calculated as the fold increase at different time points compared with the 12th hour postinfection. The asterisk indicates significant differences of the growth kinetics between rHVT-EF-pmpD and rHVT-CMV-pmpD (P < 0.05). The values were shown as means ± SD.
Measurement of NO and IL-6
The quantity of NO and IL-6 on HD11 cells infected by rHVT-CMV-pmpD and rHVT-EF-pmpD was assayed on days 1, 2, and 4. The NO production of rHVT-EF-pmpD was significantly higher than those of rHVT-CMV-pmpD on day 2 and day 4 (P < 0.05) (Figure 4). The IL-6 level of rHVT-EF-pmpD was apparently higher than that of rHVT-CMV-pmpD on day 4 (Figure 5).
Figure 4.
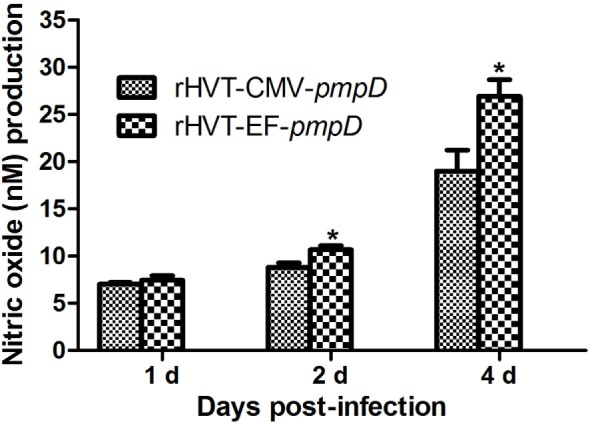
Nitric oxide (NO) responses of HD11 cells to rHVT-EF-pmpD. HD11 cells were subjected to 400 plaque-forming unit recombinant viruses for 1, 2, and 4 days. The supernatants were collected for NO analysis. Data were expressed as mean ± SE (n = 3) and were analyzed by Student’s t-test. Significance (*) was considered as P < 0.05 when compared with rHVT-CMV-pmpD.
Figure 5.
IL-6 responses of HD11 cells to rHVT-EF-pmpD. HD11 cells were subjected to 400 plaque-forming unit recombinant viruses for 1, 2, and 4 days. The cells were collected for IL-6 analysis using relative quantitative real time RT-PCR. The IL-6 increase fold of the recombinant viruses relative to chicken embryo fibroblast (CEF) cells was analyzed. Data were expressed as mean ± SE (n = 3) and were analyzed by Student’s t-test. Significance (*) was considered as P < 0.05 when compared with rHVT-CMV-pmpD.
Discussion
Chlamydia psittaci, an obligate intracellular Gram-negative bacterium, often causes avian chlamydiosis and influenza-like symptoms in humans. At present, five serovars of C. psittaci have been found in chickens, which are genotypes B, C, D, F, and E/B (3, 21–23). The development of an efficacious vaccine against C. psittaci is needed as a high seropositivity rate was found in chickens (3–6). In this study, a recombinant HVT vaccine expressing PmpD-N based on EF-1α promoter was developed and was proved to be more efficient than that of CMV-based promoter.
To stimulate better immune protection, the constructed recombinant virus can deliver the target protein to the surface of the cell. Confocal analysis showed that the HVT vector could deliver PmpD-N protein to the surface of the cell in the rHVT-EF-pmpD, same as rHVT-CMV-pmpD. Plaque assay further showed that compared with rHVT-CMV-pmpD, the rHVT-EF-pmpD did not change the lesion status, plaque size and proliferation rate.
