Abstract
Triple negative breast cancer (TNBC), which constitutes 10%–20% of all breast cancers, is associated with aggressive progression, a high rate of metastasis, and poor prognosis. The treatment of patients with TNBC remains a great clinical challenge. Preclinical reports support the combination immunotherapy of cancer vaccines and immune checkpoint blockades in non-immunogenic tumors. In this study, we constructed nanoparticles (NPs) to deliver an mRNA vaccine encoding tumor antigen MUC1 to dendritic cells (DCs) in lymph nodes to activate and expand tumor-specific T cells. An anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) monoclonal antibody was combined with the mRNA vaccine to enhance the anti-tumor benefits. In vivo studies demonstrated that the NP-based mRNA vaccine, targeted to mannose receptors on DCs, could successfully express tumor antigen in the DCs of the lymph node; that the NP vaccine could induce a strong, antigen-specific, in vivo cytotoxic T lymphocyte response against TNBC 4T1 cells; and that combination immunotherapy of the vaccine and anti-CTLA-4 monoclonal antibody could significantly enhance anti-tumor immune response compared to the vaccine or monoclonal antibody alone. These data support both the NP as a carrier for delivery of mRNA vaccine and a potential combination immunotherapy of the NP-based mRNA vaccine and the CTLA-4 inhibitor for TNBC.
Keywords: mRNA nano-vaccine, CTLA-4 blockade, triple negative breast cancer
Graphical Abstract

Nanoparticle (NP)-based MUC1 mRNA vaccine could induce a strong antigen-specific immune response against TNBC, and combined treatment with anti-CTLA-4 monoclonal antibody could significantly enhance the immune response of the vaccine, indicating the NP as a carrier for delivery of the mRNA vaccine and a potential combination immunotherapy of the vaccine and CTLA-4 inhibitor.
Introduction
Breast cancer is the most common cancer among women around the world, and 30%–40% of breast cancer cases will progress to metastatic disease.1 Triple negative breast cancer (TNBC)—defined by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression—is associated with aggressive growth, a high rate of metastasis, and the poorest prognosis of all breast cancer sub-types.2, 3, 4 Due to the lack of therapeutic targets, TNBC is unresponsive to typical endocrine therapies and HER2-targeted therapy.2 The treatment of patients with TNBC remains a great clinical challenge. Cancer vaccines based on tumor-associated antigens represent an attractive therapeutic strategy. They can induce a specific immune response toward the tumor and establish long-term immune memory, reducing the likelihood of toxic side effects and preventing tumor recurrence.5
Major histocompatibility complex (MHC)-I-restricted cytotoxic T lymphocytes (CTLs) are important for eradicating the growth of tumor cells and preventing the recurrence of cancer.6 T lymphocyte activation requires distinct signals from an antigen-presenting cell. Recognition of the antigen-MHC complex by the T cell antigen receptor is insufficient for activation of naive T cells. Additional costimulatory signals are required, which are provided by the engagement of CD28 on the T cell surface with B7 molecules (CD80 and CD86) on the antigen-presenting cell.7 T cell activation also induces an inhibitory pathway that could eventually attenuate and terminate T cell responses. Expression of CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), which has very high homology to CD28, is induced after activation of T cells. Just like CD28, CTLA-4 binds B7 molecules at the T cell-APC interface to block costimulation and abrogate the activated T cell response.7 Antibodies that restore T cell responses against tumors represent a rapidly emerging anti-cancer strategy. The US Food and Drug Administration (FDA) approved an antibody against CTLA-4, ipilimumab, in 2011 for the treatment of melanoma.8 Ipilimumab has been successfully combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) cell-based vaccines for pancreatic cancer treatment, with the potential for clinical benefit.9
MUC1 is a heavily glycosylated type 1 transmembrane mucin, which was ranked second of the 75 tumor-associated antigens by the National Cancer Institute.10 A large number of carcinomas of the breast, ovary, colon, rectum, pancreas, and prostate exhibit striking overexpression of MUC1.11 In recent years, MUC1 has been utilized in immunotherapeutic approaches for the development of peptide-, carbohydrate-, DNA-, and dendritic cell (DC)-based vaccines.12 RNA-based vaccines have only recently received attention, but they may offer certain advantages over peptide, carbohydrate, and DNA vaccines. As a vaccine modality, RNA has two major advantages over DNA.13, 14 First, RNA needs only to be delivered into the cytoplasm of the host cell to be translated into protein, whereas DNA must enter the nucleus to be transcribed into mRNA, which is then transported into the cytoplasm for translation.13 In fact, mRNA can mediate higher levels of protein expression in vivo when compared with DNA, and in a shorter time frame.14 Second, using RNA obviates the potential integration of foreign DNA into the host genome, which may induce mutations. Moreover, gene expression via mRNA is relatively transient, and is therefore safer to use when compared with DNA.14 An mRNA vaccine is also more suitable than a peptide vaccine. Peptide antigens usually contain only one epitope, whereas mRNA vaccines can express several epitopes from the same sequence. Carbohydrate vaccines induce antibodies against cancer,15 whereas mRNA vaccines trigger T cell immune responses.16 Despite many advantages, it is difficult to administer mRNA in vivo, mainly because of its instability under physiological conditions and difficulty for delivering into the cytoplasm of DCs. Improvements in the stability, durability, and expression levels of mRNA-based vaccines have been achieved through nanoparticle (NP) delivery.17
Lipid/calcium/phosphate (LCP) NPs were previously developed in our laboratory as a new class of intracellular delivery systems for impermeable drugs.6, 18 The sub-cellular distribution indicated that LCP could efficiently release their cargo into the cytoplasm,6, 18 which would be beneficial for the expression of delivered mRNA encoding MUC1. The surface of LCP can be modified with mannose, a ligand for mannose receptors that is expressed on DCs, which promotes delivery of an exogenous antigen/mRNA to the cytosol of DCs to induce an MHC-I-restricted CTL response.19 LCP, as a peptide vaccine delivery system, has been successfully applied to the delivery of tyrosinase-related protein 2 (Trp 2) peptide for melanoma treatment.6 In this study, an LCP modified with mannose was employed to deliver mRNA encoding MUC1 to DCs in the lymph nodes. Therapeutic efficiency after vaccination with the mRNA-loaded NP was evaluated in an orthotopic TNBC model. The anti-CTLA-4 monoclonal antibody was combined with the mRNA vaccine to enhance the anti-tumor immune response by targeting regulatory pathways in T cells.
