Abstract
Replication-competent retrovirus/lentivirus (RCR/L) and insertional oncogenesis are potential safety risks with integrating viruses in gene-modified cell therapies. As such, the Food and Drug Administration guidances outline RCR/L-monitoring methods throughout the entire gene therapy treatment cycle. We present data for 17 vector lots, 375 manufactured T cell products, and 308 patients post-infusion across both HIV and oncology indications, showing no evidence of RCR/L. Given our data, a Poisson probability model estimates that a single patient, or a group of patients, would need to be followed for at least 52.8 years to observe one positive RCR/L event, highlighting the unlikelihood of RCR/L development. Additionally, we estimate the median time for lentivirus-modified T cell products to fall below the 1% vector sequence threshold in peripheral or whole blood that would trigger vector integration site analysis. These estimated times are 1.4 months in hematologic malignancies, 0.66 month in solid tumors, and 0.92 month in HIV. Based on these considerable safety data in HIV and oncology and recent Biologics License Applications filed for lentiviral-modified T cell products for hematologic malignancies, this may be an opportune time to re-evaluate the current guidelines for T cell gene therapy product testing and long-term patient monitoring.
Keywords: clinical gene therapy, immunotherapy, lentivirus, retrovirus, replication-competent virus, safety, HIV, hematologic malignancy, viral vector
Replication-competent retrovirus/lentivirus (RCR/L) and insertional oncogenesis are potential safety risks in gene therapy. Marcucci et al. present negative RCR/L data for 17 vector lots, 375 T cell products, and 308 infused patients. Poisson probability estimates at least 52.8 patient follow-up years to observe an RCR/L event, highlighting the unlikelihood of RCR/L.
Introduction
For nearly 30 years, investigators have been conducting clinical trials of cell and gene therapies for conditions ranging from rare orphan diseases to common chronic illnesses. Although many delivery systems are available, retroviral and lentiviral gene transfer allows integration and long-term gene expression, which may in turn promote a durable therapeutic effect.1, 2, 3, 4 The first results of a study using retroviral-mediated gene transfer to treat patients with advanced melanoma were published in 1990.5 Since that time, continual advancements have been made in the field of gene therapy, which have expanded the methods and safety of gene delivery systems (retroviral vectors, adenoviral vectors, and lentiviral vectors), target cells (T cell, stem cell, dendritic cell, etc.), and disease indications (infectious diseases, malignancies, and hereditary diseases). Despite all of the innovations in therapeutic gene transfer and the potential it holds, the focus has been and continues to be ensuring patient safety.
Although retroviral and lentiviral vectors are designed to be replication incompetent,6, 7, 8 one theoretical safety risk related to their use in gene therapy is a recombination event leading to generation of a novel replication-competent retrovirus or lentivirus (RCR/L) during cell product manufacturing or in patients post-infusion. This concern was originally rooted in discussions during the Asilomar Conference and results of a gene transfer study in which non-human primate hematopoietic stem cells transduced with retroviral vector were used for bone marrow transplantation. Three of eight animals developed a rapidly progressing T cell neoplasm due to replication-competent virus contamination of the retroviral vector.9 To address this concern, the first recommendations related to RCR testing in both cell products and patients were released by the Food and Drug Administration (FDA) in 1993 and updated in the 2001 guidance for industry.10 This guidance recommends monitoring the following: (1) clinical vector lots, (2) manufactured cell products, and (3) patients post-infusion by suitable biological and/or molecular test methods for RCR/L detection.11, 12 Thus far, there has been no reported evidence in the literature of confirmed positive RCR/L results in clinical vector lots, cell products transduced with those vector lots, or patients infused with those cellular products.13, 14
Insertional oncogenesis is another post-infusion safety risk related to integrating viral vectors for cell modification. Clonal T cell leukemias driven by gammaretroviral integration at the LMO2 locus of hematopoietic stem cells in X-linked severe combined immunodeficiency disorder (X-SCID)15, 16 and Wiskott-Aldrich syndrome (WAS)17 and myelodysplastic syndrome (MDS) driven by retroviral integration in hematopoietic stem cells in X-linked chronic granulomatous disease (X-CGD)18, 19 have been described clinically. As a result of the X-SCID events, the FDA developed a guidance20 outlining monitoring subjects receiving cellular products modified using integrating vectors for the presence of ≥1% vector sequence in a surrogate sample (e.g., whole blood) for up to 15 years. Detection of vector sequences at or above the 1% marking threshold would subsequently prompt analysis to determine vector integration patterns. To date, there have been no additional reports of a clonal malignancy resulting from an integrating gene therapy vector in modified T cells.
In this paper, we describe RCR/L test results for 17 clinical vector lots, 375 manufactured T cell products, and 308 infused patients (Figure 1), analyzed across both oncology and HIV clinical trials infusing retroviral- or lentiviral-transduced T cells from a total of 194.8 post-infusion person years of RCR/L follow-up. Moreover, long-term monitoring for vector sequences in 305 patients infused with lentiviral-modified T cell products revealed that the probability of modified T cells being above the 1% threshold continued to decrease over time for both oncology and HIV subjects. Combined, our data add to the growing safety profile for retroviral- and lentiviral-modified T cells in the literature, and they prompt re-evaluation of current safety-monitoring guidelines for the testing of integrated virus products and subjects post-infusion.
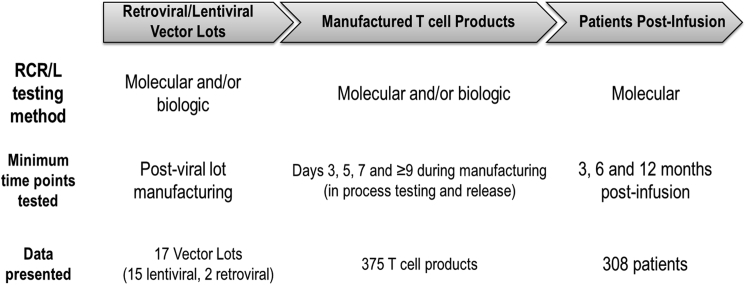
Figure 1.
Overview of RCR/L Results Presented
The three main components, vector lots, manufactured T cell products, and patients post-infusion, monitored for RCR/L during the viral vector gene therapy treatment cycle are highlighted in this paper. Test methods, time points, and total data presented are summarized for each of the three components.