Macrophage, which belongs to phagocyte, participates in innate and cellular immunity in the body of animals. Macrophages display remarkable plasticity and can change their physiology in response to environmental cues. These changes can give rise to different populations of cells with distinct functions. Macrophages play an important role in monitoring, phagocytosis, uptake of antigens, secretion of various proinflammatory cytokines, and control and elimination of infections (24). In birds, macrophages play a central role in resistance to microbial infections and the pathogenesis of viruses, bacteria, and parasitic infections. Research shows that avian macrophages exposed to pathogens can be activated to produce proinflammatory cytokines, chemokines, reactive oxygen species (ROS), and NO, while ROS and NO are considered as antimicrobial arsenal that can control and remove pathogens. The HD11 cell line, which is obtained from avian macrophage like cell lines transformed by the avian encephalomyelitis virus, has the ability of phagocytosis and expressing Fc receptors and macrophage surface antigens (25). HD11 cells, as chicken macrophages, are widely used in the study of immune function in vitro. In this study, HD11 cells were used to analyze the macrophage that was impacted by the recombinant virus rHVT-EF-pmpD and rHVT-CMV-pmpD, respectively. Our results have shown that rHVT-EF-pmpD can significantly stimulate the macrophage to produce more proinflammatory cytokines IL-6 than rHVT-CMV-pmpD from day 2, and significantly stimulate the macrophage to produce more NO than rHVT-CMV-pmpD from day 4. Furthermore, the rHVT-EF-pmpD can stimulate the macrophage to kill more pathogens and promote more immune response than rHVT-CMV-pmpD.
In summary, the recombinant HVT expressing PmpD-N based on EF-1α promoter is more effective than our former constructed HVT based on CMV promoter in vitro. Further study is needed to analyze whether the rHVT-EF-pmpD could produce better protective immune response than rHVT-CMV-pmpD in vivo.
Author Contributions
SL and CH designed the study. SL, WS, and XH performed experiments. SL, WZ, CJ, JL, and YS collected test data. SL and WS performed the date analysis and drafted the manuscript. CH and SE-A revised the manuscript. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by Tongren science and technology project (Grant No. copper science research [2016] 17-5), National major innovation ability construction project of emerging industries in the west area (Grant No. Fagaibangaoji [2016] 2195), the National Natural Science Foundation of China (Grant No. 31172305 and 31372420), Ministry of Science and Technology (MoST) (Grant No. 2012BAD31B05), and Beijing Natural Science Foundation (Grant No. 6122018).
References
- 1.Friis RR. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol (1972) 110(2):706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou J, Qiu C, Cao XA, Lin G. Construction and immunogenicity of recombinant adenovirus expressing the major outer membrane protein (MOMP) of Chlamydophila psittaci in chicks. Vaccine (2007) 25(34):6367–72. 10.1016/j.vaccine.2007.06.031 [DOI] [PubMed] [Google Scholar]
- 3.Yin L, Kalmar ID, Lagae S, Vandendriessche S, Vanderhaeghen W, Butaye P, et al. Emerging Chlamydia psittaci infections in the chicken industry and pathology of Chlamydia psittaci genotype B and D strains in specific pathogen free chickens. Vet Microbiol (2013) 162:740–9. 10.1016/j.vetmic.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Chu J, Zhang Q, Sun W, Zhang T, He C. Development of a novel PmpD-N ELISA for Chlamydia psittaci infection. Biomed Environ Sci (2016) 29(5):315–22. 10.3967/bes2016.041 [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Wang H, Lei L. Epidemiological investigation of avian chlamydiosis in Tianjin area. Heilongjiang Anim Sci Vet Med (2016) 10:148–50. [Google Scholar]
- 6.Li J, Wang M, Liu X, Li Z, Li F. Investigation of infection of avian Chlamydia psittaci in some regions of Shandong province. Chin J Prev Vet Med (2015) 37(7):514–7. [Google Scholar]