Results
Characterization of LCP NPs Containing mRNA Encoding MUC1 and HA Tag
MUC1 is a transmembrane glycoprotein normally expressed on the apical surface of ductal epithelia.11 In order to differentiate between exogenously and endogenously expressed MUC1 in tumor cells and normal tissue, a hemagglutinin (HA) tag-encoding sequence was designed at the 3′ terminal of MUC1 mRNA. The Kozak consensus sequence plays a major role in the initiation of the translation process. The untranslated region (UTR), including the Kozak sequence (GCCACC), was designed at the 5′ terminal of MUC1 mRNA. RNA-encoding MUC1 and the HA tag were synthesized by the in vitro transcription system. To impart desirable mRNA characteristics, such as increased stability against nucleases, increased translation, or reduced innate immune stimulation, modified ribonucleotides were used for synthesis of RNA.20, 21
Our previous studies have shown that LCP can efficiently encapsulate nucleic acids and peptides,6, 18, 22 which could be applied to the mRNA vaccine in this study. The mRNA-loaded LCP was prepared in a water-in-oil micro-emulsion. The high PEG density on the LCP surface significantly increased the in vivo colloidal stability of the NP and thus improved the pharmacokinetic and pharmacodynamics profiles of the therapeutics.23 The surface of LCP was modified with mannose to target the mannose receptor that is highly expressed on DCs.24 The encapsulation efficiency of mRNA into LCP was about 50%. CaP cores and final LCP were about 15 nm and 25 nm in diameter, respectively, as determined by transmission electron microscopy (TEM) (Figures 1C and 1D), whereas the hydrodynamic size of LCP was 58 nm in diameter, as determined by dynamic light scattering, with a surface charge of 38 mV (Figures 1A and 1B).
Figure 1.
Characterization of mRNA-Loaded LCP
(A) Dynamic light scattering (DLS) size of LCP. (B) Zeta potential of LCP. (C) TEM images of LCP cores. (D) Final NPs encapsulating mRNA-encoding MUC1. Three curves in Figure 1B showed three measurements of the same sample.
Expression of MUC1 Fusion Protein with HA Tag in 4T1 Cell Line and Lymph Node
In order to distinguish the exogenous from endogenous expression of MUC1, we have synthesized an HA-tagged MUC1 gene. The recombinant plasmid and transcribed mRNA encoding MUC1 and HA tag were respectively transfected into the 4T1 cell line. Expression of the MUC1 fusion protein was detected by western blot assay with a peroxidase-labeled anti-HA antibody. The result showed that the HA tag was expressed in 4T1 cells after transfection. Because the HA tag was co-expressed with MUC1 and the HA tag was located downstream of MUC1, western blot analysis indicated that exogenous MUC1 was successfully expressed in 4T1 cells (Figure 2A). Draining lymph nodes were harvested from mice immunized with LCP loaded with mRNA encoding MUC1 fusion protein on day 7 after vaccination. Western blot analysis detected HA-tagged MUC1, indicating that LCP could release mRNA in the lymph nodes and mRNA was correctly translated into the target protein (Figure 2B).
Figure 2.
Expression of HA-Tagged MUC1 Fusion Protein in the 4T1 Cell Line and Lymph Nodes
(A) Expression of MUC1 fusion protein in cells detected by western blot assay. (B) Expression of MUC1 fusion protein detected by western blot analysis in lymph nodes from immunized mice, with LCP loaded with mRNA-encoding MUC1 fusion protein. UN, untreated; 1 and 2, two mice immunized with mRNA-loaded LCP NPs.
In Vivo CTL Assay
To assess the NP-based mRNA vaccine’s ability to activate CTLs, an in vivo CTL assay was performed. In many approaches, mRNA encoding for tumor-associated antigens is applied to induce specific CTL responses.25, 26 In the in vivo CTL and ELISPOT assays, MUC1 peptide was typically used for the evaluation of MUC1-specific response. But the potential MHC class I binding epitopes derived from MUC1 are mostly associated with H2b molecules,27, 28 and the binding epitopes to H2d MHC class I have hardly been reported in previous studies. Therefore, MUC1 was analyzed for predicted binding epitopes to H2d MHC class I molecules using algorithms available online.29, 30 MUC1 peptide, including a highly scored MHC class I epitope based on in silico analysis, and OVA peptide were synthesized and then evaluated for an in vivo MUC1-specific CTL response and interferon-γ (IFN-γ) production, but the MUC1 peptide could not work in these assays (Figure S3). For this purpose, MUC1 mRNA was transfected into cells for production of sufficient recombinant MUC1 protein to pulse the splenocytes. The lysate of the transfected cells provided a sufficient amount of antigens for splenocyte loading. Splenocytes from naive mice were pulsed with 4T1 cell lysates transfected with MUC1 mRNA and untreated CT26 (colorectal cancer) cell lysates, and then the transfected cell lysate-pulsed cells and CT26 cell lysate-pulsed cells were labeled with high and low concentrations of carboxyfluorescein succinimidyl ester (CFSE), respectively. The mice were intravenously injected with a mixture containing equal amounts of CFSEhigh (transfected 4T1 cell lysate-pulsed cells) and CFSElow (CT26 cell lysate-pulsed cells) 7 days after immunization with empty LCP NP, naked mRNA, PBS, or mRNA-loaded LCP NP. MUC1-specific lysis was analyzed using flow cytometry 18 hr after injection. As seen in Figure 3, the mice immunized with either PBS or empty LCP NP could not generate any detectable MUC1-specific CTL response, mice receiving naked mRNA exhibited a weak MUC1-specific CTL killing compared to the LCP-mRNA-treated group, and mice treated with LCP-based mRNA vaccine could induce the strongest CTL response. The result demonstrated that the killing efficiency of antigen-specific CD8+ cells was elicited by immunization with target antigen MUC1.