Results
Lentiviral and Retroviral Vector Design and Manufactured Lots
Eight distinct transgenes were used for viral vector lot manufacturing (Table 1). Six transgenes were chimeric antigen receptors (CARs), one transgene encoded an endoribonuclease, and one transgene was an HIV-1 envelope antisense gene. CAR targets include CD19, BCMA, EGFRvIII, mesothelin, and CD4. For the 8 distinct cell products under analysis, 5 used a third-generation, self-inactivating lentiviral vector system; one used a two-plasmid lentiviral system (intact 3′ HIV LTR); and two used second-generation retroviral vector systems for manufacturing. The vesicular stomatitis virus envelope glycoprotein (VSV-G) was used for lentiviral vector pseudotyping, and amphotropic murine leukemia virus (aMLV) and gibbon ape leukemia virus (GaLV) envelopes were used for retroviral vector genome pseudotyping. Five of the six lentiviral vectors used the human Elongation Factor-1 alpha (EF-1a) promoter to drive transgene expression, while a conditional HIV-1 LTR drove VRX496 transgene expression. Similarly, MazF, a transgene in a retroviral vector, was driven by the conditional HIV-1 LTR, with CD4z, the other transgene in a retroviral vector, driven by a phosphoglycerol kinase (PGK) promoter.
Table 1.
Lentiviral and Retroviral Vector Lots, RCL/R Monitoring
| Investigational Product | Vector Type | Envelope | Promoter | Transgene | Number of Vector Lots |
NCT Number(s) | |||
|---|---|---|---|---|---|---|---|---|---|
| Produced | Tested for RCR/L via Biological Method | Tested for RCR/L via Molecular Method | Used for T Cell Product Manufacture | ||||||
| CTL019 | lentivirus, third generation, SIN | VSV-G | EF-1a | murine anti-CD19 scFv with 4-1BB and CD3ζ signaling domains | 9 | 9 | 8 | 7 | NCT02030847, NCT01029366, NCT01626495, NCT01747486, NCT02135406, NCT02030834, NCT02465983 |
| CTL119 | lentivirus, third generation, SIN | VSV-G | EF-1a | humanized anti-CD19 scFv with 4-1BB and CD3ζ signaling domains | 2 | 2 | 1 | 3 | NCT02374333, NCT02640209, NCT02030834 |
| CART-meso | lentivirus, third generation, SIN | VSV-G | EF-1a | murine anti-mesothelin scFv with 4-1BB and CD3ζ signaling domains | 1 | 1 | 1 | 2 | NCT02465983, NCT02159716 |
| CART-BCMA | lentivirus, third generation, SIN | VSV-G | EF-1a | human anti-BCMA scFV with 4-1BB and CD3ζ signaling domains | 1 | 1 | 0 | 1 | NCT02546167 |
| CART-EGFRvIII | lentivirus, third generation, SIN | VSV-G | EF-1a | humanized anti-EGFRvIII scFV with 4-1BB and CD3ζ signaling domains | 1 | 1 | 1 | 1 | NCT02209376 |
| VRX-496 | lentivirus, third generation, SIN | VSV-G | HIV-1 LTR | antisense sequence targeting HIV env | 1 | 1 | 0 | 1 | NCT00295477 |
| CD4zeta | retrovirus, second generation | aMLV | PGK | human CD4 transmembrane and extracellular domains fused with CD3ζ signaling domain | 1 | 1 | 0 | 1 | NCT01013415 |
| MazF | retrovirus, second generation | GaLV | HIV-1 LTR | MazF endoribonuclease | 1 | 1 | 0 | 1 | NCT01787994 |
| Lentivirus totals | 15 | 15 | 11 | 15 | |||||
| Retrovirus totals | 2 | 2 | 0 | 2 | |||||
SIN, self-inactivating; VSV-G, vesicular stomatitis virus glycoprotein; LTR, long terminal repeat; PGK, phosphoglycerol kinase; aMLV, amphotropic murine leukemia virus; GaLV, gibbon ape leukemia virus.
A total of 15 lentiviral and 2 retroviral vector lots were manufactured at various manufacturing facilities, both academic and commercial. All vector lots underwent RCR/L testing as part of release testing according to FDA guidance (Table 1). The VRX496 lot testing was previously reported.14 RCR/L was tested by molecular (residual VSV-G transfer to target cells, lentiviruses only) assays and/or biological (C8166 cells with amplification) assay on either end of product (EOP) cells, supernatant, or both. For those with both EOP and supernatants tested, this provides two negative tests of the same lot. While one CTL019 lentiviral vector lot tested positive for RCL by molecular (qPCR) assay, all vector lots, including the molecular RCL-positive lot, tested negative for RCR/L by the biological assay. Fifteen of the seventeen vector lots described here were used for cell product manufacturing of patient T cell product lots for HIV or oncology indications.
Monitoring T Cell Product Lots for RCR/L
A total of 375 gene-modified T cell product lots were manufactured in the University of Pennsylvania Clinical Cell and Vaccine Production Facility (CVPF) between 2001 and 2017 for eight different investigational products used in clinical trial subjects with hematological malignancies, solid tumors, or HIV (Table 2). Subjects’ final manufactured product lots were tested for RCR/L by qPCR specific for envelope sequences and/or by biological assay using subculture viral amplification followed by focus assay in permissive cells (e.g., C8166) at the time of harvest for product release (Figure 2A). For VSV-G-pseudotyped vectors, gene-modified cells were tested by qPCR for envelope sequences throughout the manufacturing process as an indicator of potential RCL (Figure 2B). Molecular RCR/L release criteria required either that qPCR results for envelope sequences must not be detectable (EGFRVIII CAR, CTL119, CTL019, BCMA CAR, muCART-MESO, and VRX496) or must be declining from day 7 to post-harvest of the culture (MazF). Any molecular RCR/L results out of specification required subsequent biological RCR/L testing. A negative biological RCR test was required for the release of CD4zeta T cell products; molecular testing was not required for the release of these products.
Table 2.
