Main Text
Oncolytic viruses (OVs) are a class of anti-cancer agents that mediate therapeutic effects through some combination of direct infection of tumor cells and tumor lysis as well as through enhancement of tumor-specific immune responses. Multiple mechanisms, both inherent to tumor cells and the tumor immune microenvironment, put constraints on the efficacy of OV therapy. In this issue of Molecular Therapy, Selman and colleagues1 explore combinatorial therapy of OVs with vanadium compounds, a group of non-specific protein phosphatase inhibitors, as a strategy to overcome these limitations. They demonstrate that, within the context of OV therapy, vanadate compounds exert pleiotropic effects, which, in the case of RNA viruses, work by independently enhancing replication and OV-mediated tumor lysis and potentiating T cell-mediated immunity (Figure 1). Notably, the same compounds led to profound inhibition of the replication of some of the known oncolytic DNA viruses. These findings add a new agent to the armamentarium of combinatorial treatment strategies using oncolytic RNA viruses but also highlight the complexity of the interactions of OVs with the different arms of the immune system and caution against the extrapolation of findings with one OV to the broad category of OV therapeutics class as a whole.
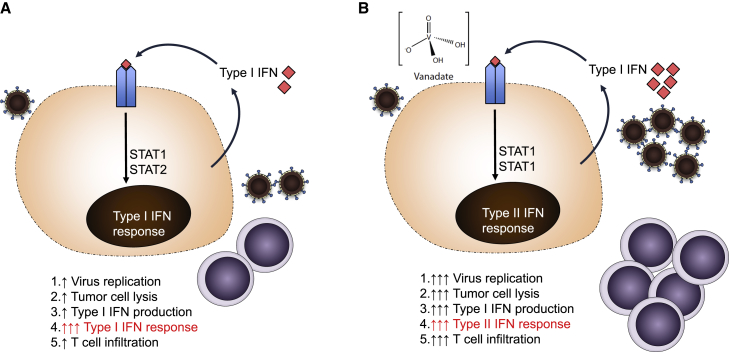
Figure 1.
Pleiotropic Effects of Vanadium Compounds on Oncolytic Immunotherapy
(A) Infection with an RNA oncolytic virus leads to virus replication with resultant production of type I IFN, which acts in an autocrine and paracrine fashion to limit viral replication and spread. This leads to limited viral replication, direct tumor cell lysis, and T cell-mediated immunity in vivo. (B) Vanadium compounds in the setting of infection with an RNA oncolytic virus re-wire intracellular signaling in response to type I IFN to induce transcriptional activation consistent with type II IFN response and result in increased virus replication, tumor cell lysis, and T cell recruitment to tumors.
With enhanced understanding of the cellular processes governing the interaction of OVs with cancer cells and the immune system, recent years have seen a flurry of activity in the field. The notion of converting “cold” tumors to “hot” has become colloquial in all oncology fields, with OVs serving as the best examples of the therapeutic potential of this principle. As an example of the latter, T-Vec, an oncolytic herpes simplex virus (HSV) expressing granulocyte-macrophage colony-stimulating factor (GM-CSF), was approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) based on its local and systemic efficacy seen following intratumoral delivery for advanced melanoma.2 Recent studies have demonstrated additive or even synergistic activity of this agent in trials in advanced melanoma when used in combination with systemic CTLA-4 or PD-1 blockade.3, 4
Multiple mechanisms can drive resistance to OV therapy. While the cardinal constraints to therapy with OVs have been primarily based on the challenges posed by systemic administration, there are multiple processes that block viruses from exerting their effects even after they make it to the tumor. First of all, resistance of cancer cells to OV infection may prevent adequate viral replication to generate sufficient lysis and induction of tumor-specific immunity. Second, OV-induced anti-tumor immune responses are a result of multiple processes driven by virus replication and tumor lysis, innate immune responses, and anti-viral and anti-tumor adaptive immune responses. For example, while type I interferon (IFN), induced by many viruses, can promote dendritic cell maturation and antigen presentation, it can also limit viral replication and tumor lysis. On the other hand, robust virus replication might not be necessary for some viruses and, in fact, excessive viral replication or expression of particular therapeutic transgenes carries the potential to impact generation of tumor-specific, as opposed to virus-specific, immune responses.5, 6, 7, 8, 9