- 7.Vanrompay D, Cox E, Volckaert G, Goddeeris B. Turkeys are protected from infection with Chlamydia psittaci by plasmid DNA vaccination against the major outer membrane protein. Clin Exp Immunol (1999) 118(1):49–55. 10.1046/j.1365-2249.1999.01024.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun (1981) 31(3):1161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatch TP, Vance DW, Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol (1981) 146(1):426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Sun W, Chu J, Huang X, Wu Z, Yan M, et al. Construction of recombinant HVT expressing PmpD, and immunological evaluation against Chlamydia psittaci and Marek’s disease virus. PLoS One (2015) 10(4):e0124992. 10.1371/journal.pone.0124992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia RC, Tan C, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci U S A (2006) 103(6):1894–9. 10.1073/pnas.0508983103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out – processing of the chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Mol Microbiol (2004) 51(2):319–34. 10.1046/j.1365-2958.2003.03838.x [DOI] [PubMed] [Google Scholar]
- 13.Gerdts V, Mutwiri GK, Tikoo SK, Babiuk LA. Mucosal delivery of vaccines in domestic animals. Vet Res (2006) 37:487–510. 10.1051/vetres:2006012 [DOI] [PubMed] [Google Scholar]
- 14.Verminnen K, Loock MV, Cox E, Goddeeris BM, Vanrompay D. Protection of turkeys against Chlamydophila psittaci challenge by DNA and rMOMP vaccination and evaluation of the immunomodulating effect of 1 alpha,25-dihydroxyvitamin D(3). Vaccine (2005) 23:4509–16. 10.1016/j.vaccine.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Reddy K, Reid SM, Cox WJ, Brown IH, Britton P, et al. Recombinant herpesvirus of turkeys as a vector-based vaccine against highly pathogenic H7N1 avian influenza and Marek’s disease. Vaccine (2011) 29(46):8257–66. 10.1016/j.vaccine.2011.08.115 [DOI] [PubMed] [Google Scholar]
- 16.Gao H, Cui H, Cui X, Shi X, Zhao Y, Zhao X, et al. Expression of HA of HPAI H5N1 virus at US2 gene insertion site of turkey herpesvirus induced better protection than that at US10 gene insertion site. PLoS One (2011) 6(7):e22549. 10.1371/journal.pone.0022549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palya V, Tatar-Kis T, Mato T, Felfoldi B, Kovacs E, Gardin Y. Onset and long-term duration of immunity provided by a single vaccination with a turkey herpesvirus vector ND vaccine in commercial layers. Vet Immunol Immunopathol (2014) 158(1–2):105–15. 10.1016/j.vetimm.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto K, Saito S, Saeki S, Sato T, Tanimura N, Isobe T, et al. Complete, long-lasting protection against lethal infectious bursal disease virus challenge by a single vaccination with an avian herpesvirus vector expressing VP2 antigens. J Virol (2002) 76(11):5637–45. 10.1128/JVI.76.11.5637-5645.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Raybourne RB, Babu US, Lillehoj HS, Heckert RA. CpG-induced immunomodulation and intracellular bacterial killing in a chicken macrophage cell line. Dev Comp Immunol (2003) 27(9):823–34. 10.1016/S0145-305X(03)00079-X [DOI] [PubMed] [Google Scholar]
- 20.Nang NT, Lee JS, Song BM, Kang YM, Kim HS, Seo SH. Induction of inflammatory cytokines and toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet Res (2011) 42:64. 10.1186/1297-9716-42-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaede W, Reckling KF, Dresenkamp B, Kenklies S, Schubert E, Noack U, et al. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health (2008) 55(4):184–8. 10.1111/j.1863-2378.2008.01108.x [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Li S, Yang J, Pang W, Yang L, He C. Isolation and characterization of Chlamydophila psittaci isolated from laying hens with cystic oviducts. Avian Dis (2008) 52(1):74–8. 10.1637/8019-051207-Reg [DOI] [PubMed] [Google Scholar]
- 23.Dickx V, Geens T, Deschuyffeleer T, Tyberghien L, Harkinezhad T, Beeckman DS, et al. Chlamydophila psittaci zoonotic risk assessment in a chicken and turkey slaughterhouse. J Clin Microbiol (2010) 48(9):3244–50. 10.1128/JCM.00698-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He H, Genovese KJ, Kogut MH. Modulation of chicken macrophage effector function by T(H)1/T(H)2 cytokines. Cytokine (2011) 53(3):363–9. 10.1016/j.cyto.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 25.Beug H, Kirchbach A, Doderlein G, Conscience JF, Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell (1979) 18(2):375–90. 10.1016/0092-8674(79)90057-6 [DOI] [PubMed] [Google Scholar]