Characteristics of Gene-Modified Cell Products and RCR/L Results
| Cell Product (Vector) | Disease Category | No. of Clinical Trials | No. of Clinical Cell Products Manufactured | Transduction Efficiency-Flow Cytometry (% Range) (% Mean ± SD) | Vector Integration Transgene Copies/Cell (Range) (Mean ± SD) | Total Cell No. in Dose (Range, No. of WBCs) | Average Dose (Mean No. of WBCs ± SD) | Molecular (qPCR) RCR/L Results (No. out of Specification/No. Tested) | Biological RCR/L Results (No. Positive/No. Tested) |
|---|---|---|---|---|---|---|---|---|---|
| EGFRvIII CAR (lentivirus) | solid tumor-glioblastoma | 1 | 15 | 4.81–25.6 (16.5 ± 6) | 0.11–0.47 (0.29 ± 0.12) | 2.37E8–4.05E9 | 2.51E9 ± 1.21E9 | 0/15 | test not performed; not required for release |
| CTL119 (lentivirus) | hematologic malignancy (pediatric leukemia/lymphoma, CLL, NHL), solid tumor (metastatic pancreatic cancer) | 5 | 71 | 2–47 (22 ± 10) | 0.09–1.21 (0.48 ± 0.22) | 1.19E8–7.85E9 | 1.5E9 ± 1.33E9 | 0/71 | 0/11 |
| BCMA CAR (lentivirus) | multiple myeloma | 1 | 15 | 9.57–33.3 (23 ± 6) | 0.21–1.22 (0.29 ± 0.12) | 1.57E8–5.52E9 | 1.71E9 ± 1.32E9 | 0/15 | test not performed; not required for release |
| muCART-MESO (lentivirus) | solid tumor (pancreatic, mesothelioma, ovarian) | 2 | 18 | 15.5–49.4 (27 ± 9) | 0.21–0.82 (0.39 ± 0.27) | 1.26E8–3.50E9 | 9.93E8 ± 1.14E9 | 0/18 | test not performed; not required for release |
| muCART19 (lentivirus) | hematologic malignancy (pediatric leukemia/lymphoma, CLL, NHL, multiple myeloma) | 6 | 213 | 1.22–37.7 (16 ± 9) | 0.02–1.12 (0.35 ± 0.23) | 1.3E8–9.49E9 | 2.78E9 ± 1.88E9 | 5/213 | 0/179 |
| VRX496 (lentivirus) | HIV | 1 | 19 | not applicable | 0.25–3.4 (1.34 ± 0.87) | 1.08E10–7.0E10 | 4.82E10 ± 1.86E10 | 0/19 | test not performed; not required for release |
| CD4zeta (retrovirus) | HIV | 1 | 12 | not available | 0.017–0.25 (0.13 ± 0.09) | 4.14E9–1.13E10 | 7.78E9 ± 2.13E9 | test not performed; not required for release | 0/12 |
| MazF (retrovirus) | HIV | 1 | 12 | not applicable | 2.32–3.7 (3.0 ± 0.4) | 4.0E9–1.0E10 | 6.7E9 ± 2.7E9 | 3/12 | 0/12 |
| Total | 18 | 375 | 8/363 | 0/214 |
WBC, white blood cell.
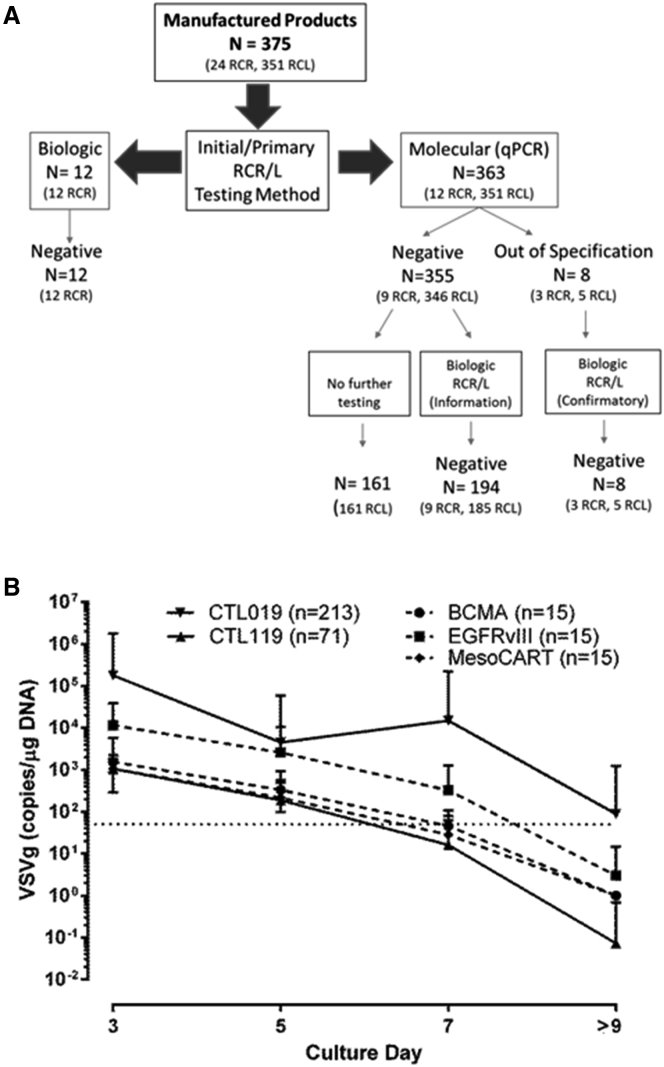
Figure 2.
Monitoring Patient Product Lots for RCR/L
(A) A total of 375 patient product lots were tested at harvest for RCR/L. Twelve products were tested solely by biological RCR assay. The remaining 363 patient product lots were tested by molecular qPCR assay for RCR/L. Eight of those product lots tested out of specification, necessitating biological RCR/L testing, which subsequently found no evidence of replication-competent virus. (B) VSV-G levels decline throughout the cell manufacturing process. Results of 329 lentivirus-transduced product lots reveal vector transduction of the activated T cells is demonstrated early on, with detection of 1,000–100,000 copies/μg VSV-G DNA within the first 3 days of culture. Sampling of cultures occurred at days 3, 5, and 7 post-activation, as well as at harvest. Throughout the manufacturing process, vector DNA copies progressively declined, reaching undetectable levels by the day of harvest. The dotted line indicates the limit of quantitation for the VSV-G qPCR assay (50 VSV-G copies/1 μg genomic DNA). The error bars represent the SD of the mean values graphed.
The qPCR-based RCR/L assay was performed as a release test on 363 T cell product lots; 208 of these cell product lots were also tested by biological RCR/L assay as release criteria (MazF) or for information-only purposes (CTL119 and CTL019). Eight T cell products tested had results that were out of specification for molecular RCR/L. Both cells and harvest supernatants were submitted from each of the 8 failed T cell products for biological RCL/R testing, and all 8 were found to be negative. Despite the presence of VSV-G DNA in the T cells at harvest, 5 products manufactured with replication-incompetent lentiviral vectors had declining VSVG copies, HIV-gag copies, and p24 values during the manufacturing process. This is consistent with the carryover of residual vector plasmid DNAs, and it is inconsistent with bona fide RCL, which would be expected to show steady or increasing VSVg, gag, or p24.