Thus, there is a strong rationale for exploring rational combinations of immunomodulatory compounds and antibodies with OV therapy. In their very detailed study, Selman and colleagues1 go outside the box of the usual immunomodulatory targets and instead explore combinatorial therapy between OV and vanadium compounds, a rather unfamiliar class of agents to the majority of those working in the cancer field. Vanadium is a transitional element, the discovery of which, in the early 1800s, is credited to the Spanish-Mexican scientist Andrés Manuel del Río and the Swedish chemist Nils Gabriel Sefström, who named the new element after the Norse goddess of love, beauty, and fertility, Vanadís. Since then, the use of vanadium and vanadium compounds has been widely implemented in metallurgy but has been also explored as a dietary supplement. The first reports of the use of vanadium compounds appeared late in the 1800s in patients with diabetes, with additional early trials conducted in the early 2000s.10 Studies have demonstrated the ability of vanadium-based compounds to nonspecifically block protein phosphatases, presumably secondary to their ability to act as phosphate analogs.11, 12 Due to involvement of tyrosine phosphorylation in multiple innate and adaptive immune pathways, the molecules have been shown to impact the immune system.13
Given the latter, the authors explored the interaction between vanadate compounds and OVs, using vesicular stomatitis virus (VSV) as a model. The authors show that the mechanisms of action of vanadate in improvement of OV efficacy are pleiotropic. First of all, it promotes multicycle virus replication in cancer cells, but not normal cells. Second, even in the absence of viral replication, vanadate could potentiate cell killing, an effect that is likely mediated through activation of type I IFN and reactive oxygen species-mediated activation of apoptosis. Third, in immunocompetent tumor models, vanadate augmented VSV-induced T cell infiltration, with the degree of T cell infiltration directly correlating with anti-tumor activity, but not viral replication, although viral replication was still required for anti-tumor efficacy. The combinatorial effect was completely abrogated in nude mice, suggesting that vanadate-mediated potentiation of VSV activity in vivo is primarily mediated by its enhancement of T cell-mediated immunity. Interestingly, the authors explored several additional transition metal salts and found the effects to be specific to vanadium complexes.
The authors next demonstrate that, in cancer cells, vanadium alters immune signaling pathways, leading to increased production of type I IFN, but reduced responses to type I IFN stimulation and subsequently decreased expression of type I IFN-inducible genes. Interestingly, they observe enhanced expression of genes typically associated with IFN-γ signaling and demonstrate the predominant activation of STAT1, rather than both STAT1 and STAT2, in response to type I IFN stimulation in the presence of vanadate, suggesting that vanadium compounds may act by “converting” a type I IFN response into a type II IFN response. Overall, at least in cancer cells, vanadium appears to alter responses to virus infection to promote T cell immunity, while limiting the negative impact of innate immune responses on viral replication (Figure 1). Of note, vanadium compounds did not appear to augment the effect of VSV in normal cells, which is certainly an encouraging sign that application of these agents might not alter the safety of OVs.
With these findings, vanadium compounds could constitute a panacea for the resistance mechanisms to OV therapy and appear to promote all pathways that have been previously demonstrated to be necessary for successful cancer immunotherapy. One may even speculate that, with systemic OV strategies, vanadium could decrease the OV dose requirements so as to rely more on intratumoral viral spread. The findings certainly merit further exploration within the setting of OV therapy as well as other immunotherapeutic modalities, especially given the intriguing finding of vanadate-mediated IFN signaling “rewiring.”