Figure 3.
In Vivo CTL Response after Vaccination
(A) In vivo CTL response after different treatments. Splenocytes from naive mice were pulsed with 4T1 cell lysates transfected with MUC1 mRNA and CT26 cell lysates. The transfected cell lysate-pulsed cells and CT26 cell lysate-pulsed cells were labeled with high and low concentrations of CFSE, respectively, and injected intravenously into the 4 groups of mice. After 18 hr, splenocytes were separated from the spleens of treated mice and subjected to flow cytometry analysis. (B) The specific lysis activity of CTL that was calculated using the equation described in the Materials and Methods section. Data are shown as mean ± SD. *p < 0.05; n = 3.
Production of IFN-γ by Lymphocytes from Vaccinated Mice
IFN-γ production induced by the tumor antigen was measured with a BD ELISPOT assay system. Spleens were sterilely harvested from each treated mouse at 7 days post immunization and dissociated into single cell suspensions. Cells were stimulated with either 2 μg/μL cell lysates transfected with MUC1 mRNA or CT26 cell lysates as a control. Production of IFN-γ was detected using a BD ELISPOT substrate set. Positive responses were manually enumerated. As shown in Figure 4, splenic cells isolated from mice in the PBS group and the empty LCP group generated low levels of IFN-γ, and the highest IFN-γ production was observed in the LCP-mRNA-treated group. The group immunized with naked mRNA stimulated modest IFN-γ secretion. The data indicated that only MUC1-specific induction produced IFN-γ. Again, neither the in silico generated MUC1 peptide nor the lysate of naive 4T1 cells could induce the IFN-γ production.
Figure 4.
Production of Mouse IFN-γ Detected by ELISPOT Assay
(A) Representative images of the IFN-γ ELISPOT assay. Spleens were harvested from each treated mouse for 7 days, and single cell suspensions were stimulated for 18 hr with either cell lysates transfected with MUC1 mRNA or with CT26 cell lysates as a control. Production of IFN-γ was detected using the BD ELISPOT substrate set. (B) Quantitation of the ELISPOT assay. Data are shown as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001; n = 3.
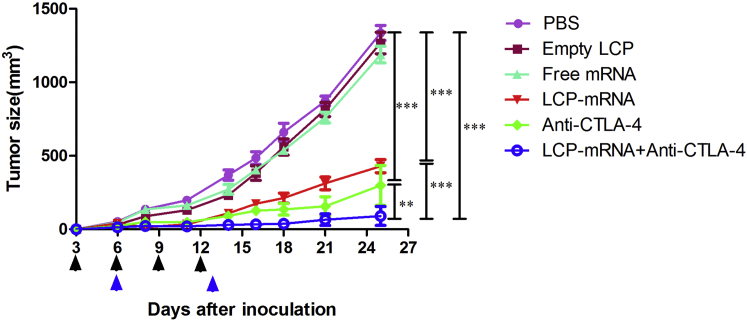
Combined Therapy with Anti-CTLA-4 Monoclonal Antibody and Vaccine Improved Inhibition of Tumor Growth
MUC1 is a widely used target for antitumor immunotherapy.31 However, a NP-based RNA vaccine containing MUC1-encoding mRNA in the central core and an asymmetric lipid outer membrane for TNBC therapy is a novel approach. An in vivo CTL assay and an IFN-γ production assay demonstrated that a MUC1 vaccine could induce antigen-specific antitumor immunity. However, T cell activation through the T cell receptor and CD28 leads to increased expression of CTLA-4, an inhibitory receptor for B7, and eventually the inhibitory pathway attenuates and terminates T cell responses.32 In order to enhance T cell function and improve the vaccine’s efficacy, the NP-based mRNA vaccine was combined with anti-CTLA-4 monoclonal antibody for TNBC therapy. As is shown in Figure 5, both the MUC1 vaccine group (p < 0.001) and anti-CTLA-4 antibody group (p < 0.001) showed strong antitumor activity. The blockade of CTLA with antibody further potentiates the efficacy of the MUC1 vaccine, which showed a superior inhibitory effect when compared with the vaccine group (p < 0.001) and the anti-CTLA-4 antibody alone group (p < 0.01). The result suggested that the MUC1 vaccine could induce an antitumor immune response and that therapeutic effects of the vaccine on TNBC were greatly enhanced by additional treatment with anti-CTLA-4 monoclonal antibody. It is noteworthy that the endogenous expression level of MUC1 on 4T1 cells successfully induced an antigen-specific T cell response, which resulted in tumor growth inhibition, even though the lysate of naive 4T1 cells failed to pulse splenocytes for antigen presentation in the in vivo CTL assay or induce the production of IFN-γ in vitro. The amount of MUC1 protein in the 4T1 cell lysates was apparently not sufficient for antigen loading and induction of IFN-γ production.
Figure 5.
Tumor Growth Inhibition Experiment
Female BALB/c mice received 1 × 105 4T1 tumor cells in the mammary fat pad on day 0. The LCP vaccine with mRNA, empty LCP, naked mRNA, and PBS were subcutaneously injected into the contralateral side of the lower flank on days 6 and 13 (blue arrows), respectively. Tumor-bearing mice were intraperitoneally injected with anti-CTLA-4 antibody on days 3, 6, 9, and 12 (black arrows). For combination therapy, mice received both the mRNA vaccine and repeated injections of the anti-CTLA-4 antibody. Tumor size was measured every 2 to 3 days for 25 days. Data are shown as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001; n = 6–10.