Post-infusion Patient RCR/L Monitoring
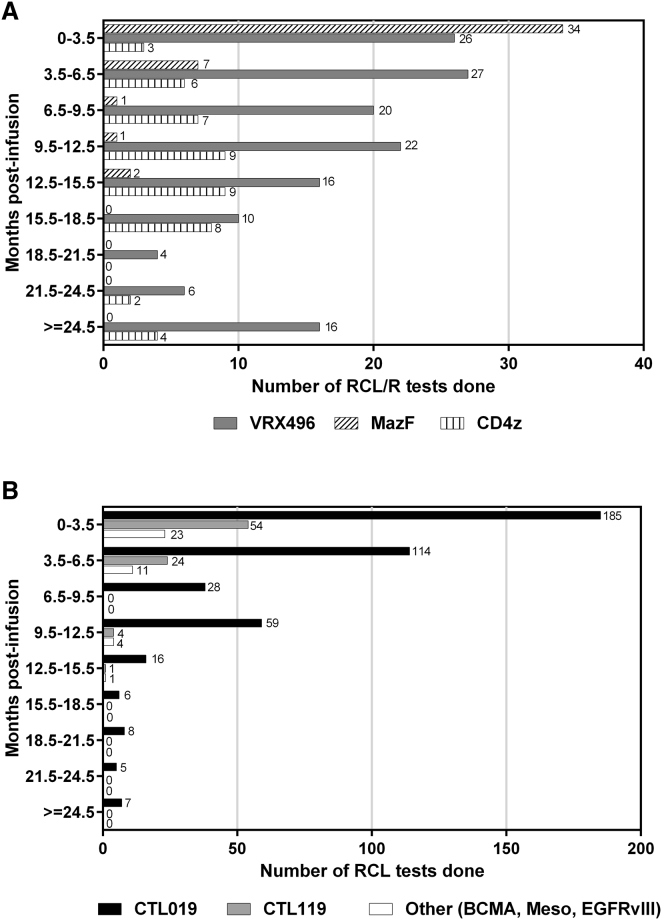
Subjects were monitored per protocol for RCL/R by molecular qPCR for relevant viral genes (i.e., VSVG, HIV gag, and GaLV env). Protocol time points for monitoring were designed based on the FDA guidance documents current at the time. For example, in studies for CTL019 or CTL119, subjects were monitored at months 3, 6, and 12 post-infusion based on the November 2006 FDA guidance for monitoring subjects for long-term adverse events.20 For the purposes of this analysis, all time points where a molecular qPCR test was performed are included. Figure 3 shows the number of RCL tests performed in 3-month intervals for infused HIV (3A) and oncology (3B) patients. All time points tested for all patients were negative. Table 3 summarizes the number of patients and molecular RCR/L tests performed post-infusion and the cumulative test follow-up time under observation grouped by vector type (lentivirus or retrovirus) for the 8 cell products. The test follow-up time was calculated from the time of infusion to the time that the last RCR/L test was performed. The cumulative test follow-up is 194.8 years for all products, with the majority of follow-up comprising the lentiviral cell product-treated patients (171.2 years).
Figure 3.
Post-infusion RCR/L Patient Monitoring
(A and B) The number of molecular RCL/R tests performed for HIV (A) and oncology (B) patients grouped by months post-infusion. Lentiviral cell products (solid bars) and retroviral cell products (patterned bars) are indicated.
Table 3.
Post-infusion Patient RCR/L Monitoring
| Cell Product | No. of Patients Tested | Total No. of Tests Performed (Range per Patient) | Cumulative Follow-up Time (Years) | 95% CI Upper Bound for the Predicted Rate of Positive RCL/R Test per Follow-up Years | Estimated No. of Patient Follow-up Years to Observe a Positive RCL/R Event |
|---|---|---|---|---|---|
| Lentivirus | |||||
| CTL019 | 188 | 422 (0–11) | 107.7 | 0.034 | 29.2 |
| CTL119 | 49 | 83 (0–4) | 18.2 | 0.203 | 4.9 |
| Other (CART-BCMA, CART-meso, CART-EGFRvIII) | 34 | 39 (0–3) | 9.9 | 0.372 | 2.7 |
| VRX496 | 17 | 147 (4–13) | 35.4 | 0.104 | 9.6 |
| Lentivirus total | 288 | 691 (0–13) | 171.2 | 0.022 | 46.4 |
| Retrovirus | |||||
| MazF | 10 | 45 (2–6) | 6.1 | 0.606 | 1.7 |
| CD4z | 10 | 58 (3–9) | 17.5 | 0.211 | 4.7 |
| Retrovirus total | 20 | 103 (2–9) | 23.6 | 0.156 | 6.4 |
| Overall total | 308 | 794 (0–13) | 194.8 | 0.019 | 52.8 |
Although no RCR/L event was detected during the available follow-up period, we cannot exclude the possibility that the true RCR/L event rate may be non-zero (>0). To quantify this uncertainty, we computed a 95% exact confidence interval (CI) of the true RCR/L event rate based on the observed data using the Poisson distribution. The Poisson distribution is typically used to model the number of events observed within a given time interval, often in cases where the event is rare. With our observed data, we estimated the exact CI to be (0, 0.019) events per year. The upper bound of the CI indicates that it is highly unlikely the true event rate is above this upper bound. Therefore, this upper bound value also represents the most conservative estimate of the true RCR/L event rate, based on the cumulative evidence so far. If we assume the true positive RCR/L rate is indeed 0.019/year, then it suggests, on average, only one positive RCR/L event will be observed in every 52.8 years of patient follow-up. In the absence of observing any RCR/L event, the number of years required to observe an event would only increase as the cumulative subject follow-up time increases by either longer follow-up duration of a single subject or treatment and follow-up of several additional subjects.
Post-infusion Patient Monitoring for ≥1% Marking
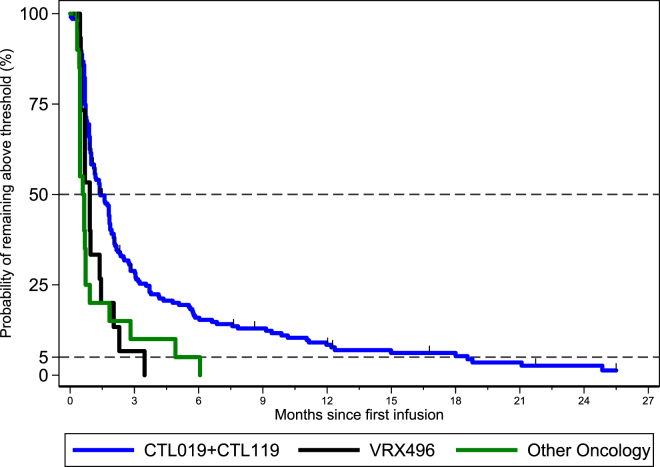
In addition to monitoring RCL/R in subjects, persistence of the gene-modified cells was also monitored. The November 2006 FDA guidance on delayed adverse events20 also recommends subject monitoring for ≥1% gene marking in a surrogate sample as a way to monitor for potential insertional oncogenesis. While ≥1% marking in the short term is desired for potential therapeutic benefit, it is in the long term when a progressive increase in cell marking could indicate a vector integration-driven expansion, either malignant or non-malignant. In the studies summarized in Figure 4, the surrogate sample was either DNA extracted from peripheral blood mononuclear cells or whole blood. Persistence for each product was monitored by either vector- or transgene-specific qPCR primer/probe sets. Table 4 shows the number of subjects analyzed, tests performed, and test results >1% threshold. A Kaplan-Meier curve for the probability of the first average marking per cell value <0.01 (1%) is grouped by lentiviral T cell product type; CTL019 + CTL119 for hematologic malignancies, Other Oncology (BCMA, Meso CART, and EGFRvIII) for solid tumors, and VRX496 for HIV (Figure 4). CTL019 and CTL119 are grouped together and represent the greatest number of patients (n = 207) exposed to a lentivirus-modified T cell product described here. The median survival time to fall below the threshold of 1% was estimated to be 1.4 months (CTL019 + CTL119, hematologic malignancies) and 0.66 months (Other Oncology, BCMA, Meso, and EGFRvIII; solid tumors) in oncology indications and 0.92 months (VRX496) in HIV.