However, the study also highlights the complexity of action of these agents, which warrants further exploration. While the authors demonstrate the alteration of transcriptional and signaling programs by vanadate in mouse and human cancer cells lines, one needs to explore the effects in vivo, particularly on its effects upon antigen-presenting cells and T cells within the context of OV therapy. Furthermore, while intratumoral administration of vanadium compounds may alter local virus replication and tumor lysis, systemic administration might yield different results. For example, inhibition of the response to type I IFN stimulation might inhibit antigen cross-presentation by CD8+ dendritic cells, which are dependent upon type I IFN receptor signaling,14, 15 and one would need to evaluate whether lack of response to type I IFN seen in cancer cells treated with vanadium is also observed in antigen-presenting cells (APCs). On the other hand, vanadium can exert stimulatory effects in T cells. For example, Src homology 2 domain-containing protein phosphatase 1 (SHP-1), a target of vanadium, leads to inhibition of antigen-dependent activation and proliferation in T cells, essentially acting as an immune checkpoint.16 Thus, systemic administration of vanadium compounds in combination with intratumoral or even systemic OVs might serve as an oncolytic vaccine and a systemic T cell immunomodulator. We need to understand potential toxicities that could be associated with systemic protein phosphatase inhibition, but early studies with vanadate compounds in diabetic patients do provide some comfort in that regard.10
Finally, we must exercise caution before extrapolating these findings to other OVs. In their study, the investigators clearly demonstrate that, while vanadium compounds substantially enhance the propagation of RNA viruses, they equally substantially inhibit replication of DNA viruses, such as vaccinia and HSV, possibly secondary to the dependence of these viruses on phosphatases in their replication cycle. It remains to be seen whether this effect upon DNA virus replication in vitro manifests itself in vivo, where the potentiation effect appears to be more dependent upon adaptive immunity rather than viral replication. This finding highlights the marked differences in biology of different OVs and cautions against lumping OVs together as a class of cancer therapeutics, when they clearly represent many different agents that are unique in their mechanisms of interaction with cancer cells and the immune system.
Acknowledgments
D.Z. received funding from the Department of Defense Ovarian Cancer Research Academy (OC150111) and is a member of the Parker Institute for Cancer Immunotherapy, which supports the MSKCC Cancer Immunotherapy Program.
References
- 1.Selman M., Rousso C., Tzelepis F. Multi-modal Potentiation of Oncolytic Virotherapy by Vanadium Compounds. Mol Ther. 2017;26:56–69. doi: 10.1016/j.ymthe.2017.10.014. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 3.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J., Hamid O., Ross M., Friedlander P., Garbe C. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 2017 doi: 10.1200/JCO.2017.73.7379. Published online October 5, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pol J.G., Zhang L., Bridle B.W., Stephenson K.B., Rességuier J., Hanson S., Chen L., Kazdhan N., Bramson J.L., Stojdl D.F. Maraba virus as a potent oncolytic vaccine vector. Mol. Ther. 2014;22:420–429. doi: 10.1038/mt.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galivo F., Diaz R.M., Wongthida P., Thompson J., Kottke T., Barber G., Melcher A., Vile R. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galivo F., Diaz R.M., Thanarajasingam U., Jevremovic D., Wongthida P., Thompson J., Kottke T., Barber G.N., Melcher A., Vile R.G. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum. Gene Ther. 2010;21:439–450. doi: 10.1089/hum.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai P., Wang W., Yang N., Serna-Tamayo C., Ricca J.M., Zamarin D., Shuman S., Merghoub T., Wolchok J.D., Deng L. Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic antitumor immunity via STING and Batf3-dependent dendritic cells. Sci. Immunol. 2017;2:eaal1713. doi: 10.1126/sciimmunol.aal1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prestwich R.J., Ilett E.J., Errington F., Diaz R.M., Steele L.P., Kottke T., Thompson J., Galivo F., Harrington K.J., Pandha H.S. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson K.H., Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006;100:1925–1935. doi: 10.1016/j.jinorgbio.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Crans D.C., Smee J.J., Gaidamauskas E., Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004;104:849–902. doi: 10.1021/cr020607t. [DOI] [PubMed] [Google Scholar]
- 12.McLauchlan C.C., Hooker J.D., Jones M.A., Dymon Z., Backhus E.A., Greiner B.A., Dorner N.A., Youkhana M.A., Manus L.M. Inhibition of acid, alkaline, and tyrosine (PTP1B) phosphatases by novel vanadium complexes. J. Inorg. Biochem. 2010;104:274–281. doi: 10.1016/j.jinorgbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Mustelin T., Vang T., Bottini N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 14.Fuertes M.B., Kacha A.K., Kline J., Woo S.R., Kranz D.M., Murphy K.M., Gajewski T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8alpha+ dendritic cells. J. Exp. Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond M.S., Kinder M., Matsushita H., Mashayekhi M., Dunn G.P., Archambault J.M., Lee H., Arthur C.D., White J.M., Kalinke U. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson H.A., Wehenkel S., Matthews J., Ager A. SHP-1: the next checkpoint target for cancer immunotherapy? Biochem. Soc. Trans. 2016;44:356–362. doi: 10.1042/BST20150251. [DOI] [PMC free article] [PubMed] [Google Scholar]