Tumor-Infiltrating Lymphocytes Analyzed by Flow Cytometry Assay
When an antitumor immune response is raised, a subset of CD8+ antitumor T cells infiltrates tumors.33 These tumor-infiltrating lymphocytes were analyzed by flow cytometry. As shown in Figure 6, the vaccine group, monoclonal antibody group, and combination group all induced a significant increase in tumor-infiltrating CD8+ T cells when compared to the PBS, naked mRNA, empty LCP, and isotype control groups. The combination group induced significantly more tumor-infiltrating CD8+ T cells than the vaccine and monoclonal antibody groups. The result showed that while treatment with either MUC1 vaccine or anti-CTLA-4 monoclonal antibody could increase the number of tumor-infiltrating CD8+ T cells, combination therapy was most effective at promoting the infiltration of CD8+ T cells compared to single treatments.
Figure 6.
Flow Cytometry Analysis of CD8+ T Cells in Tumors
At the endpoint of the tumor growth inhibition study (day 25), tumor tissues were harvested and digested for the flow cytometry assay. Data are shown as mean ± SD. *p < 0.05; **p < 0.01; ns, no significant difference; n = 3.
Toxicity Studies
Mice were sacrificed at the end of treatment and organs and blood were removed for analysis. Serum markers for kidney and liver toxicity were measured (alanine aminotransferase [ALT], aspartate aminotransferase [AST], blood urea nitrogen [BUN], and creatinine). White blood cells (WBCs), red blood cells (RBCs), platelets (PLTs), hemoglobin (HGB), hematocrit (HCT), and mean cell volume (MCV) were counted for the detection of myelosuppression. H&E staining of the heart, liver, spleen, lung, and kidneys showed that there were no noticeable morphological changes in any of the 6 groups (Figure 7). Serum markers for kidney and liver toxicity remained in the normal range after treatment (Table 1). The combination therapy with the MUC1 vaccine and anti-CTLA-4 monoclonal antibody significantly reduced the levels of WBCs inside the normal range compared to the PBS group (Table 2). Because of treatment, anti-CTLA-4 monoclonal antibody, free mRNA, and empty LCP all reduced platelet count below the normal range, and the combination group showed a slight increase in hemoglobin above the normal range (Table 2).
Figure 7.
Toxicity Study of Organs of the Treated Mice
Mice were sacrificed at the end of treatment and organs were dissected. Morphologies of the heart, liver, spleen, lung, and kidneys after H&E staining were compared to evaluate organ-specific toxicity. Scale bar, 50 μm.
Table 1.
Serum Protein Values after Treatments
| Samples | BUN (mg/dL) | Creatinine (mg/dL) | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|
| PBS | 15 ± 1 | 0.2 ± 0 | 144.3 ± 15 | 37.7 ± 1.5 |
| Empty LCP | 14.67 ± 0.58 | 0.10 ± 0 | 114.00 ± 7 | 26.67 ± 3.51 |
| Free mRNA | 16.33 ± 1.53 | 0.13 ± 0.06 | 170.33 ± 53.05 | 30.00 ± 4.36 |
| LCP mRNA | 15.3 ± 3.5 | 0.2 ± 0 | 149.7 ± 20.6 | 39 ± 3.5 |
| Anti-CTLA-4 | 14.3 ± 2.3 | 0.2 ± 0 | 130 ± 22.6 | 37 ± 1 |
| LCP-mRNA+ anti-CTLA-4 | 18.7 ± 5.5 | 0.2 ± 0 | 170.7 ± 47.1 | 42.7 ± 2.3 |
| Normal range | 8–33 | 0.1–0.9 | 54–298 | 17–77 |
Table 2.
Whole Blood Analysis after Treatments
| Samples | WBC (103/μL) | HCT (%) | MCV (fL) | RBC (106/μL) | HGB (g/dL) | PLT (103/μL) |
|---|---|---|---|---|---|---|
| PBS | 33 ± 19.3 | 56.4 ± 4.7 | 58.2 ± 0.9 | 9.7 ± 0.8 | 16 ± 0.9 | 916.7 ± 231.4 |
| Empty LCP | 26.5 ± 19.8 | 41.5 ± 6.2 | 47.5 ± 1.5 | 8.72 ± 1.0 | 15 ± 1.7 | 571.7 ± 76.9 |
| Free mRNA | 32 ± 21.0 | 52.4 ± 11.1 | 53.1 ± 5.4 | 9.89 ± 2.0 | 16.3 ± 3.0 | 637.3 ± 54.4 |
| LCP mRNA | 19.8 ± 5.8 | 55.6 ± 3.6 | 55.1 ± 0.9 | 10 ± 0.6 | 16.1 ± 0.9 | 712.3 ± 118.5 |
| Anti-CTLA-4 | 37.5 ± 5.3 | 56.7 ± 3.6 | 57 ± 0.9 | 9.9 ± 0.6 | 16.5 ± 0.9 | 672 ± 38 |
| LCP-mRNA+ anti-CTLA-4 | 5.9 ± 0.8 | 57.1 ± 1.4 | 54.8 ± 1.3 | 10 ± 0.3 | 17.4 ± 0.6 | 883 ± 136.5 |
| Normal range | 2.6–10.1 | 32.8–48.0 | 42.3–55.9 | 6.5–10.1 | 10.1–16.1 | 780–1,540 |
Discussion
The lack of targeted therapies and poor prognosis of patients with TNBC have greatly promoted the need to discover molecular targets and develop new therapeutic approaches.34 Immunotherapy slows TNBC progression and represents an attractive approach to treating TNBC.35 In this study, LCP was used as a carrier and adjuvant system for the development of mRNA vaccine encoding tumor-associated antigen MUC1. In addition, the vaccine was combined with anti-CTLA-4 monoclonal antibody to enhance the immune response of the vaccine against TNBC through improvement of T cell function. In vivo studies demonstrated that the NP-based mRNA vaccine, modified with a mannose targeting DC surface receptor, could drain into the lymph node, and the encapsulated mRNA could be successfully expressed in DCs of the lymph node. In addition, in vivo studies demonstrated the NP vaccine could induce a strong, antigen-specific CTL response against 4T1 TNBC cells, and that combined treatment with an anti-CTLA-4 monoclonal antibody could significantly enhance the anti-tumor immune response compared to the vaccine or monoclonal antibody alone.