Figure 4.
Post-infusion Patient Monitoring for ≥1% Vector Sequences
The Kaplan-Meier curve for the probability of the average marking per cell remaining above threshold is shown for CTL019 + CTL119 (blue line), Other Oncology (green line), and VRX496 (black line).
Table 4.
Patient Monitoring for ≥1% Vector Sequences Risk Table
| Days, Monthsa |
Monthsa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤15 | ≥16–<32 | ≥32–<2.5 | ≥2.5–<3.5 | ≥3.5–<6.5 | ≥6.5–<9.5 | ≥9.5–<12.5 | ≥12.5–<15.5 | ≥15.5–<18.5 | ≥18.5–<21.5 | ≥21.5–<24.5 | ≥24.5 | |
| CTL019 + CTL119 | ||||||||||||
| Subjects analyzed | 239 | 232 | 201 | 151 | 134 | 83 | 63 | 37 | 43 | 37 | 26 | 24 |
| Total number of tests performed | 1,435 | 646 | 428 | 249 | 495 | 234 | 152 | 49 | 58 | 40 | 27 | 51 |
| Total number of tests above threshold | 607 | 322 | 91 | 27 | 47 | 28 | 15 | 2 | 10 | 2 | 1 | 1 |
| Other Oncology (BCMA, Meso, EGFRvIII) | ||||||||||||
| Subjects analyzed | 40 | 38 | 28 | 18 | 13 | 9 | 2 | 3 | 1 | 1 | 0 | 0 |
| Total number of tests performed | 274 | 78 | 37 | 19 | 23 | 11 | 2 | 4 | 1 | 1 | 0 | 0 |
| Total number of tests above threshold | 58 | 12 | 8 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VRX496 | ||||||||||||
| Subjects analyzed | 17 | 17 | 17 | 17 | 16 | 17 | 14 | 14 | 10 | 4 | 9 | 15 |
| Total number of tests performed | 32 | 31 | 76 | 58 | 61 | 24 | 22 | 16 | 11 | 4 | 9 | 51 |
| Total number of tests above threshold | 20 | 11 | 10 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Timepoints are reflected in months after day 32.
Discussion
The viral vector lot, manufactured T cell product, and the patient post-infusion represent the three components of gene therapy studies monitored for integrated viral vector safety. Our program’s extensive negative dataset for RCR/L and vector sequence cell marking ultimately decreasing below the 1% FDA guidance threshold (in lentiviral- and retroviral-modified T cell products across HIV and oncology indications) support the continued safety of lentivirus- and retrovirus-modified T cell therapy products.
Safety enhancements reducing the risk for RCR/L have been made throughout the evolution from first-,21 second-,22 and now third-generation lentiviral production systems.7 At each step of viral vector design evolution, vital elements of the HIV genome have been separated onto different plasmids or eliminated, including long terminal repeat (LTR) U3 deletion in self-inactivating vectors preventing full-length vector transcript expression. These design choices rooted in HIV molecular virology have reduced the risk in the third-generation systems7 by requiring at least three recombination events among the four plasmids to occur in order for RCL formation. Promoter optimization has also increased overall viral vector safety for both genotoxicity considerations23 and for desired cell type expression.24, 25 For the majority of the T cell trials described here, EF-1a was selected to drive vector transgene expression as a result of pre-clinical experiments for the anti-CD19 CAR.24 Thus, viral vector design has continued to evolve with a focus on patient safety.
Viral vector lot production methods have also been refined over time to reduce the risk of RCR/L formation. Supernatant treatment with benzonase decreases residual plasmid DNA post-production,26 thus reducing the risk for packaging plasmid carryover in the patient cell lots. Additionally, retroviruses produced with PG13 packaging cell lines have been RCR negative.13 Here we demonstrated the HEK293 cell lines used for production do not produce RCL-positive lots. Our negative RCR/L results across 15 lentiviral and 2 retroviral vector lots augment what has previously been reported in the literature. For example, 10 lentiviral lots with no RCL detected in 3.5 × 107 up to 5.3 × 109 transducing units have been reported.27
One lentiviral vector lot included in our analysis tested positive by qPCR, but not by the reference biological RCL assay. Moreover, CTL019 T cell products manufactured with the molecular-positive/biological-negative RCL vector lot did not result in any positive RCL tests in the subjects infused. Just as the one lentiviral lot tested positive, albeit at low levels for VSV-G DNA by the molecular assay, there are instances reported by others of a single retrovirus lot testing positive by S+/L− assay, with 114 negative lots prior to and 57 negative lots after the positive RCR event.28 Thus, positive RCR/L tests are likely due to viral vector DNA carryover from the transient plasmid transfection used for viral vector production and not replication-competent virus.
Of the 375 gene-modified T cell product lots described herein, 12 were released solely on a negative biological RCR result, while the other 363 were tested via molecular qPCR for pseudotyped envelope sequences. All products were tested for RCR/L since the ex vivo cultures were carried out for longer than the 4-day threshold outlined in the FDA guidance.10 Of those product lots tested by qPCR, 98% had passing molecular RCR/L results. Nevertheless, the 8 product lots that failed molecular RCR/L were subsequently subjected to further testing using the biological RCR/L method, and they were confirmed to be negative. Six of the 8 RCR/L-negative product lots that did not meet the molecular specification had declining VSV-G or GaLV copy numbers over the course of the manufacturing process. The continuous decline of the vector envelope DNA was observed for several products throughout the ex vivo culture (Figure 2B), correlating with a several log-fold drop in vector DNA levels due to the multiple cell washes and media perfusions that dilute residual vector throughout the manufacturing process. If replication-competent virus was present, the VSV-G copy numbers would be expected to increase over time. Therefore, none of the transduced T cell products we have manufactured have had evidence of RCR/L.