The use of tumor-specific mRNA as a vaccine is a focus of current research and shows several advantages, including feasibility, applicability, safety, and efficacy, in generating immune responses.16, 36, 37 A variety of NPs have been investigated for effective gene delivery in vivo, including lipid-based and polymer-based NPs, in which the negatively charged nucleic acid molecules are encapsulated within the hydrophilic core or adsorbed on the cationic surface.38, 39 LCP, which consists of a calcium phosphate core and an asymmetrical lipid bilayer, was first developed in our laboratory and has been applied to the delivery of small interfering RNA (siRNA) and peptides.6, 18, 22 When MUC1 mRNA was packaged into LCP, RNA molecules were condensed and encapsulated into the calcium phosphate core. An in vivo delivery study indicated that LCPs could successfully deliver and release MUC1 mRNA into the cytoplasm of targeted cells in lymph nodes (Figure 2B). In the present study, the NP-based mRNA vaccine was first developed for TNBC treatment. It meant that LCP is also a powerful system for more tumor-specific RNA delivery.
Many MUC1-based immunotherapies have been reported in clinical studies.40 There are two MUC1-based therapeutic vaccines in clinical development, and these vaccines are long non-glycosylated polypeptides derived from the variable number tandem repeats (VNTR) sequence of MUC1.41, 42 Most potential CTL epitopes on the MUC1 molecule lie outside of the VNTR;40 therefore, it is important to incorporate the entire MUC1 molecule into therapeutic vaccines. Two such vaccines utilize poxviruses as vectors for the MUC1 gene sequence, but improved DC uptake and T cell recruitment are deemed necessary.40 MUC1 is abnormally overexpressed on a variety of epithelial tumors.43 It has also been shown to be expressed on the epithelial cells of several healthy tissues, acting as a protective lubricant of bacterial infections.44 Our western blot analysis showed that MUC1 was highly expressed on a series of tumor cell lines, NIH/3T3 and the murine immature DC line JAWSII (Figure S1). Overexpressed MUC1 is hypo-glycosylated and not restricted to the cell surface.40
Tumor-infiltrating cytotoxic T cells can be inhibited by the CTLA-4 co-inhibitory signal.45 Antibody blockade of CTLA-4 has been shown to enhance antitumor immune responses in both murine preclinical models and clinical trials.46, 47 Tumor inhibition was significantly more effective when vaccination with MUC1 mRNA-loaded LCP was combined with antibody blockade of CTLA-4 (Figure 5). Enhanced T cell infiltration of tumors was observed following simultaneous blockade of the CTLA-4 pathway (Figure 6). CTLA-4 functions by binding to phosphatase PP2A, leading to the inhibition of Akt phosphorylation and ultimately the inhibition of T cell activation.48 The antibody targeting CTLA-4 appears to restore anti-tumor immunity in the priming phase of the immune response.49 Blocking CTLA-4 leads to Akt phosphorylation, which likely promotes effective T cell activation and ultimately an increased therapeutic effect when combined with the vaccine.32 Various cancer vaccines combined with inhibitory pathway blockade have been validated in preclinical models, and enhanced T cell infiltration of various tumors has been demonstrated following this combination therapy.50
Conclusions
The MUC1-based mRNA vaccine induces a potent CTL response against TNBC. The combination of an mRNA vaccine with an anti-CTLA-4 monoclonal antibody can greatly enhance T cell immune response significantly better than treatment with either the mRNA vaccine or the anti-CTLA-4 monoclonal antibody alone. LCP is a powerful system for tumor-specific RNA delivery.
Materials and Methods
Reagents
Dioleoylphosphatydic acid (DOPA) and 1,2-dioleoyl-3-trimethylammonium-propane chloride salt (DOTAP) were purchased from Avanti Polar Lipids. 1,2-Distearoryl-sn-glycero-3-phosphoethanolamine-N-(methoxy[polyethyleneglycol-2000]) ammonium salt (DSPE-PEG) and DSPE-PEG-NHS were purchased from NOF. Cholesterol, cyclohexane, and IGEPAL-CO-520 were purchased from Sigma-Aldrich. Mannose-PEG-DSPE was synthesized using DSPE-PEG-NHS and 4-amino phenyl-mannopyranoside (Sigma-Aldrich, St. Louis, MO) according to the previously established protocol in our laboratory. Nucleotides (ATP and guanosine triphosphate [GTP]) were purchased from Affymetrix, and modified nucleotides (5-methylcytidine-5′-triphosphate and pseudouridine-5′-triphosphate) were purchased from Trilink Biotechnologies. 3′-O-Me-m7G (5′) ppp (5′) G RNA cap structure analog was purchased from NEB. All other chemicals were purchased from Sigma-Aldrich if not specifically mentioned.
Cell Line, Mice, and Antibodies
The TNBC 4T1 cell line, which is derived from a spontaneous mammary carcinoma in a BALB/c mouse, was obtained from ATCC and cultured in a Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). 6- to 8-week old female BALB/c mice were obtained from Charles River Laboratories. Animals were raised in the Center for Experimental Animals (an AAALAC-accredited experimental animal facility) at the University of North Carolina (UNC) at Chapel Hill. All animal handling procedures were approved by the Institutional Animal Care and Use Committee at UNC at Chapel Hill. Anti-CTLA-4 (9D9) and mouse immunoglobulin G2b (IgG2b) isotype control used in vivo were obtained from Bio X Cell. Dosing per injection was 100 μg 9D9 and 100 μg mIgG2b. Primary antibodies used for western blot analysis included glyceraldehyde 3-phosphate dehydrogenase and anti-HA-peroxidase (Sigma-Aldrich). Fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD8α and FITC-conjugated κ isotype control were obtained from BD Biosciences. Rabbit anti-mouse IgG-horseradish peroxidase, used for western blot analysis, was purchased from Santa Cruz Biotechnology.
Expression of MUC1 Fusion Protein with HA Tag
Total RNA was extracted from the 4T1 cell line using an RNeasy kit (QIAGEN). The cDNA was reverse transcribed using the First-Strand Synthesis System for PCR. The whole MUC1 gene was amplified from cDNA by PCR with specific oligonucleotide primers. The PCR products were cloned into the Hind III and EcoR V sites of the eukaryotic expression vector pcDNA3.1 (+) (Invitrogen). The open reading frame of the MUC1 gene was flanked by the Kozak consensus sequence in the 5′ of the UTR. Recombinant plasmid pcDNA3.1-MUC1 was used as a PCR template to amplify the whole MUC1 gene and HA tag-encoding sequence. The HA tag-encoding sequence was designed in the reverse primer. Then, the PCR product was cloned into plasmid pcDNA3.1 (+). Subsequently, the recombinant plasmid was transfected into the 4T1 cell line with Lipofectamine 2000 reagent (Invitrogen).
In Vitro Transcription and Transfection of mRNA Encoding MUC1 and HA Tag
The template for in vitro transcription was prepared by PCR amplification, followed by agarose gel electrophoresis purification. RNA was synthesized in vitro by using a MEGAscript T7 Transcription kit (Ambion). 1.6 μg amplicon was incubated in 40 μL total volume at 37°C for 4 hr with 4 μL reaction buffer (10 × ), 4.8 μL 3′-O-Me-m7G(5′)ppp(5′)G RNA Cap Structure Analog (50 mM), 3 μL 5-methylcytidine-5′-triphosphate (100 mM), 3 μL pseudouridine-5′-triphosphate (100 mM), 0.6 μL GTP (100 mM), 3 μL ATP (100 mM), and 4 μL T7 Enzyme Mix. The obtained RNA was polyadenylated using a Poly(A) Tailing kit (Ambion) and then purified with a MEGAclear Transcription Clean-Up kit (Ambion). The purified mRNA encoding MUC1 and HA tag fusion protein was transfected into the 4T1 cell line using a TransIT-mRNA transfection kit (Mirus Bio) according to the manufacturer’s instructions.
Preparation of LCP-Based mRNA Vaccine NP
The mRNA-loaded LCP was prepared in a water-in-oil microemulsion. Briefly, 600 μL 2.5 M CaCl2 and 50 μg mRNA were dispersed in 20 mL cyclohexane/Igepal CO-520 (71:29, V:V) oil phase to form the calcium phase. At the same time, 600 μL 12.5 mM Na2HPO4 (pH = 9.0) was dispersed in a separate 20-mL oil phase. After separate stirring at room temperature for 5 min, the two oil phases were mixed and stirred at room temperature for 20 min. Subsequently, 400 μL 20 mM DOPA was added. After the microemulsion was stirred for another 15 min, 40 mL ethanol was added to precipitate the calcium phosphate cores. The calcium phosphate cores with encapsulated mRNA were collected using centrifugation at 10,000 × g for 20 min and washed with 40 mL ethanol. The pellets were dissolved in chloroform. For final LCP preparation, the cores were mixed with 140 μL 20 mM DOTAP, 140 μL 20 mM cholesterol, 100 μL 20 mM DSPE-PEG-2000, and 80 μL 5 mM DSPE-PEG-mannose. After chloroform evaporation, the LCP was rehydrated in 250 μL of a 5% glucose solution. Both the calcium phosphate cores and the final LCP were observed using TEM (JEOL 100CX II TEM). Particle size and zeta potential were measured in water with a Malvern Zetasizer Nano ZS.
Expression of MUC1 Fusion Protein in Lymph Node after Vaccination
Female BALB/c mice were immunized with mRNA-loaded LCP and sacrificed 7 days later. Vaccine-draining lymph nodes were harvested from immunized mice, and the lymph node in the same position was harvested from the untreated mouse. Expression of MUC1 fusion protein was detected using western blot analysis.
Western Blot Analysis
The transfected cells and lymph nodes of mice in the control group and the immunized group were harvested, and the total proteins were extracted using radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich). After the total protein concentrations were determined using a BCA protein assay system (Thermo Fisher Scientific), the same amount of extracted proteins were separated by SDS-PAGE, transferred onto polyvinylidene fluoride membranes (Millipore), and respectively incubated with primary antibodies glyceraldehyde 3-phosphate dehydrogenase and anti-HA-peroxidase. Subsequently, the membranes were incubated in horseradish-peroxidase-conjugated secondary antibodies. Bands were visualized using the Pierce ECL Western Blotting Substrate according to the manufacturer’s instructions (Thermo Fisher Scientific).