In all, we have monitored 308 subjects for RCR/L for a total of 194.8 post-infusion follow-up years. The estimated worst-case scenario and minimum patient follow-up years expected to observe a positive RCR event appear to be shorter for retroviral-modified cell products than for lentiviral-modified cell products, however, this mostly reflects the limited statistical evidence from a smaller number of patients observed (20 versus 288) for fewer cumulative years (23.6 versus 171.2) rather than biological risk factors. Moreover, the two retroviral-modified cell products, MazF and CD4z, were infused in HIV patients. The hypothetical risk for RCR would be greater in these patients, given their established infection with the HIV retrovirus. If HIV were actively replicating due to uncontrolled viremia or analytical treatment interruption, the theoretical possibility exists for recombination between HIV and the retroviral transgene vector, and thus the creation of a novel RCR. Despite this, all HIV patients were negative post-infusion for RCR and RCL. One HIV trial subject was determined to have a false-positive VSV-G test 52 weeks post-infusion. An investigation at the testing site (VIRxSYS Corporation) indicated that the likely cause of the false-positive result was contamination of samples at the time of sample preparation, and upon retest the result was confirmed to be negative. Thus, there are no data to support that retroviral-modified T cell products have an increased risk for RCR compared to the lentiviral-modified T cell products.
As we have not observed a positive RCR/L event in any patient, the true rate could be zero. However, assuming the rate is non-zero, using a Poisson CI calculation, the combined negative RCR/L test results we have collected from over 194.8 years of patient follow-up data indicate that, on average, a study would only observe one positive RCR/L event in at least 52.8 person years. This is a conservative estimate as the number of years required to observe a positive event would only increase with additional patients treated and/or with longer post-infusion follow-up, assuming all future test results are negative. As an RCR/L event remains a theoretical possibility, the use of the Poisson distribution is conceptually appropriate, even though it cannot be validated given the cumulative negative data. Thus, the favorable safety profile established by the data presented here as well as those in the literature13, 14, 29, 30, 31 supports re-evaluation of the current T cell product and post-infusion RCR/L monitoring frequency, particularly if the viral vector lot is negative by the biological RCR/L assay at the end of manufacturing. It is useful to note that treatment-related acute myeloid leukemia and myelodysplastic syndrome occur in at least 5% of the long-term survivors from chemotherapy for breast and ovarian cancer.32, 33, 34
Monitoring subjects for vector sequences at or above the 1% threshold is another step in post-infusion safety monitoring focused on insertional oncogenesis surveillance. Modified T cell persistence is desired for function and has been demonstrated long term by our group in oncology1, 3 and HIV4, 30 trials, as well as by others for an adenosine deaminase deficiency severe combined immunodeficiency (ADA-SCID) trial.2, 35 In CTL019 for oncology indications, long-term persistence, even below the 1% threshold, leads to sustained clinical remissions.1, 3 Previous analysis of lentiviral-modified T cells from our VRX496 study revealed a polyclonal integration pattern with no indication of integration near oncogenes.36 Moreover, insertion-driven clonal expansion that led to malignant (Six et al., 2017, Mol Ther., abstract)16, 17 or pre-malignant pathogenesis18, 19 occurred in stem cell products modified with retroviral, not lentiviral, vectors. Given that T cells are a terminally differentiated cell type, it is possible that there is less risk for vector integration-driven pathogenesis than that seen in early hematopoietic stem cells. In a study directly comparing the predisposition of mature T cells and hematopoietic stem cells to transformation after retroviral transduction with known T cell oncogenes, it was found that, even with multiple insertion sites, no transformation events were observed in the T cells and the integration pattern was polyclonal.37 Thus, the insertional oncogenesis risk is likely based on differentiation state of the modified cell type as well as the type of viral vector used.
From the 244 subjects infused with lentiviral-modified T cells and assessed for vector sequences, the median time to gene marking falling below the 1% threshold with 50% probability was estimated to be 1.4 months (CTL019 + CTL119, hematologic malignancies) and 0.66 months (BCMA, Meso, EGFRvIII; solid tumors) in oncology indications and 0.92 months (VRX496) in HIV. The medians are influenced by the number of subjects and follow-up duration analyzed. Moreover, this probability continued to decrease over time, as viewed from the overall product profiles. These data are supported by the previously reported experience with CD4zeta,30 a retroviral-modified T cell product. Therefore, our post-infusion monitoring for vector sequences provides a favorable post-infusion safety profile from the lentiviral- and retroviral-modified T cell product level.
It is worth noting that a 1% gene marking threshold fails to take into account the virus vector system, differentiation state of the transduced cell type, and proliferative or homeostatic nature of the gene therapy product being administered. Differential risk profiles for vector-driven insertional oncogenesis appear to be emerging for gammaretroviral-modified hematopoietic stem cells compared to lentiviral-modified hematopoietic stem cells and retroviral- or lentiviral-modified T cells. The X-SCID15, 16 and WAS17 vector-derived T cell malignancies occurred in gene therapy products originating from hematopoietic stem cells modified with a gammaretrovirus vector. This is in contrast to the benign vector-driven expansion of lentiviral-modified hematopoietic stem cells in β-thalassemia.38 Notably, vector-driven insertional oncogenesis has not been observed to date in retroviral- and lentiviral-transduced terminally differentiated T cells. It should be noted that both retroviruses described here were gammaretroviral pseudotypes (GaLV and amphotropic MLV); however, transgene expression was driven by non-gammaretroviral promoters. Based on these data, both the molecular virology of the vector system used for transduction and the target cells’ differentiation state contribute to the vector-driven insertional oncogenesis risk. Therefore, the safety profile described in this paper is applicable to retroviral- and lentiviral-transduced T cells.
Although intended as a trigger for detection of possible vector-derived malignancies, modification in greater than 1% of cells is not necessarily a cause for alarm. This and other considerations regarding insertional oncogenesis have been highlighted in a reflection paper from the European Medicines Agency (EMA).39 In the context of our data, gene-modified T cell products expressing a CAR or T cell receptor (TCR) on the cell surface can expand upon exposure to target antigen, directly influencing engraftment and persistence. In the case of CARs in oncology, a target antigen-positive relapse event may cause gene marking to be above the 1% threshold from the product’s inherent biological activity and not a vector-driven event. In contrast, T cells modified for gene disruption or editing, either through direct modification or editing enzyme expression, such as those for HIV, do not expand in response to target antigen but rather are influenced by initial cell dose and modification levels (e.g., vector copy number) as well as initial engraftment levels. It is also possible that clonal T cell expansion could be driven by foreign antigen exposure where the increase in gene marking would be a bystander effect of the immune system. A more relevant indicator to prompt clonality assessment or integration analysis may be a steady or clinically unexplained increase in gene marking after a period of stability or decline.