In Vivo CTL Assay
The CTL assay was performed following the previous protocol with slight modifications.6, 51 Briefly, female BALB/c mice were immunized with empty LCP, naked mRNA, PBS, and mRNA-loaded LCP, respectively. 7 days later, splenocytes were separated from the spleens of naive BALB/c mice and immediately pulsed with either 2 μg/μL lysates of 4T1 cells transfected with MUC1 mRNA or CT26 cell (MUC1 negative; see Figure S1) lysates for 1.5 hr in a complete RPMI 1640 medium at 37°C. Both kinds of pulsed splenocytes were stained with 2 μM PKH-26 (Sigma-Aldrich) according to the manufacturer’s instructions. The transfected cell lysate-pulsed cells and CT26 cell lysate-pulsed cells were then incubated for 15 min with 4 and 0.4 μM of CFSE, respectively. Equal numbers of CFSEhigh (transfected cell lysate-pulsed cells) and CFSElow (CT26 cell lysate-pulsed cells) were mixed together and injected intravenously into the four groups of mice. After 18 hr, splenocytes were separated from the spleens of treated mice and subjected to flow cytometry analysis. The numbers of CFSEhigh and CFSElow were calculated, and the in vivo MUC1-specific lysis percentage was enumerated according to the following equation:51
Production of Mouse IFN-γ Detected by ELISPOT Assay
Female BALB/c mice were immunized with empty LCP, naked mRNA, PBS, and mRNA-loaded LCP, respectively. 7 days later, spleens were sterilely harvested from each treated mouse and separated into single cell suspensions. IFN-γ production was measured with the BD ELISPOT assay system (BD PharMingen) according to the manufacturer’s instructions. Briefly, cells were seeded at 2 × 106 per well in a capture antibody-coated 96-well plate. The single cell suspensions were then cocultured with either 2 μg/μL 4T1 cell lysates transfected with MUC1 mRNA or CT26 cell lysates at 37°C for 18 hr. Subsequently, cells were removed and the production of IFN-γ was measured by adding a detection antibody, followed by an enzyme conjugate. Red dot signals were developed using the BD ELISPOT substrate set and enumerated manually.
Tumor Growth Inhibition
Female BALB/c mice received 1 × 105 4T1 tumor cells in the mammary fat pad on day 0. The LCP vaccine with mRNA, empty LCP, naked mRNA, and PBS were subcutaneously injected into the contralateral side of the lower flank on days 6 and 13, respectively. For antibody therapy studies, tumor-bearing mice were intraperitoneally injected with either anti-CTLA-4 antibody (9D9) or, as a control, mIgG2b on days 3, 6, 9, and 12. For combination therapy, mice received both the mRNA vaccine and repeated injections of anti-CTLA-4 antibody. Tumor size was measured every 2 to 3 days using digital calipers (Thermo Fisher Scientific), and tumor volume was calculated as 0.5 × length × width2. After completion of the experiment on day 25, all mice were euthanized.
Flow Cytometry Assay
Tumor-infiltrating lymphocytes were analyzed by flow cytometry. In brief, tumor tissues were harvested and digested with collagenase A and DNase I at 37°C for 40 min. After lysis of red blood cells, cells were dispersed in 1 mL PBS. Immune lymphocytes (5 × 106 cells/mL) were stained with FITC-conjugated anti-mouse CD8α. Analysis was performed using a FACS Caliber flow cytometer and analyzed using Cell Quest software (BD Biosciences).
Toxicity Studies
The vaccinated mice, control mice, antibody-treated mice, and mice co-treated with both the vaccine and antibody were all subjected to toxicity studies. Blood and organs were dissected. Serum levels of AST, ALT, BUN, and creatinine were measured as indicators of renal and liver function. Organs, including the hearts, livers, spleens, lungs, and kidneys, were stained with H&E to evaluate organ-specific toxicity. Whole blood was measured for changes in WBC count, HCT, MCV, RBC count, HGB, and PLT.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 Software. A two-tailed t test and one-way ANOVA were performed when comparing two groups and more than two groups, respectively. Two-way ANOVA with multiple comparison was used to statistically analyze the data of the tumor growth curve. Differences were considered statistically significant if p < 0.05. Data are shown as mean ± SD.
Author Contributions
L.L. and L.H. designed the experiments. L.L., Y.W., and J.L. prepared LCP NP. L.L., L.M., and Q.L. performed in vivo studies. L.L. wrote the manuscript with the help of Q.L., S.M., and L.H., who reviewed and edited it. Q.L. designed the graphical abstract.
Conflicts of Interest
The authors declare that they have no conflicts of interest for this work.
Acknowledgments
The work was supported by NIH grants CA149363, CA151652, and CA149387. L.L. was supported by the State Scholarship Fund of China Scholarship Council(CSC) (201308525104) and the Science and Technology Foundation of Guizhou Province (LH-2015-7318, J-2016-1118). L.H. was a Senior Visiting Scholar at the State Key Laboratory of Molecular Engineering of Polymers, Fudan University, China.
Footnotes
Supplemental Information includes three figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2017.10.020.
Supplemental Information
References
- 1.Kim S.H., Castro F., Paterson Y., Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer K.R., Brown M., Cress R.D., Parise C.A., Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Ismail-Khan R., Bui M.M. A review of triple-negative breast cancer. Cancer Contr. 2010;17:173–176. doi: 10.1177/107327481001700305. [DOI] [PubMed] [Google Scholar]
- 4.Castro N.P., Fedorova-Abrams N.D., Merchant A.S., Rangel M.C., Nagaoka T., Karasawa H., Klauzinska M., Hewitt S.M., Biswas K., Sharan S.K. Cripto-1 as a novel therapeutic target for triple negative breast cancer. Oncotarget. 2015;6:11910–11929. doi: 10.18632/oncotarget.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn O.J. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z., Ramishetti S., Tseng Y.C., Guo S., Wang Y., Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Control. Release. 2013;172:259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 8.Melero I., Gaudernack G., Gerritsen W., Huber C., Parmiani G., Scholl S., Thatcher N., Wagstaff J., Zielinski C., Faulkner I. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 9.Le D.T., Lutz E., Uram J.N., Sugar E.A., Onners B., Solt S., Zheng L., Diaz L.A., Jr., Donehower R.C., Jaffee E.M. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheever M.A., Allison J.P., Ferris A.S., Finn O.J., Hastings B.M., Hecht T.T., Mellman I., Prindiville S.A., Viner J.L., Weiner L.M. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshminarayanan V., Thompson P., Wolfert M.A., Buskas T., Bradley J.M., Pathangey L.B., Madsen C.S., Cohen P.A., Gendler S.J., Boons G.J. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. USA. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machluf N., Arnon R. Therapeutic MUC1-based cancer vaccine expressed in flagella—efficacy in an aggressive model of breast cancer. World J. Vaccines. 2012;2:109–120. [Google Scholar]