The negative RCR/L test results for 17 viral vector lots, 375 manufactured T cell products, and 308 patients post-infusion, as well as the overall probability of lentiviral-modified T cell products falling below the 1% vector sequence threshold, enhance the growing evidence for the favorable safety profile of lentiviral- and retroviral-modified T cell products in oncology and HIV. These data provide support for the RCL/R vector lot testing results as indicator of manufactured T cell products and study subjects post-infusion. Given the recent approval of Kymriah (tisagenlecleucel) and additional pending Biologics License Applications for gene-modified T cells to treat hematologic malignancies, these gene therapies will soon be clinically available. With the accumulated safety data in several hundred study subjects presented here, along with the experience of other groups, now is an opportune time to re-evaluate the current guidelines for retroviral-/lentiviral-modified T cell products, which have remained unchanged for over a decade.
Materials and Methods
Data Collection
Data described here were obtained from October 2001 to December 2016 from the following clinical trials at ClinicalTrials.gov: NCT02030847, NCT01029366, NCT01626495, NCT01747486, NCT02135406, NCT02030834, NCT02374333, NCT02640209, NCT02465983, NCT02159716, NCT02209376, NCT00295477, NCT01013415, NCT01787994, and NCT02546167. Additional data were obtained on associated non-therapeutic long-term follow-up protocols. All samples were collected on institutional review board (IRB)-approved protocols in which subjects had provided informed consent.
Biological RCR/L Assays
These were performed as previously described11 by the NIH-funded National Gene Vector Biorepository (NGVB) at the Indiana University School of Medicine, BioReliance, and WuXi AppTec.
Molecular RCR/L Assays
qPCR for pseudotype envelope sequences was performed with the primer and probe sets for VSV-G (primers: 5′-TCAAAGGCTCAGGTGTTCGA-3′ and 5′-CATCAGGAAGTTGCGAAGCA-3′; probe: 5′-FAM-CATCCTCACATTCAAGACG-MGBNFQ-3′) and GaLV (primers: 5′-GCCCTTTACCCATCAGCATCT-3′ and 5′-TACTGATGGTCTCCGGAGGA-3′; probe: 5′-6FAM-CAATCAGACCCTATCCAT-3′).
Persistence of Gene-Modified Cells
qPCR assays were performed as previously described for VRX4964 and CD4zeta.40 The following primer and probe sets were used for the specified transgenes: human anti-CD19, human anti-BCMA, and humanized anti-EGRFvIII (primers: 5′-CTGCTGCTTTCACTCGTGATCACT-3′ and 5′-ATGAAGGGTTGCTTAAAGATGTACAG-3′; probe: 5′-VIC-ACTCTCAGTTCACATCCTC-MGBNFQ-3′); murine anti-CD19 and murine anti-mesothelin (primers: 5′-TGCCGATTTCCAGAAGAAGAAGAAG-3′ and 5′-GCGCTCCTGCTGAACTTC-3′; probe: 5′-VIC-ACTCTCAGTTCACATCCTC-MGBNFQ-3′); and MazF (primers: 5′-GGCAAGAGGAGCAACGAAGA-3′ and 5′-TGGCTTTAATGAGCTGCAGTTC-3′; probe: 5′-FAM- AGGAACCGTTGCCC-MGBNFQ-3′).
Statistical Methods
Summary statistics were computed for the number of patients infused, RCR/L and persistence tests performed, and the follow-up duration across all trials, and they are presented according to vector type (lentivirus or retrovirus) and product type (CTL019 + CTL119 for hematologic malignancies; Other Oncology [BCMA, Meso CART, and EGFRvIII] for solid tumors; and VRX496, MazF, and CD4z for HIV). The follow-up time for a subject was calculated from the time of infusion to the time that the last test was performed. If needed, data were grouped into 3-month follow-up intervals, which was the frequency with which tests were scheduled for most trial protocols.
To estimate the true-positive RCR/L event rate, we computed the 95% exact CI based on Poisson probability model, which is appropriate for rare events. The RCR/L event rate of one event every 52.8 years was calculated by dividing 1 by the upper bound CI value, rounded to a single decimal place. The upper bound of the 95% CI provides us the worst-case scenario for the true-positive event rate given the cumulative (negative) test results and follow-up to date. To describe the probability of having detectable markings as a function of time post-infusion, Kaplan-Meier curves were generated for the time-to-event variables defined as months from infusion to the first time when persistence test results fall and stay below the threshold thereafter. The threshold was set to be 1% for all products. Median survival time, the probability that the number of markings fell below the threshold before this time point is 50%, was reported.
Author Contributions
K.T.M., J.K.J., W.-T.H., and M.S.-D. wrote the manuscript, with S.F.L. and J.J.M. contributing to writing and A.C., G.P., S.F.L., J.J.M., B.L.L., and C.H.J. providing feedback. W.-T.H. performed the statistical analyses. I.K. and M.G. processed and analyzed samples. S.F.L. and J.J.M. designed assays for transgene and molecular RCL/R testing. V.E.G. provided data quality control for transgene and molecular RCL/R testing.
Acknowledgments
We thank the patients for their participation in the clinical trial from which research samples were obtained and FDA product reviewers. Additionally, we thank all study investigators and clinical research staff, including the Clinical Trials Unit (CTU) at the University of Pennsylvania. We also acknowledge John Scholler, the Translational and Correlative Studies Laboratory staff, Dr. Linda Jagodzinski, and Dr. Naomi Aronson for research support. We would also like to thank Dr. Ken Cornetta and the National Gene Vector Biorepository (NGVB) at the Indiana University School of Medicine for biological RCL/R testing. We also thank Lentigen Inc., Center for Advanced Retinal and Ocular Therapeutics Clinical Vector Core (CAROT CVC) at the University of Pennsylvania’s Perelman School of Medicine, the Children’s Hospital of Philadelphia Clinical Vector Core (CHOP CVC), the Indiana University Vector Production Facility, Takara Bio Center for Cell and Gene Therapy Facility, and City of Hope’s Center for Biomedicine and Genetics for virus production. Funding for the clinical trials described here was provided by the Leukemia and Lymphoma Society, Takara Bio, and Novartis Pharmaceuticals. C.H.J. and B.L.L. have sponsored research grants from Novartis and receive royalties from the University of Pennsylvania for IP licensed to Novartis. C.H.J., B.L.L., and A.C. are scientific founders of and have equity interests in Tmunity Therapeutics.