- 13.Geall A.J., Mandl C.W., Ulmer J.B. RNA: the new revolution in nucleic acid vaccines. Semin. Immunol. 2013;25:152–159. doi: 10.1016/j.smim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Phua K.K., Leong K.W., Nair S.K. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J. Control. Release. 2013;166:227–233. doi: 10.1016/j.jconrel.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston P.O., Ragupathi G. Carbohydrate vaccines that induce antibodies against cancer. 2. Previous experience and future plans. Cancer Immunol. Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann A., Held S.A., Heine A., Brossart P. RNA vaccines in cancer treatment. J. Biomed. Biotechnol. 2010;2010:623687. doi: 10.1155/2010/623687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamara M.A., Nair S.K., Holl E.K. RNA-based vaccines in cancer immunotherapy. J. Immunol. Res. 2015;2015:794528. doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Yang Y., Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J. Control. Release. 2012;158:108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacken P.J., de Vries I.J., Torensma R., Figdor C.G. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 20.Kormann M.S., Hasenpusch G., Aneja M.K., Nica G., Flemmer A.W., Herber-Jonat S., Huppmann M., Mays L.E., Illenyi M., Schams A. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 21.Warren L., Ni Y., Wang J., Guo X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci. Rep. 2012;2:657. doi: 10.1038/srep00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Chen Y.C., Tseng Y.C., Mozumdar S., Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y., Huo M., Xu Z., Wang Y., Huang L. Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials. 2015;68:54–66. doi: 10.1016/j.biomaterials.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoerr I., Obst R., Rammensee H.G., Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur. J. Immunol. 2000;30:1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Weide B., Carralot J.P., Reese A., Scheel B., Eigentler T.K., Hoerr I., Rammensee H.G., Garbe C., Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 2008;31:180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee P., Pathangey L.B., Bradley J.B., Tinder T.L., Basu G.D., Akporiaye E.T., Gendler S.J. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picco G., Beatson R., Taylor-Papadimitriou J., Burchell J.M. Targeting DNGR-1 (CLEC9A) with antibody/MUC1 peptide conjugates as a vaccine for carcinomas. Eur. J. Immunol. 2014;44:1947–1955. doi: 10.1002/eji.201344076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreatta M., Nielsen M. Gapped sequence alignment using artificial neural networks: application to the MHC class I system. Bioinformatics. 2016;32:511–517. doi: 10.1093/bioinformatics/btv639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen M., Lundegaard C., Worning P., Lauemøller S.L., Lamberth K., Buus S., Brunak S., Lund O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003;12:1007–1017. doi: 10.1110/ps.0239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roulois D., Grégoire M., Fonteneau J.F. MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. BioMed Res. Int. 2013;2013:871936. doi: 10.1155/2013/871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X.Y., Zuo D., Sarkar D., Fisher P.B. Blockade of cytotoxic T-lymphocyte antigen-4 as a new therapeutic approach for advanced melanoma. Expert Opin. Pharmacother. 2011;12:2695–2706. doi: 10.1517/14656566.2011.629187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koneru M., Monu N., Schaer D., Barletta J., Frey A.B. Defective adhesion in tumor infiltrating CD8+ T cells. J. Immunol. 2006;176:6103–6111. doi: 10.4049/jimmunol.176.10.6103. [DOI] [PubMed] [Google Scholar]
- 34.Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grima D., Marshall D., Weinstein M., Wong J., Kleinman S. Immunotherapy slows TNBC progression. Cancer Discov. 2015;5:570. doi: 10.1158/2159-8290.CD-NB2015-059. [DOI] [PubMed] [Google Scholar]
- 36.Palamà I.E., Cortese B., D’Amone S., Gigli G. mRNA delivery using non-viral PCL nanoparticles. Biomater. Sci. 2015;3:144–151. doi: 10.1039/c4bm00242c. [DOI] [PubMed] [Google Scholar]
- 37.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 38.Kim I.S., Lee S.K., Park Y.M., Lee Y.B., Shin S.C., Lee K.C., Oh I.J. Physicochemical characterization of poly(L-lactic acid) and poly(D,L-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrier. Int. J. Pharm. 2005;298:255–262. doi: 10.1016/j.ijpharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Woodrow K.A., Cu Y., Booth C.J., Saucier-Sawyer J.K., Wood M.J., Saltzman W.M. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acres B., Lacoste G., Limacher J.M. Targeted immunotherapy designed to treat MUC1-expressing solid tumour. Curr. Top. Microbiol. Immunol. 2015;405:79–97. doi: 10.1007/82_2015_429. [DOI] [PubMed] [Google Scholar]
- 41.Apostolopoulos V., Pietersz G.A., Tsibanis A., Tsikkinis A., Drakaki H., Loveland B.E., Piddlesden S.J., Plebanski M., Pouniotis D.S., Alexis M.N. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835] Breast Cancer Res. 2006;8:R27. doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell E., Chow L.Q. BLP-25 liposomal vaccine: a promising potential therapy in non-small-cell lung cancer. Expert Rev. Respir. Med. 2008;2:37–45. doi: 10.1586/17476348.2.1.37. [DOI] [PubMed] [Google Scholar]
- 43.Jonckheere N., Van Seuningen I. The membrane-bound mucins: from cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 2010;92:1–11. doi: 10.1016/j.biochi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Peat N., Gendler S.J., Lalani N., Duhig T., Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–1960. [PubMed] [Google Scholar]
- 45.Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peggs K.S., Quezada S.A., Allison J.P. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol. Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 47.Okazaki T., Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 48.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., Riley J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philips G.K., Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int. Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 50.Karaki S., Anson M., Tran T., Giusti D., Blanc C., Oudard S., Tartour E. Is there still room for cancer vaccines at the era of checkpoint inhibitors. Vaccines (Basel) 2016;4:E37. doi: 10.3390/vaccines4040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byers A.M., Kemball C.C., Moser J.M., Lukacher A.E. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J. Immunol. 2003;171:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.