References
- 1.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muul L.M., Tuschong L.M., Soenen S.L., Jagadeesh G.J., Ramsey W.J., Long Z., Carter C.S., Garabedian E.K., Alleyne M., Brown M. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood. 2003;101:2563–2569. doi: 10.1182/blood-2002-09-2800. [DOI] [PubMed] [Google Scholar]
- 3.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tebas P., Stein D., Binder-Scholl G., Mukherjee R., Brady T., Rebello T., Humeau L., Kalos M., Papasavvas E., Montaner L.J. Antiviral effects of autologous CD4 T cells genetically modified with a conditionally replicating lentiviral vector expressing long antisense to HIV. Blood. 2013;121:1524–1533. doi: 10.1182/blood-2012-07-447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg S.A., Aebersold P., Cornetta K., Kasid A., Morgan R.A., Moen R., Karson E.M., Lotze M.T., Yang J.C., Topalian S.L. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 6.Cesana D., Ranzani M., Volpin M., Bartholomae C., Duros C., Artus A., Merella S., Benedicenti F., Sergi Sergi L., Sanvito F. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014;22:774–785. doi: 10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacein-Bey-Abina S., Pai S.Y., Gaspar H.B., Armant M., Berry C.C., Blanche S., Bleesing J., Blondeau J., de Boer H., Buckland K.F. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donahue R.E., Kessler S.W., Bodine D., McDonagh K., Dunbar C., Goodman S., Agricola B., Byrne E., Raffeld M., Moen R. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidance for Industry. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research (CBER) Supplemental guidance on testing for replication-competent retrovirus in retroviral vector-based gene therapy products and during follow-up of patients in clinical trials using retroviral vectors. Hum. Gene Ther. 2001;12:315–320. doi: 10.1089/10430340150218440. [DOI] [PubMed] [Google Scholar]
- 11.Cornetta K., Yao J., Jasti A., Koop S., Douglas M., Hsu D., Couture L.A., Hawkins T., Duffy L. Replication-competent lentivirus analysis of clinical grade vector products. Mol. Ther. 2011;19:557–566. doi: 10.1038/mt.2010.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sastry L., Cornetta K. Detection of replication competent retrovirus and lentivirus. Methods Mol. Biol. 2009;506:243–263. doi: 10.1007/978-1-59745-409-4_17. [DOI] [PubMed] [Google Scholar]
- 13.Bear A.S., Morgan R.A., Cornetta K., June C.H., Binder-Scholl G., Dudley M.E., Feldman S.A., Rosenberg S.A., Shurtleff S.A., Rooney C.M. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol. Ther. 2012;20:246–249. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGarrity G.J., Hoyah G., Winemiller A., Andre K., Stein D., Blick G., Greenberg R.N., Kinder C., Zolopa A., Binder-Scholl G. Patient monitoring and follow-up in lentiviral clinical trials. J. Gene Med. 2013;15:78–82. doi: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]
- 15.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 16.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 17.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D. Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 18.Ott M.G., Schmidt M., Schwarzwaelder K., Stein S., Siler U., Koehl U., Glimm H., Kühlcke K., Schilz A., Kunkel H. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 19.Stein S., Ott M.G., Schultze-Strasser S., Jauch A., Burwinkel B., Kinner A., Schmidt M., Krämer A., Schwäble J., Glimm H. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. (2006). Guidance for industry: gene therapy clinical trials – observing subjects for delayed adverse events. Guidance from the Center for Biologics Evaluation and Research. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm078719.pdf.
- 21.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 22.Zufferey R., Nagy D., Mandel R.J., Naldini L., Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 23.Zychlinski D., Schambach A., Modlich U., Maetzig T., Meyer J., Grassman E., Mishra A., Baum C. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- 24.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hock R.A., Miller A.D., Osborne W.R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989;74:876–881. [PubMed] [Google Scholar]
- 26.Sastry L., Xu Y., Cooper R., Pollok K., Cornetta K. Evaluation of plasmid DNA removal from lentiviral vectors by benzonase treatment. Hum. Gene Ther. 2004;15:221–226. doi: 10.1089/104303404772680029. [DOI] [PubMed] [Google Scholar]
- 27.Escarpe P., Zayek N., Chin P., Borellini F., Zufferey R., Veres G., Kiermer V. Development of a sensitive assay for detection of replication-competent recombinant lentivirus in large-scale HIV-based vector preparations. Mol. Ther. 2003;8:332–341. doi: 10.1016/s1525-0016(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 28.Otto E., Jones-Trower A., Vanin E.F., Stambaugh K., Mueller S.N., Anderson W.F., McGarrity G.J. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum. Gene Ther. 1994;5:567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- 29.Mohanlal R., Qiu Y., Zheng M., Mirkou A., Sridharan K., Keir C. Long-Term Safety Follow-Up of Subjects Previously Treated with Non-Replicating Retroviral Vector-Based Gene Therapies. Mol. Diagn. Ther. 2016;20:591–602. doi: 10.1007/s40291-016-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hege K.M., Bergsland E.K., Fisher G.A., Nemunaitis J.J., Warren R.S., McArthur J.G., Lin A.A., Schlom J., June C.H., Sherwin S.A. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J. Immunother. Cancer. 2017;5:22. doi: 10.1186/s40425-017-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis R.E., Boice J.D., Jr., Stovall M., Bernstein L., Greenberg R.S., Flannery J.T., Schwartz A.G., Weyer P., Moloney W.C., Hoover R.N. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N. Engl. J. Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 33.Kaldor J.M., Day N.E., Pettersson F., Clarke E.A., Pedersen D., Mehnert W., Bell J., Høst H., Prior P., Karjalainen S. Leukemia following chemotherapy for ovarian cancer. N. Engl. J. Med. 1990;322:1–6. doi: 10.1056/NEJM199001043220101. [DOI] [PubMed] [Google Scholar]
- 34.Travis L.B., Holowaty E.J., Bergfeldt K., Lynch C.F., Kohler B.A., Wiklund T., Curtis R.E., Hall P., Andersson M., Pukkala E. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N. Engl. J. Med. 1999;340:351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 35.Aiuti A., Vai S., Mortellaro A., Casorati G., Ficara F., Andolfi G., Ferrari G., Tabucchi A., Carlucci F., Ochs H.D. Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement. Nat. Med. 2002;8:423–425. doi: 10.1038/nm0502-423. [DOI] [PubMed] [Google Scholar]
- 36.Wang G.P., Levine B.L., Binder G.K., Berry C.C., Malani N., McGarrity G., Tebas P., June C.H., Bushman F.D. Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells. Mol. Ther. 2009;17:844–850. doi: 10.1038/mt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newrzela S., Cornils K., Li Z., Baum C., Brugman M.H., Hartmann M., Meyer J., Hartmann S., Hansmann M.L., Fehse B., von Laer D. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 38.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aiuti A., Cossu G., de Felipe P., Galli M.C., Narayanan G., Renner M., Stahlbom A., Schneider C.K., Voltz-Girolt C. The committee for advanced therapies’ of the European Medicines Agency reflection paper on management of clinical risks deriving from insertional mutagenesis. Hum. Gene Ther. Clin. Dev. 2013;24:47–54. doi: 10.1089/humc.2013.119. [DOI] [PubMed] [Google Scholar]
- 40.Mitsuyasu R.T., Anton P.A., Deeks S.G., Scadden D.T., Connick E., Downs M.T., Bakker A., Roberts M.R., June C.H., Jalali S. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]